Abstract
In this work, a techno-economic assessment of carbon dioxide capture from limekiln flue gas of a pulp and paper mill (Mill A) and a linerboard mill (Mill B) using a Monoethanolamine (MEA) absorption desorption process was carried out. We coupled the ASPEN Plus simulator with a derivative-free optimization (DFO) tool to identify the optimal configuration for minimizing the total capture cost. The capture costs were calculated using CAPCOST, a modular program for equipment cost estimation, and appropriate coefficients. Eight degrees of freedom, the direct contact cooler stages, the absorber stages, the stripper stages, the solvent lean loading, the solvent weight concentration, the stripper inlet temperature, the flue gas inlet temperature, and the amount of CO2 captured, were selected for process and flowsheet optimization. Additionally, we evaluated the effect of steam integration and Sect. 45Q of the existing federal tax credit for carbon capture and sequestration on CO2 capture costs. The total capture costs per tonne of CO2 were $64.9 for Mill A and $69.7 for Mill B. When steam integration and Sect. 45Q are considered, the costs dropped to − $2.5 and $2.6 for Mill A and Mill B, respectively. The sensitivity of CO2 capture cost to changes in the inlet flue gas flowrate, flue gas CO2 mol%, and the electricity and MEA prices were investigated. The sensitivity analysis results revealed that the capture costs vary from − $5.9 to $5.9 per tonne of CO2 captured.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
In the United States, carbon dioxide (CO2) accounted for almost 79% of all U.S. greenhouse gas emissions in total of 5981 million metric tons from human activities in 2020 [1]. Industrial processes contributed to about 16% of total U.S. CO2 emissions, including around 1.2% from the pulp and paper industry [1, 2].
The pulp and paper sector produces pulp, paper, board, and other cellulose-based products [3], with the primary sources of CO2 emissions from the recovery boiler, biomass boiler, and limekiln [4]. The CO2 emissions from the pulp and paper industry are mainly of biogenic origin, accounting for over 80% of the total emissions. Depending on the mill type, the pulp and paper mills also utilize CO2 as raw material for bioproducts.
Scattered studies on the applications of CO2 in pulp and paper mills have been reported in the literature. Kuparinen et al. [5] state that the application of CO2 depends on the mill-specific details, chosen practices, and the type of woody raw material. Also, CO2 has been used for multiple processes, including neutral papermaking, near-neutral bleaching, brown stock washing, and effluent treatment [6,7,8,9]. As a result, the pulp and paper mills could be a site for negative CO2 emissions by utilizing the captured CO2 [5]. Most strikingly, Sect. 45Q of the Internal Revenue Code (IRC) intends to incentivize investment in carbon capture and sequestration and doesn’t distinguish between biogenic and non-biogenic sources of CO2 [10]. Therefore, the pulp and paper mills utilizing CO2 could be eligible for Sect. 45Q utilization tax credits.
Limited research has been reported on the economic feasibility of CO2 capture from the pulp and paper industry. Onarheim et al., in the first part of their work [4], showed that it is technically feasible to retrofit post-combustion CO2 capture to an existing pulp and pulp and board mill. In another study [11], Onarheim et al. performed a techno-economic analysis for CO2 capture from a pulp mill and an integrated pulp mill. For 90% CO2 capture from the limekiln flue gas, the capture cost was calculated as $91 per tonne of CO2. However, the study didn’t focus on the process and flowsheet optimization and didn’t explore the in-mill applications of CO2. Recently, Sagues et al. [12] have assessed the techno-economic aspects of MEA solvent-based CO2 capture for the US pulp and paper industry. The study also considered pathways for CO2 utilization at pulp and paper mills, considering the 45Q utilization tax credit. However, the study evaluated only lignin precipitation and calcium carbonate filling applications of CO2 and its economic impact on CO2 capture costs without considering process and flowsheet optimization.
Recently, we performed a techno-economic analysis, evaluating the CO2 capture costs from the lime kiln section of a hypothetical pulp and paper mill [13]. The amine-based absorption–desorption process was employed for capturing CO2. The study used CAPCOST modular program for calculating the capital costs, provided a basis for cost calculations, and compared the estimated costs to published CO2 capture cost data for CO2 capture from pulp and paper mill limekiln [4, 11].
The present study extends the analysis in [13] by process flowsheet optimization of capturing CO2 from the limekiln flue gas using actual mill data from two different mills and performing the techno-economic analysis using the optimum process flowsheets. To achieve this, we couple a process simulator, ASPEN Plus, with a derivative-free optimization (DFO) solver, which identifies the optimum process design and operating conditions that minimize the CO2 capture cost. Additionally, we analyze possible steam integration from within the mill and explore potential in-mill CO2 applications and their impact on the total CO2 capture costs. A sensitivity analysis is carried out to examine the changes in the CO2 capture cost with changing flue gas conditions and utility costs. Kraft pulping, ASPEN plus modeling of the MEA solvent-based process with the optimization framework, and economic analysis are introduced in the next section. The results and discussion section analyzes the results of the process flowsheet optimization using the DFO solver, the impact of steam integration on the cost analysis, the in-mill CO2 application, and sensitivity analysis. Finally, the last section states the conclusions and future directions of this study.
