Abstract
Approximately 50 million ton of municipal waste is generated in Pakistan per annum and most of this waste does not reach final deposit sites. In this research, Silvia gas technology for municipal solid waste (MSW) steam gasification is studied to produce high energy density product gas. A detailed simulation model is developed with the help of Aspen Plus®. Catalyst coal bottom ash along with lime (CaO) as sorbent is employed for tar reduction and improving the hydrogen (H2) yield in the product gas. The effect of gasification operating temperature and the ratio of steam to feedstock on synthetic gas composition, hydrogen (H2) yield and heating values of synthesis gas was studied. Coal bottom ash along with CaO had a substantial effect on hydrogen (H2) yield and synthesis gas production. Rise in steam–MSW ratio increased the hydrogen (H2) from 58 to 74.9% (vol.). The maximum value of hydrogen (H2) production, i.e., 74.9% by vol. was achieved at a steam–feedstock ratio of 1.9. A maximum of 79.8% by vol. hydrogen (H2) was attained at 680 °C gasification operating temperature with 1.3 ratio of steam to feedstock and coal bottom ash 0.07% by wt. High value of 13.1 MJ/Nm3 of hydrogen-rich synthetic gas was achieved at 680 °C. The acquired results lay the foundation for the economic feasibility study and pilot plant for MSW usage for hydrogen production.
Highlights
A simulation model is developed with the help of Aspen Plus® to study the municipal solid waste steam gasification for hydrogen production.
The maximum value of hydrogen (H2) production i.e., 74.9% by vol. was achieved at a steam-feedstock ratio of 1.9.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The energy consumption has been increasing progressively and by 2040 it is expected to increase by 50% because of industrialization, rapid economic growth, and population thus resulting in the rise of fuel demand [1]. Conventional fuels e.g., petroleum, natural gas etc. are used to meet this high demand. Such non-renewable sources are limited in amount and going to deplete soon [2]. Thus, it is imperative to find and develop alternate energy sources. Secondary sources (hydrogen) or renewable energy like biomass, hydropower, solar, wind etc. are best to replace these conventional ones [3].
Municipal solid waste (MSW) is one of the significant issues we are experiencing due to, urbanization and industrialization. Other than its non-renewable constituents MSW is a major source of biomass [3, 4]. In 2015, global MSW generation was estimated about 1.3 billion ton per annum, with an annual growth rate estimated at 4–5.6% in developed countries and 2–3% in developing countries [5]. In 2010, roughly 0.64 kg of MSW per person (0.68 billion ton per year) was resulted by an urban population of 2.9 billion. According to the World Bank’s “Urban Development and Local Government Unit” report, by 2025 current waste produced by 1.2 kg/capita per day increased up to 1.4 kg/capita per day (2.2 billion ton per anum) [6] and thus overstraining the existing waste disposal system.
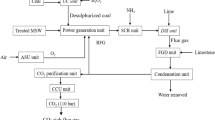
For renewable energy, MSW can be converted into solid fuels (char or carbon), liquid fuels (tars, bio-oil, and heavier hydrocarbons) and gaseous fuel (CO, H2, CH4, C2H4, C2H6, and C6H6) with the help of any of the route mentioned in Fig. 1. Among these process gasification is an efficient auto-thermal process [7]. The energy recovery and product gas specific heat is higher because of the high syngas (CO, H2) yield, while in liquefaction and pyrolysis the yield is lower because of complex nature and occurrence of secondary reactions between volatiles and hot solid particles [8]. Furthermore the conversion of the liquid fuels obtained from pyrolysis and liquefaction in the form of bio-oil is not economically feasible, whereas synthetic gas from gasification is readily convertible into synthetic natural gas by using CO and CO2 catalytic methanation process [9]. Additionally, the synthesis gas from gasification can directly lead to the electricity generation and reduce the waste disposal expenditures. All these factors suggest gasification the most feasible process either for energy production or petrochemical derivatives [10].
By 2010 approximately 122,106 MW of energy was being produced utilizing gasification technology all across the globe, and the contribution of biomass including MSW was 0.33% of the total energy produced while the rest of thermal energy is produced by coal, gas, petroleum, and coke [10]. Yang et al. outlined the recent development in the field of gasification and its capacity growth all-around the world. By 2030, the usage of refuse-derived fuel for energy purposes is forecasted to reach 2.6 billion metric ton [11]. Arafat et al. reported the recent development in the field of gasification and its capacity growth all-around the world [12].