2 Process simulation and optimization framework
2.1 Kraft pulp and paper mill
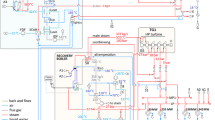
The process of reducing the fibrous raw material to a fibrous mass is called pulping. As shown in Fig. 1, wood logs are debarked and converted to chips in the mill’s wood preparation section. Along with chip fines, the wood bark is burned in the bark boiler for steam generation, in which CO2 is formed. The chips are fed to the digester for cooking, usually at about 170 °C, for up to 3 h to complete reactions. The cooking chemicals are sodium hydroxide (NaOH) and sodium sulfide (Na2S) in a water solution called white liquor. Residual liquor and the cooked pulp are then separated by brown stock washing, typically using a multi-stage counter-current washing sequence [14]. Weak black liquor leaving the brown stock washers, with a solids content between 13 and 17%, is concentrated in a multi-effect evaporator. The concentrated black liquor, with a solid content of between 60 and 80% solids, is burned in a recovery boiler for chemical and energy recovery—this burning of concentrated black liquor results in CO2 emissions from boiler flue gases. The reboiler smelt is dissolved in water to form green liquor and causticized with the reburned lime (CaO) to form white liquor used in the cooking process, completing the cycle [15].
In an integrated pulp and paper mill, the pulp is processed into the stock used for papermaking, either directly or after a bleaching process to take the pulp to the desired brightness level. The papermaking process includes beating and refining, pulp blending specific to the desired paper product, dispersion in water,, and any necessary wet additives. Then, the pulp goes to the paper machine from the headbox, and after a series of drying operations, the pulp is formed into paper [16].
For a linerboard mill, the pulp, after cooking, is refined and washed. In some cases, fibers recovered from previous corrugated boxes get added to the washed pulp’s mixer. The brown-colored pulp is then formed into a sheet by the paper machine by pressing the paper against pair of heated rolls in the calendaring section of the mill [17, 18].
Carbon dioxide emissions from the bark boiler and recovery boiler fall under biogenic CO2, as the CO2 is released from burning wood-derived fuels. The wood bark is burned in the bark boiler, and strong black liquor from the multiple-effect evaporators is burned in the recovery boiler. In the limekiln, CO2 is released during the calcination of limestone [4]. Figure 1 also identifies primary CO2 emission sources from the pulp and paper industry.
In this study, the limekiln is selected as a case for CO2 capture because the limekiln is the only source of fossil fuel-based CO2 and has the highest concentration of CO2 in the flue gases [19]. Also, it is shown earlier that the CO2 capture costs decrease as the CO2 concentration increases [20, 21].
2.2 ASPEN plus process simulation of the CO2 capture process
The absorption-based CO2 capture process that was simulated utilizes monoethanolamine (MEA) as the solvent. Two southern United States softwood-based kraft pulp mills, a pulp and paper mill (Mill A) and a linerboard mill (Mill B), provided their lime kiln flue gas process conditions and composition (Table 1). The flue gas CO2 (weak acid) is absorbed via a temperature-dependent acid–base reaction by a solvent (weak base) in the absorption column. After reacting with CO2, the “CO2 loaded” solution (rich MEA) is regenerated to reverse the reaction, thus liberating gaseous CO2 in the desorption cycle [22]. The reactions are given in Eq. 1 for primary amines, such as MEA [23].
The ASPEN Plus process simulation flow diagram for solvent-based CO2 capture is shown in Fig. 2. A direct contact cooler (DCC) is used to cool and quench the lime kiln flue gas. The cooled flue gas is fed to the bottom of the absorber column, and the lean MEA, regenerated solvent from the stripper column, enters the absorber from the top. Solvent MEA absorbs the CO2 from the flue gas via the absorption reaction in Eq. (1). This CO2-rich stream (rich MEA solution) is heated in a rich/lean heat exchanger with the lean MEA stream and sent to the stripper column. The rich MEA solution is stripped of its CO2 in the stripper column. The stripper column top product, CO2OUT stream, contains water vapor and CO2. The CO2 product is compressed to 110 bar at 33 °C via a four-stage compressor train. The lean MEA stream is mixed with make-up water and make-up amine after the rich/lean heat exchanger. This mixed lean MEA is further cooled with cooling water at an exchanger and sent back to the absorber.
The amine-based CO2 capture process using the absorption and desorption cycle is simulated in ASPEN Plus V10.0. The KEMEA (Kent-Eisenberg) data package is used to model the thermodynamic and transport properties. The package utilizes the electrolyte-NRTL model with the appropriate kinetics and rate constants, enabling the modeling of the MEA system accurately. Absorber and stripper columns are modeled using the RadFrac unit operation in ASPEN Plus. The absorber is modeled without a condenser and a reboiler, while the stripper column has both. The Rate-based model is employed to model both columns. Piping and equipment pressure drops are neglected. The stripper column operates at 1.9 bar pressure to prevent potential solvent degradation due to high pressure and bottom temperatures [24]. COMPR block is used in Aspen plus to simulate the CO2 compressors.
2.3 Optimization environment
Derivative-free optimization (DFO) is a framework for solving optimization problems that do not have an explicit mathematical expression for their objective function and/or its derivative. Instead, this framework utilizes the objective function values for sets of decision variables with an algorithm-specific search strategy to gradually improve the objective function value to locate the best set of decision variables. A general procedure for a DFO framework is given in Fig. 3. In a DFO framework, the model that is used to estimate the objective function value given a set of decision variables is treated as a black-box model. The framework begins by providing the black-box model a set, or sets, of decision variable values to obtain the corresponding objective function value(s). The set(s) of decision variable and objection function value pair(s) are then passed back to the DFO algorithm, where new set(s) of decision variable values are determined and passed back to the black-box model for evaluation. This process is repeated until a termination criterion is met. There are different termination criteria; however, one of the most commonly used is the maximum number of black-box model evaluations [25]. DFO is a natural choice for process flowsheet optimization because it can be coupled with the ASPEN Plus process simulation of the amine-based CO2 capture from the limekiln flue gas and the ECON software for locating the minimum capture cost by adjusting the decision variable values.
For this work, the objective function of the multivariant optimization problem to be minimized within the DFO framework is the CO2 capture cost given by Eq. 4. The decision variables determined by the DFO algorithm for minimizing the CO2 capture cost are (1) DCC stages, (2) flue gas temperature, (3) absorber stages, (4) stripper stages, (5) MEA solvent lean loading, (6) MEA solvent wt.%, (7) stripper inlet temperature, and (8) amount of CO2 captured. We constrained the search space to the following ranges of decision variables, from 2 to 20 DCC stages, flue gas temperature from 35 to 50 °C, from 2 to 40 absorber stages, from 6 to 8 stripper stages, MEA solvent lean loading from 0.15 to 0.3, MEA solvent wt.% of 25–50%, stripper inlet temperature from 90 to 100 °C, and at least 85% of incoming CO2 captured for the amount of CO2 captured. The absorber and stripper stages are treated as integer values, while the remaining variables are continuous. A Python script is used to link the ASPEN Plus simulator and the DFO solver. The solver used is glcSolve from the TOMLAB optimization suite [26], with a termination criterion of 2000 black-box model (which is the ASPEN Plus simulator and the corresponding economic analysis) evaluations. At the framework’s termination, DCC stages, flue gas temperature, absorber and stripper stages, the MEA solvent lean loading, MEA solvent wt.%, stripper inlet temperature, and the amount of CO2 captured that yield the minimum CO2 capture costs are determined.
2.4 Economic analysis
The CO2 capture costs include fixed capital costs (\({C}_{Capital}\) ) and operating costs (\({C}_{Operating}\)). Equations and data from the module factor-based software CAPCOST are used to evaluate the fixed capital costs for the columns (DCC, absorber, and stripper), heat exchangers (condenser, reboiler, rich/lean heat exchanger, and solvent cooler), and the pump [27]. The cost data is adjusted for inflation using the 2020 Chemical Engineering Plant Cost Index value, CEPCI, 596.2 [28]. The total annualized cost (\(TAC\)) for the system is calculated using Eq. 2,
The Annualization Factor, \(AF\), is calculated in Eq. 3,
where \(i\) is the interest rate, and \(n\) is the plant operation year.
The total CO2 capture cost (\(C{O}_{2} capture costs\)) used for flowsheet optimization is estimated using Eq. 4,
where \(Captured C{O}_{2}\) is the amount of CO2 captured.
The overall assumptions used for the calculation of capture costs are:
-
A Grassroots facility with 8400 h of operation per year is considered.
-
Twenty years (\(n\)) of plant operation and a 20% interest rate (\(i\)) are considered with no salvage value.
-
Maintenance and repair costs are at 6% of the fixed capital investment.
-
Operating supplies are at 0.9% of the fixed capital cost investment.
-
Insurance and taxes are taken at 2% of the fixed capital investments.
-
Thirteen operators are required as operating labor with an annual salary of $ 66,910 was determined using the method defined in Turton et al. [27].
-
Direct supervisory and clerical level costs are taken at 18% of the labor costs.
-
Laboratory charges are taken at 15% of the labor costs.
-
Plant overhead cost is a summation of 70.8% of the labor costs and 3.6% of the fixed capital cost investment.
-
General and Administrative expense cost is a summation of 17.7% of the labor costs and 0.9% of the fixed capital costs.
-
Startup and MEA costs are taken at 10% of fixed capital investment.
-
Make-up water costs are $0.177/1000 kg, and make-up MEA costs are taken from [23] and inflated as per CEPCI value.
-
The steam (5 barg) costs, process cooling water, and electricity are $9.45/1000 kg, $15.7/1000 m3, and $0.0674/kWh, respectively [27].
The absorption setup’s equipment costs primarily include the costs of the absorber column, the stripper column, and the exchanger costs. The absorber and stripper columns are packed bed columns with the packed height per stage in meters (\(HETP\)) calculated using Eq. 5 [29]:
where \({a}_{p}\) is the surface area per volume of the packing.
3 Results and discussions
3.1 Derivative-Free optimization (DFO) parametric study
The optimal process configuration identified by the DFO solver along with MEA solvent flowrate and reboiler heat duty, two critical process parameters, are shown in Table 2 for Mill A and B. Above 90% of CO2 in the flue gas was captured for both mills. Due to the slightly higher CO2 composition, the optimum number of absorber stages is lower for Mill A than Mill B. The higher CO2 composition and stack gas flowrate that should be processed by the capture facility lead to a slightly higher solvent flowrate with a higher loading for Mill A.
Table 3 summarizes the equipment costs for the optimized flowsheets. The costs are rounded to the nearest 1000. The difference in the cost of the absorber columns is due to the higher number of absorber stages for Mill B. However, Mill A absorber column has a slightly larger diameter than Mill B absorber column because Mill A solvent circulation rate is almost 1.3 times higher than that of Mill B. Furthermore, a higher capital cost for Mill A stripper column is attributed to the amount of CO2 stripped in the Mill A stripper column being almost 1.2 times higher than Mill B. This higher amount of CO2 stripping and the higher solvent regeneration leads to a stripper column with a larger diameter and, thus, a higher cost for Mill A stripper column. Also, the higher MEA circulation rate for Mill A leads to a higher exchanger cross-sectional area, increasing the equipment capital costs for Mill A.
Table 4 provides the cost evaluation results for the optimized process flowsheet for both mills. The total annualized capital costs and the operating costs are higher for Mill A than for Mill B. The equipment costs are shadowed by the total operating costs, which include the reboiler steam, cooling water, electricity, and make-up chemicals. Steam contributes to almost 53% of the total operating costs for both mills. The impact of steam consumption on the total capture costs was compared with literature data [20, 30] and made up the most significant portion of the operating costs. Although the percentage of CO2 capture from both mill flue gases is similar for the optimized flowsheets, the flue gas for Mill A has a higher CO2 mol% entering the capture system. The higher CO2 concentration in the flue gas leads to a higher amount of CO2 captured for Mill A. The higher amount of CO2 captured from Mill A flue gas leads to a lower CO2 capture cost per tonne of CO2 captured for Mill A.