In the gasification process, the feed is converted into gaseous fuel which is used to produce energy, or the biomass even coal is converted into chemical feedstock, to produce useful chemical products [13]. Gasification is carried out under controlled gasifying medium/agent, which can be oxygen, air or steam [14]. To achieve an optimum H2 to CO ratio post-gasification cleaning of synthetic gas is required. Inorganic impurities are best removed in successive order as their removal might generate other undesired products. Initially quenching with the help of water removes ash and residual char. Followed, by COS and HCN hydrolysis. The NH3 and halides are removed via water scrubbing before adsorption of H2S. For tar removal either (1) nickel-based catalytic cracking or (2) high temperature cracking or (4) solvent (methyl ester) scrubbing is utilized. Catalytic cracking completely get rid of tar from the synthesis gas but can cause catalyst deactivation and poisonous gas buildup [15].
The catalyst utilized in gasifier promotes the cracking of higher hydrocarbons into small chain hydrocarbons and gases improves gas quality. It also tends to improve reaction kinetics making lower temperature gasification a possibility [15]. Several researchers have reported various cost-effective and efficient catalysts for the gasification process. Some of them are discussed below in Table 1.
Akhil et al. analyzed the influence of various integration options for biofuels systems based on gasification on the greenhouse gas (GHG) emissions reduction potential, annual net profit and fuel production price [26].
Along with suitable catalyst, fluidized bed gasifier has been reported to achieve about 60% (vol.) hydrogen [27]. Besides, the cost of hydrogen production via MSW gasification is competitive with natural gas steam reforming. Taking issues of conventional waste disposal system and environmental benefits into account, MSW gasification is a promising option for hydrogen synthesis.
Simulations studies of biomass gasification provide rigorous information about the process. Such studies can be categorized into steady-state and dynamic models and steady-state models can be further categorized into kinetic systems and kinetic-free system [28,29,30].
To design and simulate the unit operations and processes without carrying rigorous calculations several software packages e.g. Aspen Plus®, Aspen HYSYS® are available. In Aspen Plus®, fluidized bed reactor library model exists [31]. Such problem-oriented package can be used for gasifier modeling, prediction of synthesis gas composition, hydrogen yield for specific feed and to identify best-suited gasification agent for feedstock, gasifier operating conditions. Previously, researchers have utilized Aspen Plus® to understand the principles of gasification. They simulated different types of coal gasifier, indirect liquefaction process, methanol synthesis, power plants based on integrated coal gasification combined cycle (IGCC) and their contributions are discussed in below Table 2.
Hydrogen from synthetic gas offers an encouraging energy alternative for developing countries like Pakistan where the MSW production rates are high and other energy resources are limited. Lahore is the densely populated city in Pakistan (9788/km2 in 2020) [32]. Almost 7200 ton MSW is generated daily which presents itself with a concerning dileman of waste disposal management [33]. Additionally, National Electric Power Regulatory Authority (NEPRA), Pakistan is willing to provide a competitive upfront tariff (U.S. $ 10,007/kWh) for the application of gasification in waste-to-energy systems however, only a few studies have been reported in Pakistan for MSW gasification [34]. Even with theses studies, no tangible results has been reported for the Lahore-based MSW. All these factors itself presents an ideal opportunity to explore the possibility of Lahore’s MSW as potential energy source.
The primary objective of this work is to evaluate the efficiency of municipal solid waste gasification system designed by Battelle Columbus Laboratories i.e., Silvia Gas Technology under varying operating conditions to assess the exergetic proficiency of the feedstock to synthetic gas conversion with the help of simulation model. The findings of this case study will lay down the foundation for the environmental feasibility and socio-economic recommendation of Silvia gas technology in the context of Pakistan. Coal bottom ash is used as a catalyst. The influence of steam to feedstock ratio (S/F) on the synthetic gas composition and effect of temperature is investigated. For simulation studies temperature range of 500–700 °C and 0.5–2 (wt./wt.) S/F was selected. Altogether, the overall performance of the pilot plant is computed. It is not only focused on the overall energy production feasibility but also evaluates the performance efficiency of dual bed circulating fluidized gasifier in Aspen Plus®.