Few studies evaluated CO2 capture from the limekiln section of the pulp and paper industry. Onarheim et al. [4] studied CO2 capture from pulp and paper mill lime kiln section, and for 90% CO2 capture from the limekiln flue gas, the capture cost was calculated at $91 per tonne CO2. Detailed comparative cost analysis between the analysis and results of Onarheim et al. [4] and the method used in this work was carried out in a previous publication [13] using the flue gas and mill data of Onarheim et al. The results revealed a significant difference between the capital cost estimates of Onarheim et al. and our method, which was mainly due to the differences in costing equations and methods used for evaluating the base equipment, EPC, construction, and fixed operating costs. The operating cost estimates were found to be similar for both approaches.
Accounting for the 45Q tax credit, a $50 per tonne CO2 cost reduction can be incorporated into each cost estimate. W.J. Sagues et al. [12] estimated limekiln CO2 capture costs to be between $2.1 per tonne CO2 to $5 per tonne CO2, considering the impact of the 45Q tax credit. The Sagues et al. study estimated levelized capital and operating expenses for CO2 capture using chemical process models and AspenTech process simulation software. We discuss the impact of 45Q tax credit on CO2 capture costs in Sect. 3.2.2.
The following subsections summarize the CO2 capture costs for all simulation runs from the DFO solver and discuss how the total capture costs vary with changing decision variable values. The DFO solver determines the minimum CO2 capture cost by changing the decision variable values systematically according to its algorithm (Fig. 3). Hence, multiple sets of numbers of absorber and stripper stages, MEA solvent lean loading, MEA solvent wt.%, stripper inlet temperature, and amount of CO2 captured were simulated, and the corresponding economic analysis carried out during a DFO solver run. For a particular decision variable value, the CO2 capture costs vary depending on the other five decision variable levels, leading to multiple CO2 capture costs being evaluated at a particular level of a single decision variable value. The y-axis in Sub-Sects. 3.1.1 to 3.1.6 represents CO2 capture costs in $ per tonne of CO2. The x-axis corresponds to the numerical values of decision variables. Each dot in Figs. 4, 5, 6, 7, 8, 9, 10, 11 represents individual runs of the process simulation model.
3.1.1 Effect of absorber stages
Figure 4 shows capture cost variation by changing the number of absorber stages. The absorber column is the equipment that facilitates the absorption reaction between the solvent and CO2. The absorber column would be treating a large amount of flue gas in the CO2 capture system [24], making the absorber column a critical piece of equipment contributing to capital costs. The absorber column’s height was varied by changing the number of stages in the column; as the absorber stages increase, the capital cost increases. The minimum value of the capture cost is at 10 and 12 stages for Mill A and Mill B, respectively. There is a decrease in absorber capital cost by almost 77% as the stages decrease from 39 to 10 for both mills. The DFO algorithm changes other decision variable values for a particular absorber stage, leading to different capture cost values at a specific absorber stage. For Mill B, the absorber column’s lowest capital cost is at ten stages; however, the values of other decision variables result in process configuration with higher overall capture costs for those number of stages, as seen from the higher capture cost values for stages 10 to 23.
3.1.2 Effect of stripper stages
The impact of the stripper stages on the capture costs is shown in Fig. 5. The lowest value of the capture cost is at the stripper stage 7 for both mills. The stripper stages above 8 are not explored by the algorithm because of the stripper column flooding, causing a convergence problem.
3.1.3 Effect of stripper inlet temperature
Figure 6 shows the variation of the capture costs with varying stripper inlet temperature. The capture cost decreases slightly as the stripper inlet temperature increases from 91 °C to 100 °C, with the lowest capture costs at 98.7 °C for Mill A and 99.4 °C for Mill B. As the stripper inlet temperature increases, the fraction of reboiler heat required to increase feed temperature to the stripper bottom temperature decreases. A slight increase in capture costs above 98.7 °C for Mill A is mainly due to the process configurations leading to higher overall capture costs.
3.1.4 Effect of the solvent lean loading
Figure 7 gives the variation of capture costs with solvent lean loading. The lowest capture cost was at a solvent lean loading of 0.281 for Mill A and 0.275 for Mill B, respectively. The lower the value of solvent loading, the lower the solvent circulation rate required to capture the desired CO2 from flue gas. Also, at a lower value of lean loading, the solvent has more MEA, boosting the absorption rate. However, a large amount of heat duty required for solvent regeneration tends to overshadow the accelerated absorption at a lower value of lean loading. The reboiler duty decreases by almost 36% as the MEA solvent lean loading increases from 0.17 to 0.281 for Mill A, and Mill B reboiler heat duty decreases by almost 38% as the solvent lean loading increases from 0.17 to 0.275. The higher solvent regeneration duty at lower values of solvent lean loading increases the overall capture costs, as steam is the major contributor to the total utility costs. The higher solvent lean loading value is also associated with reduced CO2 absorption rates, increasing the solvent circulation rate and equipment costs. The reboiler heat duty changes and the associated steam costs with changes in solvent lean loading are also recorded by [31,32,33]. Due to its estimation of possibly higher capture costs, the DFO solver did not explore the range between 0.17 and 0.225 for both mills.
3.1.5 Effect of MEA weight percentage
Figure 8 shows the capture cost variation with the changes in MEA solvent concentration. The lowest capture cost was at a solvent concentration of 45 wt.%. The change in solvent concentration affects the reboiler heat duty in two ways:
-
(1)
As the solvent concentration increases, the amount of water evaporated in the stripper column decreases.
-
(2)
An increase in the solvent concentration leads to a lower solvent circulation rate and a lower sensible heat requirement in the reboiler.
An increase in solvent concentration from 25 to 45 wt % leads to a drop in MEA flow rate by almost 64% for Mill A and 53% for Mill B, respectively. This decrease in the solvent flow rate leads to a reduction in the reboiler energy consumption. A decrease in the solvent flow rate and the regeneration duty with increasing solvent concentration was also reported [34]. The MEA concentration change also impacts the capital cost leading to a cost reduction for the rich/lean heat exchanger by almost eight times for Mill A and almost five times for Mill B, respectively, as the MEA concentration increases from 25% to 45 wt %. The higher MEA concentration at a constant lean loading value leads to a decrease in the reboiler temperature due to the higher partial pressure of CO2. A decrease in the reboiler temperature outweighs concentration effects, reducing thermal degradation in the system [35]. The reduction in thermal degradation could reduce corrosion as the MEA degradation products have been shown to increase the corrosion rates [23, 36]. Further, with higher MEA concentrations, corrosion inhibitors are required. The MEA concentrations above 45 wt % lead to a process configuration with a rise in the fixed operating costs and consecutively higher overall capture costs.
3.1.6 Effects of the amount of CO2 capture, DCC stages, and flue-gas temperature
Figure 9 gives the variation of capture costs with the amount of CO2 captured. The total costs of CO2 capture decrease slightly with an increasing amount of CO2 captured. The minimum capture cost was seen at 94.8% of CO2 capture for Mill A and 94.4% of CO2 capture for Mill B. The capture costs tend to flatten at higher amounts of CO2 capture.
Figure 10 gives the variation of capture costs with the DCC stages. The reduction of DCC capital costs with the decrease in DCC stages results in CO2 capture cost decreases for both mills.
Figure 11 gives the variation of capture costs with the flue-gas temperature entering the absorber column. The CO2 absorption in MEA solvent is favored at lower temperatures, and higher temperatures in the absorber column also lead to higher solvent losses from the absorber column. As a result, the capture costs are lower at the lower flue gas temperatures. It should be noted that the number of DCC stages affects the flue gas inlet temperature. However, this interaction is considered by the DFO solver when adjusting these parameters. Water circulation rate may be considered as a decision variable in future studies.
3.2 Integration of the CO2 capture system within a pulp and paper mill
The possibility of using extracted steam from the pulp and paper mill for the CO2 capture system and utilizing captured CO2 for in-mill CO2 applications are studied for integrating the CO2 capture process within the mill. Sect 3.2.1 discusses the use of extracted steam from the mill’s steam island system in the stripper column reboiler of the CO2 capture process. Sect 3.2.2 discusses the in-mill application of captured CO2 with an existing federal tax credit for carbon capture and sequestration (Sect. 45Q—Internal revenue code).
3.2.1 Economic impact of the steam integration on the CO2 capture costs
A typical steam turbine island system for an integrated pulp and paper mill is shown in Fig. 12 [4]. Steam generated from the bark boiler combined with the steam generated from the recovery boiler is expanded in a steam turbine. The steam turbine plant comprises an extraction-condensing turbine and a power generator. High Pressure (HP) steam from the bark and recovery boiler at 505 °C and 103 bar(a) is expanded across an extraction-condensing set of turbines to generate power using a set of generators. In the first stage, extracted steam at 30 bar is used for soot blowing in the recovery boiler, whereas the 13 bar and 4 bar extracted steam is used in the process at various points of application. The first three stages comprise the high-pressure turbine section, and the last two constitute the low-pressure turbine section. The steam extracted from the steam turbine island is used at various locations within the mill. The process condensate generated is mixed with the turbine condensate and sent to the boiler feed water (BFW) tank [4].
COMPR block is used in ASPEN Plus to simulate the steam turbine. The values of isentropic efficiencies, the values defined by [4, 37, 38] for extracted steam pressure levels, and the amount of steam extracted from the turbine are utilized in this analysis.
The MEA-based CO2 capture process is costly because of the high-energy requirements [39], and the most significant contributor to the cost is the steam demand in the stripper column of the CO2 capture plant. Hence, there is a synergistic opportunity to reduce CO2 capture costs for a mill by steam integration with the mill. In this study, the extracted steam at 4.2 bar is investigated as a possible source for the stripper reboiler steam. In Fig. 12, the dashed red line marked “To stripper reboiler” gives the possible steam extraction point from the steam island towards the stripper column reboiler.
Steam integration would reduce the capture costs by 27% for Mill A and around 25% for Mill B, with a net reduction of 6.6 MWh of electricity export to the grid for Mill A and 5.4 MWh for Mill B, respectively. For Mill A, a decline in the electricity export to the grid results in a $12 per tonne CO2 cost penalty. However, the cost savings of $17.7 per tonne of CO2 from the use of extracted steam in the stripper reboiler exceeds the cost penalty. For Mill B, a reduction in the electricity export to the grid results in an $11.5 per tonne CO2 cost penalty. However, the cost savings of $17.1 per tonne of CO2 is higher than the electricity export loss penalty. The cost savings are similar for both mills. For Mill A, the amount of reboiler steam required for solvent regeneration is 1.2 times higher than that for Mill B. At the same time, the amount of CO2 captured for Mill A is 1.2 times higher, reducing the electricity export loss costs and balancing out the costs for reboiler steam. A decrease in CO2 capture costs using extracted steam in the stripper reboiler was also reported in [40]. Table 5 summarizes the steam turbine section changes and steam savings in the stripper column reboiler for both mills.