2 Silvia gas technology
Patented by Battelle Columbus Laboratories, the process employs dual-fluidized bed gasifier in which MSW undergo the gasification process using steam as gasification agent. Gasification occurs in indirectly heated circulated fluidized bed (CFB) reactor utilizing the heat of parallel blown CFB char combustion chamber. It produces high energy density product gas as not diluted with N2 and without the need of oxygen plant.
Gasification model has been developed by energy, chemical and mass balance principles. The hydrogen (H2) production route consists of several successive units comprising MSW gasification, synthesis gas trim cooler, carbon monoxide shift reactor, methanation reactor. Modeling is carried out by the Gibbs free energy minimization equilibrium method [41]. Input specification and process flowchart for this Silvia gas technology plant is present below in Table 3 and Fig. 2, respectively.
Coal bottom ash use as catalyst provides an efficient and environment friendly path of recycling solid waste. The higher stability of coal bottom ash provides catalytic support by impregnating of other active components for several different reactions.
3 Materials and methods
3.1 Selection of waste and its characterization
Feedstock data used for this gasification model is based on the MSW generated in Lahore city situated in Pakistan. Approximately 36,300 households were considered for the survey carried out by Lahore Waste Management Company (LWMC) in 2012 to evaluate the waste generation per house.
The survey methodology is as follows:
-
1.
Designing and conduction a survey based on a questionnaire, categorizing selected material to develop worksheets to sort out waste.
-
2.
Information collection regarding the households inside the premises of the study area.
MSW forwarded to the LWMC for characterization process is given below in Fig. 3. Results were consistent with typical developing country characteristics [45].
Lahore 2012 MWC characterization [46]
Ultimate and proximate analysis of MSW is present in below Table 4.
Based on following equations [47], and survey results as reference, MSW of approximately 36,300 houses has to be collected on a daily basis for 50 MW capacity power plant.
3.2 Bed material in gasifier
Sand is used as bed material in the primary (1st) stage of gasification while dolomite (CaO) is used in the secondary (2nd) stage. In pilot plant developed by Battelle Columbus Laboratories, nitrogen flow is varied at a rate of 7–8 Nm3/h. Dolomite (CaO) acts as an adsorbent as well as a catalyst. It increases the tar reduction and cracking process. The dolomite is composed of 88.5%CaO.
3.3 Coal bottom ash
It is a mixture of alkaline nature oxides. Aluminum oxide (Al2O3), magnesium oxide (MgO) and Fe2O3 promotes catalytic qualities of coal bottom ash. The composition of coal bottom ash used for simulation purpose is taken from Huaneng Shandong Ruyi Power Plant, Sahiwal, Pakistan and is given below in Table 5. This specific coal bottom ash is produced from coal of different origins including Australia, New Zealand, Indonesia, China and South Africa. For developing model in Aspen Plus® it is assumed that coal bottom ash is uniformly distributed in the circulating bed reactor. Catalytic activity of coal bottom ash is incorporated in the Aspen Plus® model with the help of existing adjustor model in Aspen Plus® library and by combining the FORTRAN based written code with Aspen Plus® model.
3.4 Aspen Plus® simulation model
3.4.1 Assumptions
Silvia Gas Technology modeling was carried out under following assumptions.
-
1.
Rapid devolatilization of feedstock yields volatile products i.e. CH4, CO, H2, CO2, H2O [48].
-
2.
All feedstock particles size is uniform and spherical.
-
3.
Char is composed of ash and carbon.
-
4.
The gasification reactions involving char initiates from the bed and move along in the freeboard.
-
5.
At gasification operating temperature the tar is altogether converted into fifteen different hydrocarbons therefore, higher chain hydrocarbons and tar are not considered.
3.5 Aspen Plus® model description
Gasification process carried out for hydrogen production is comprised of four main operations: biomass feeding system, gasification unit, steam generation and effluent treatment (particulate separator, moisture and impurities removal). The description of these units can be summarized in block operation for modeling in Aspen Plus® and are given in Table 6 [41].
Non-conventional solids of feedstock are those which do not participate in phase equilibrium calculations. Due to its non-defined molecular weight, MSW is considered as heterogeneous solids in Aspen Plus®. These unfamiliar components of feedstock were defined with the help of the Peng-Robinson– Boston-Mathias (PR-BM) property library. Generally, PR-BM is used for oil and gas production (OILGAS), gas processing: hydrocarbon separation (GASPROC) and coal processing: combustion (COALPROC) [49]. PR-BM established properties of pure as well as mixed component properties. DCOA-LIGHT and HCOALGEN library was used to determine the density and enthalpy of MSW.
In RYield MSW is converted into hydrogen (H), carbon (C), oxygen (O), sulfur (S), nitrogen (N), ash and inert. The whole set of gasification reaction is considered with an assumption that the char is solely comprised of carbon. The yield distribution is based on the ultimate analysis. From RYield the stream is then fed into the fluidized bed reactor block. Based on hydrodynamic parameters, fluidized reactor block consists of two sections—dense bed and freeboard. Perfect and uniform mixing inside the fluidized bed reactor block is safeguarded by the constant and stringent motion of bed material. For each reactor block the reaction kinetics are defined individually through built-in reaction models. The kinetic and equilibrium rate were used to define the gas phase reactions and to quantify the products of char gasification reactions. At 350 °C super-heated steam is used as gasification medium [41]. For simulation purpose, MSW is introduced into the system at a rate of 20.4 t/h. Steam consumption is calculated with reference to the sub-stochiometric conversion of MSW during the gasification reactions. Based on MSW proximate and ultimate analysis as shown in Table 4 steam flow rate is set at 7.5 t/h. which is found to be optimal steam consumption for the sub-stochiometric reactions in given case. MSW feedstock is pre-mixed with the coal bottom ash and then introduced into the system. The input specifications for the Aspen Plus® model are specified in Table 3.
Flowsheet schematic of the Silvia gas technology simulation model developed in Aspen Plus® is given below in Fig. 4. The MSW first entered in RYIELD block where based on feed material composition it is decomposed. This stream then entered the ideal mixing environment of CFB block. In CFB block built-in reaction models are used to define the kinetic and exiting stream composition. The stream then entered Cyc-Sep for separating particulates from the gaseous product stream. Reactions initiated in CFB block can proceed further in CFB1 block for the process to carry out efficiently. Afterwards the product gas stream can cool down in COOLER block and post-gasification purification takes place in SCRUB block.
Reactions participating in MSW gasification process are listed below in Table 7 [3].
4 Results and discussion
4.1 Model results
MSW atmospheric gasification with the help of steam was simulated for 500–700 °C temperature range and 0.5–2 steam to feedstock ratio using 1.4% sorbent-feedstock ratio and 0.07% of coal bottom ash catalyst. It was observed that for lower operating temperature higher S/F ratio was required to promote water–gas shift reaction thus increasing the hydrogen yield and ultimately reducing tar. Maximum yield of hydrogen (H2) i.e., 79.8% was observed at a temperature of 680 °C and S/F ratio of 1.3 (Tables 8, 9).
4.2 Effect of gasification operating temperature on synthetic gas yield
For simulation studies and a better understanding of the gasification process, atmospheric gasification process of MSW with the help of steam was converged in Aspen Plus® for different temperatures ranging from 500–700 °C. Based on simulation results the effect of gasification operating temperature on product gas composition and LHV and HHV is being discussed. From various studies and experimental results, it was previously established that 0.07% catalytic coal bottom and 1.4% sorbent is the optimum amount to carry out biomass specially MSW gasification [4].
With the increase in temperature, synthesis gas composition and hydrogen yield vary significantly as shown in below Fig. 5. As the temperature increases from 600–700 °C, the hydrogen yield rises from 45.8 to 79.1% (by vol.) at constant steam to biomass ratio (1.3). At the same time with the increase in temperature the CO yield in synthetic gas is reduced from 19.8 to mere 7.9% (vol.). This change in fuel gas composition and hydrogen yield can be explained due to the exothermic and endothermic nature of gasification reaction mentioned in above Table 7. From temperature ranging from 500 to 700 °C the hydrogen (H2) yield and synthetic gas composition were mainly because of the water–gas shift reaction [50].