3.2.2 In-mill CO2 utilization
There is potential for the pulp and paper mills to improve the cash flow through in-mill CO2 utilization of the captured CO2. An entity’s eligibility depends on whether the CO2 is captured and permanently isolated or displaced from the atmosphere [12]. First, we discuss seven in-mill CO2 applications where the captured CO2 may be utilized. Then, considering 2026 as the year for calculating the capture cost with $50 per tonne of CO2 captured as the 45Q sequestration tax credit levels off in 2026, the capture costs for both mills are calculated.
3.2.2.1 Tall oil manufacture
The tall oil soap rises as a top layer when the black liquor is concentrated and allowed to settle. The top layer is skimmed off and maybe subsequently acidified to convert the tall oil soaps to crude tall oil, i.e., free fatty and resin acids, which are used to make coatings, sizing paper, paints, varnishes, etc. Generally, sulfuric acid is used for acidulation purposes [41]. However, CO2, being a weak acid, can partly replace the sulphuric acid used for acidulation [5].
3.2.2.2 Lignin separation
Lignin is separated from the black liquor, as it often is a bottleneck for increasing pulp production in mills. Biofuels can be produced from the separated lignin, yielding an additional product that can be sold for revenue [5, 42]. Carbon dioxide can replace part of the mineral acid used in the acid treatment.
3.2.2.3 Precipitated calcium carbonate (PCC)
PCC is used as a filler in the paper machine section as it has a high scattering coefficient that helps increase the opacity of the paper produced [43]. PCC is manufactured by bubbling the CO2 through a calcium hydroxide solution.
3.2.2.4 Brown stock washing (BSW)
Lower washing losses are achieved in the BSW section of the pulp and paper mill by reducing the pH by CO2 addition. The pH is lowered in one or more washing stages, attaining an improved washing-out of the pulp from substances contributing to chemical oxygen demand [44].
3.2.2.5 pH control for stock preparation and near-neutral bleaching
Carbon dioxide treatment of the alkaline pulp before processing in the paper machine assembly can improve the drainage of the pulp in the wet end section of the paper machine [45]. Bleaching costs by maximizing chlorine dioxide bleaching efficiency can be reduced by carrying out the final chlorine dioxide brightening at a near-neutral condition using CO2 for pH control [7].
3.2.2.6 Effluent treatment
CO2 can be used to replace the mineral acids in the acidulation stage in the effluent treatment to maintain the pH at neutral [9].
Using captured CO2 in the mill would displace the CO2 from the environment and also make the mill self-sufficient concerning in-mill CO2 requirements. The existing federal tax credit for carbon capture and sequestration would help improve the process economics, making the system attractive for investments and incorporating carbon capture techniques in the pulp and paper mills. Considering the steam integration and the federal tax credit, the CO2 capture costs for Mill A come at − $2.5 per tonne CO2 captured and Mill B at $2.6 per tonne CO2 captured. The negative value of CO2 capture costs implies that the tax credit for Mill A acts as a source of income. A negative CO2 capture cost value for CO2 capture from the pulp and paper mill limekiln was also reported by [12].
3.3 Sensitivity analysis
The sensitivity analysis is carried out to understand the impact of flue gas CO2 composition and flow rate, and utility costs on CO2 capture costs. For both mills, the base case is defined as the optimum flow sheet identified by the DFO, i.e., the process with the lowest CO2 capture cost, considering the effects of steam integration and in-mill CO2 utilization to perform the sensitivity analysis.
Figure 13 gives the variation of CO2 capture costs with changing flue gas CO2 composition (mol%). The flue gas CO2 composition was varied from 5 mol % to 25 mol % to study its impact on the total capture costs. Initially, for lower flue-gas CO2 compositions, there is a sharp decline in capture costs as the composition increases. The capture costs decrease by almost 31% as the CO2 mol % increase from 5 to 10%. However, a moderate decline in capture costs is observed at higher flue-gas CO2 compositions. The capture costs decrease by almost 7% as the CO2 mol % increase from 20 to 25%. As the CO2 mol % increases, an increase is seen in the equipment sizes; however, the capture costs decrease because of the relatively higher change in the amount of CO2 captured. A reduction in the capture costs with increasing flue-gas CO2 composition was also reported in literature [20, 21, 46].
Figure 13 also gives the variation of CO2 capture costs with changing flue gas flowrate. The flue gas flow rate was changed from 2000 kmol/hr to 6000 kmol/hr, and the capture costs were calculated. The flow rate was varied so that the base case flow rate was around the midpoint of the lower and upper values of the flue gas flow rates. For the same CO2 composition at different flue gas flow rates, the difference in capture costs was higher at the lower flue gas flow rates. As the flue gas flow rate increased, the difference in the capture costs was reduced. The decrease in difference is due to the increased CO2 captured with increasing flue gas flow rate at the same flue gas composition. For both mills, the flue gas CO2 composition greater than 15 mol% and a flue gas flowrate of 6000kmol/hr lead to a negative value of CO2 capture costs, indicating a net earning for the mill.
The CO2 capture cost sensitivity to MEA solvent and electricity costs is evaluated by varying each one in the range of ± 50% of the base costs. MEA solvent losses occur from the absorber and the stripper column top. Furthermore, MEA degradation losses are accounted for in the calculation of the operating costs. The MEA make-up and degradation losses account for around 5% of the total CO2 capture costs for Mill A and almost 4.5% for Mill B. The sensitivity of the CO2 capture costs to changing MEA costs is illustrated in Fig. 14. As expected, the capture cost changes linearly with changes in MEA costs. An increase in MEA cost from $1000 to $3300 per ton results in a $2.14 increase (from − $3.59 to − $1.45) per ton of CO2 captured for Mill A. The increase for the same MEA cost change is $2.99 (from $1.00 to $3.99) per ton of CO2 captured for Mill B.