Similar behavior for catalytic steam biomass gasification has been discussed for the temperature range of 650–695 °C. 1.4 wt.% CaO as an absorbent plays an essential role in synthetic gas composition. The use of CaO increase the hydrogen (H2) and synthetic gas yield, giving the merest fraction of CO at 700 °C. Previously, for steam gasification researchers have discussed the use of CaO as catalyst and adsorbent on synthetic gas composition during steam gasification [37]. When the temperature is increased from 500 to 700 °C, the methane volume fraction decreases from 23.6 to 10.2% (vol.) as shown in Fig. 5. The decrease in methane fraction is due to the endothermic nature of methane reforming reaction which in turn increases hydrogen (H2) and carbon monoxide (CO) yield in synthetic gas. Temperature range of 500–700 °C is vital for hydrogen yield in dual bed fluidized gasifier along with CaO sorption [51].
In literature, for biomass steam gasification, decline in methane (CH4) fraction is reported with the rise in temperature from 650 to 700 °C using a fluidized bed gasifier [52]. The endothermic tar reduction reactions are also favorable at elevated temperatures which increases the hydrogen (H2) fraction in synthetic gas. In this study, it was observed that with temperature rise to 700 °C hydrogen (H2) yield increases while carbon dioxide (CO2), carbon monoxide (CO) and methane (CH4) fraction in synthetic gas decreased. Inayat et al. [50] validated the results as they investigated the increase in H2 contents for in-situ empty fruit branches steam gasification. Concerning temperature rise, increase in H2 and syngas composition was also reported for steam catalytic gasification of pine sawdust feedstock with CaO sorption [53, 54].
Heating values of synthetic gas mainly depends on the H2, CO and CH4 contents and can be calculated using their molar content by using following equations [55]
With increase in temperature from 500 to 700 °C LHVsyngas decreased from 17.8 to 10.9 MJ/Nm3 as shown in Fig. 6. Similar trend for heat values was reported for the semi-batch gasifier operation of palm residue [52]. Hoque et al. [56] performed experimental gasification studies of rice husk, saw dust and coconut shell in fixed-bed gasifier with air as gasification medium and found the cumulative heating value of synthetic gas approximately 8.67 MJ/Nm3.
4.3 Effect of the steam-feedstock ratio on synthetic gas yield
Steam is among the most preferred and capable gasification agent because of its higher yield of synthetic gas without diluting it with N2 and high hydrogen content [14]. Steam gasification also facilitate tar cracking and possess benefits such as optimum residence time and higher enthalpy value of synthetic gas. In this case study, steam gasification of MSW in the presence of catalytic coal bottom ash and dolomite as an adsorbent has been simulated. The effect of steam to feedstock ratio (w/w) varying from 0.5 to 2 on synthetic gas yield was reported. For this case study, the temperature at 650 °C, MSW feed rate 5.15 kg/h., CaO-MSW ratio 1.4 and 0.07% catalytic coal bottom ash was kept constant. As shown in Fig. 7 the hydrogen yield in synthetic gas was 58.9% when S/F ratio was 0.5 and it increased to 71.2% at S/F ratio of 1. The maximum hydrogen yields 74.9% was achieved at S/F ratio of 1.9. This rise in hydrogen (H2) fraction is because of the methane reforming, char gasification and water–gas shift reaction [14].
With the increase in S/F ratio, syngas yields also increased. Rise in hydrogen (H2) fraction with the increasing steam to MSW ratio. Moreover, the yield of methane (CH4), carbon monoxide (CO) and carbon dioxide (CO2) decreased with the rise in S/F ratio. The rise in steam consumption shifts the equilibrium of water–gas shift reaction in the forward direction. This behavior has already been reported in the literature [57, 58].
At S/F ratio of 2, high CO contents were observed at approximately 15.9%. Such high fraction in CO fraction can be explained by the steam methane reforming. These reactions were also observed in previous case studies of steam gasification. For MSW steam gasification, the methane fraction decreased from 11.9 to 8.2% (vol.). This is because of the rise in the steam consumption encouraged by steam reforming and lessened methane (CH4) fraction in the synthetic gas. Optimal S/F ratio is of huge importance as excessive steam can lead to the reduction of gasifier temperature [59].