Electricity costs account for 21% of the total operating costs making up to 10% of the total CO2 capture costs for both mills. Not surprisingly, the CO2 capture costs increase linearly as the electricity costs increase (Fig. 15). The liquid CO2 pump and compressors are the major electricity consumers in the compression and dehydration section and the inlet flue gas blower.
4 Conclusions and future directions
In this study, we performed a techno-economic analysis and process flowsheet optimization of the CO2 capture from a pulp and paper mill (Mill A) and a linerboard mill (Mill B) using an MEA absorption process with the objective of minimum annualized CO2 capture costs. The approach utilized an MEA-based CO2 absorption simulation in Aspen Plus and linked the simulation to a DFO tool using python scripting. We employed the CAPCOST modular program for carrying out the CO2 capture costs calculations. The results revealed that, for the optimized flowsheet, total annualized costs and utility costs were higher for Mill A. However, a higher CO2 capture from Mill A results in overall lower per ton of CO2 capture costs for Mill A compared to Mill B.
We studied the impacts of steam integration from within the mill to reduce the total capture costs and in-mill application of captured CO2 with an existing federal tax credit for carbon capture and sequestration (Sect. 45Q—Internal revenue code). A reduction in the electricity export from the mill is observed when the mill steam is used for the stripper column reboiler. However, the costs due to steam savings mask the loss of revenue from the reduced electricity exports. Steam integration and CO2 utilization enabling the use of tax credit reduced the total capture costs per tonne of CO2 to − $2.5 from $64.9 for Mill A and to $2.6 from $69.7 for Mill B. The capture cost sensitivity was investigated by varying the inlet flue gas flowrate, flue gas CO2 mol%, and the electricity and MEA prices. The results revealed that the capture costs vary from − $5.9 to $5.9 per tonne of CO2 captured.
Future studies will investigate how the total capture cost changes with changes in the carbon tax credit as it may change in the long term, and estimate the net carbon emissions of the mills with and without retrofitting them with CO2 capture plants.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
EPA, Overview of Greenhouse Gases, 2022. [Online]. Available: https://www.epa.gov/ghgemissions/overview-greenhouse-gases. Accessed 19 Mar 2023.
EPA, Greenhouse Gas Reporting Program Yearly Review (GHGRP), 2018. [Online]. Available: https://www.epa.gov/sites/default/files/2019-10/documents/ry18_ghgrp_yearly_overview.pdf. Accessed 19 Mar 2023.
Börjesson MH, Ahlgren EO. Pulp and paper industry intelligence. IEA ETSAP Technol Br. 2015;107:1–9.
Onarheim K, Santos S, Kangas P, Hankalin V. Performance and costs of CCS in the pulp and paper industry part 1: performance of amine-based post-combustion CO2 capture. Int J Greenh Gas Control. 2017;59:58–73.
Kuparinen K, Vakkilainen E, Tynjälä T. Biomass-based carbon capture and utilization in kraft pulp mills. Mitig Adapt Strateg Glob Chang. 2019;24:1213–30.
Praxair, Stock preparation and paper machine wet end pH control with carbon dioxide, 1998. [Online]. Available: https://www.praxair.co.in/-/media/corporate/praxairus/documents/specification-sheets-andbrochures/industries/pulp-and-paper/p8222.pdf?la=en. Accessed 19 Mar 2023.
Jiang Z, Richard B. Near-neutral final chlorine dioxide brightening: theory and practice. J Sci Technol Forest Prod Process. 2011;1(1):14–20.
Luthe C, Berry R, Nadeau L. How does carbon dioxide improve brownstock washing? TAPPI Fall Tech Conf. 2003;211–219.
Linde North America, When Being Green Matters - CO2 pH control, 2012. [Online]. Available: https://www.yumpu.com/en/document/read/32069072/when-being-green-matters-co2-ph-control-linde-northamerica. Accessed 19 Mar 2023.
Internal Revenue Service (IRS) and Treasury Department. Final Rule on Section 45Q Credit Regulations, 2020. [Online]. Available: https://www.irs.gov/pub/irs-drop/td-9944.pdf. Accessed 19 Mar 2023.
Onarheim K, Santos S, Kangas P, Hankalin V. Performance and cost of CCS in the pulp and paper industry part 2: economic feasibility of amine-based post-combustion CO2 capture. Int J Greenh Gas Control. 2017;66:60–75.
Sagues WJ, Jameel H, Sanchez DL. Prospects for bioenergy with carbon capture & storage (BECCS) in the United States pulp and paper industry. Energy Environ Sci. 2020;13(8):2243–61.
Parkhi A, Cremaschi S, Jiang Z. Techno-economic analysis of CO2 capture from pulp and paper mill Limekiln. IFAC Papers. 2022;55(7):284–291.
Smook GA. Handbook for pulp and paper technologists. In: Chapter 7 Kraft pulping, 3rd ed. Vancouver: TAPPI Press; 2015. pp. 75–84.
Smook GA. Handbook for pulp and paper technologists. In: Chapter 10 chemical recovery, 3rd ed. Vancouver: TAPPI Press; 2015. pp. 134–163.
EPA, Available and emerging technologies for reducing Greenhouse gas emissions from the pulp and paper manufacturing industry, 2011. [Online]. Available: https://www.epa.gov/sites/default/files/2015-12/documents/pulpandpaper.pdf. Accessed 19 Mar 2023.
AF&PA, “Containerboard,” 2019. https://www.afandpa.org/our-products/paper-based-packaging/containerboard. Accessed: 10-Mar-2021.
PCA, “How containerboard is made,” 2021. https://www.packagingcorp.com/how-containerboard-is-made. Accessed 29-Jul-2020.
Kristin O, et al. Retrofitting CO2 capture to an integrated pulp and paper mill. Nord Pulp Pap Res J. 2014;29(4):620–34.
Husebye J, Brunsvold AL, Roussanaly S, Zhang X. Techno economic evaluation of amine based CO2 capture: Impact of CO2 concentration and steam supply. Energy Procedia. 2012;23(1876):381–90.
David J, Herzog H. The cost of carbon capture, 2000. [Online]. Available: https://sequestration.mit.edu/pdf/David_and_Herzog.pdf. Accessed 19 Mar 2023.
Bhown AS, Freeman BC. Analysis and status of post-combustion carbon dioxide capture technologies. Environ Sci Technol. 2011;45(20):8624–32.
Kohl AL, Nielsen R. Gas Purification. 5th ed. Houston, Texas: Gulf Professional Publishing; 1997.
Abu-Zahra MRM, Schneiders LHJ, Niederer JPM, Feron PHM, Versteeg GF. CO2 capture from power plants. Part I. A parametric study of the technical performance based on monoethanolamine. Int J Greenh Gas Control. 2007;1(1):37–46.
Boukouvala F, Misener R, Floudas CA. Global optimization advances in mixed-integer nonlinear programming, MINLP, and constrained derivative-free optimization, CDFO. Eur J Oper Res. 2016;252(3):701–27.
Holmstr K, Anders OG, Edvall MM. User's guide for TOMLAB/CPLEX v12. 1, 2009;1–106.
Turton R, Shaeiwitz JA, Bhattacharyya D, Whiting WB. Analysis, synthesis, and design of chemical processes, 5th ed. 2018.
C. Maxwell, “Cost Indices,” towering skills, 2020. https://www.toweringskills.com/financial-analysis/cost-indices/. Accessed: 22-Feb-2022.
Wankat PC. Separation process engineering: includes mass transfer analysis, 3rd ed. 2011.
Zhang X, Song Z, Gani R, Zhou T. Comparative economic analysis of physical, chemical, and hybrid absorption processes for carbon capture. Ind Eng Chem Res. 2020;59(5):2005–12.
Oh SY, Binns M, Cho H, Kim JK. Energy minimization of MEA-based CO2 capture process. Appl Energy. 2016;169:353–62.
Duan L, Zhao M, Yang Y. Integration and optimization study on the coal-fired power plant with CO2 capture using MEA. Energy. 2012;45(1):107–16.
Hassan SMN, Douglas P, Croiset E. Techno-economic study of CO2 capture from an existing cement plant using MEA scrubbing. Int J Green Energy. 2007;4(2):197–220.
Li K, Leigh W, Feron P, Yu H, Tade M. Systematic study of aqueous monoethanolamine (MEA)-based CO2 capture process: techno-economic assessment of the MEA process and its improvements. Appl Energy. 2016;165:648–59.
Davis JD. Thermal degradation of aqueous amines used for carbon dioxide capture. PhD Thesis., Univ. Texas Austin, 2009.
Fytianos G, Ucar S, Grimstvedt A, Hyldbakk A, Svendsen HF, Knuutila HK. Corrosion and degradation in MEA based post-combustion CO2 capture. Int J Greenh Gas Control. 2016;46:48–56.
Kangas P, Kaijaluoto S, Maattanen M. Evaluation of future pulp mill concepts—reference model of a modern Nordic kraft pulp mill. Nord Pulp Pap Res J. 2014;29(4):620–34.
IEAGHG, Techno-economic evaluation of retrofitting CCS in a market pulp mill and an integrated pulp and board mill, 2016.
Zhang W, Liu H, Sun Y, Cakstins J, Sun C, Snape CE. Parametric study on the regeneration heat requirement of an amine-based solid adsorbent process for post-combustion carbon capture. Appl Energy. 2016;168:394–405.
Roussanaly S, Fu C, Voldsund M, Anantharaman R, Spinelli M, Romano M. Techno-economic analysis of MEA CO2 capture from a cement kiln—impact of steam supply scenario. Energy Procedia. 2017;114(1876):6229–39.
Lawson N.v E, Amer G. Acidulation and recovery of crude tall oil from tall oil soaps. United States Patent no.4,495,095;1985.
Kouisni L, Holt-Hindle P, Maki K, Paleologou M. The LignoForce System™: a new process for the production of high-quality lignin from black liquor. Pulp Pap Canada. 2014;115(1):18–22.
Biermann CJ. Handbook of pulping and papermaking-Chapter 8, Second. Cambridge, Massachusetts: Academic Press; 1996.
Bokstrom M, Rasimus R. Method of washing of alkaline pulp by adding carbon dioxide to the pulp. United States Patent no. 5,429,717;1995.
Hollerbach W, George H, Kleinberg Jr W. Cellulosic pulp. European Patent no. 88301311.2;1988.
Nuchitprasittichai A, Cremaschi S. Optimization of CO2 capture process with aqueous amines using response surface methodology. Comput Chem Eng. 2011;35(8):1521–31.
Acknowledgements
The authors acknowledge our industrial partners, a linerboard and a pulp and paper mill in the USA, for providing their limekiln flue gas data for this study.
Author information
Authors and Affiliations
Contributions
AP (AP), DY (DY), SC (SC), ZJ (ZJ). SC, ZJ––Conceptualization; AP, DY––Data Curation; AP, DY, SC, ZJ––Analytical Approach; AP, DY––Formal Analysis; AP, DY, SC––Methodology; AP, DY––Software; SC, ZJ––Project Administration; SC, ZJ––Supervision, AP––Validation; AP––Visualization; AP, DY––Writing—original draft; AP, DY, SC, ZJ –– Writing—review & editing. All author read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Parkhi, A., Young, D., Cremaschi, S. et al. Carbon dioxide capture from the Kraft mill limekiln: process and techno-economic analysis. Discov Chem Eng 3, 8 (2023). https://doi.org/10.1007/s43938-023-00024-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s43938-023-00024-7