The lower yield of carbon dioxide (CO2) in synthetic gas shows the sorption properties of dolomite (CaO). The higher synthetic gas yield is because of the catalytic coal bottom ash i.e. MgO, Fe2O3 and Al2O3. Iron oxide (Fe2O3) and aluminum oxide (Al2O3) in catalyst improves the char gasification yield.
By using the above Eqs. (4) and (5), LHV and HHV of product gas can be calculated [55]. With the increase in S/F ratio, the LHVsyngas decreased due to decline in CO and CH4 content.
Khan et al. [60] also observed the decline in enthalpy of product gas in case of atmospheric steam gasification. The HHVsyngsas decreased from 18.1 to 14.4 MJ/Nm3 with the increase in S/F ratio. This decline is because of the low fraction of carbon monoxide (CO) and methane (CH4) at S/F ratio of 2 as shown in Fig. 8. It was observed that the decline in methane (CH4) and carbon monoxide (CO) yield was higher than the increase in hydrogen (H2) fraction.
Xiong et al. [24] reported the significance of MgO, CaO, Fe2O3, and Al2O3 in coal bottom ash for synthetic gas yield, tar cracking and cold gas efficiency. The hydrogen yield increased while the contents of carbon dioxide (CO2), carbon monoxide (CO) and methane (CH4) decreased. Due to the water–gas shift reaction and steam reforming reaction, the fraction of hydrogen (H2) increased. For tar reduction, the use of catalytic ash (coal bottom) was also reported in coal pyrolysis case study [61]. The catalyst enhanced the carbon conversion into the gaseous product as well as the cracking of tar, due to which hydrogen yield increased in the product gas. Chin et al. [62] observed less residual amount left after the gasification process conducted in the presence of catalyst as compared to the non-catalytic process. The dolomite (CaO) used as sorbent in steam gasification encourages the steam methane reforming and water–gas shift reaction during the gasification process. The dolomite (CaO) adsorbed carbon dioxide (CO2) via the shift reaction to form CaCO3. This dolomite effect arises in two steps: an initial step (1st), rise in hydrogen fraction is because of the catalytic effect on steam reforming and tar reduction reactions; it is followed by, rise in H2 fraction because of carbon dioxide capture via dolomite [63].
The carbon dioxide (CO2) sorption moved the equilibrium of the water–gas shift reaction and steam reforming in the forward direction, which in turn results in rise of H2 fraction in the product gas. Khan et al. [60] acknowledged the significance of dolomite in the gasification process and reported the hydrogen (H2) and synthetic gas sensitivity to dolomite (CaO) in the literature [64].
5 Conclusions
Energy is an integral component for the economic and technological development of any country. The industrial growth and educational development is limited if the energy resources are not reliable. On the other hand, the global industrial revolution and dense population is causing global warming. To cater the aftereffects of the global warming there is a need to change the pattern of energy e.g., from fossil fuels to waste-to-energy. In this paper municipal solid waste (MSW) is explored as an alternate renewable energy source. The steam gasification of MSW was carried out in Aspen Plus® to determine the viability of MSW as feedstock to produce H2. In this simulation, coal bottom ash was used as a catalyst while dolomite (CaO) as adsorbent. With the rise in temperature from 500 to 700 °C, hydrogen yield increases from 45.8 to 79.8% (vol.) and at the same time the CO fraction decreased from 23.6 to 10.2%. At temperature of 650 °C, the use of dolomite (CaO) as sorbent enhanced the synthetic gas and hydrogen (H2) yield by giving the lowest fraction of carbon dioxide (CO2). The LHVsyngas declined from 17.8 to 10.9 MJ/Nm3 with the temperature rise from 500 to 700 °C. The use of steam as a gasifying agent has a significant effect on hydrogen yield in synthetic gas but at a range. As S/F increased the H2 fraction also increased from 58.9 to 74.9%. At S/F ratio of 0.5 the hydrogen (H2) yield was 58.9% which then increased to 71.2 at ratio of 1. Maximum hydrogen yield was achieved at S/F ratio of 1.9 which was 74.9% (vol.). However, with the increase in the S/F ratio, the HHVsyngsas decreased from 18.1 to 14.4 MJ/Nm3. The decline in the HHVsyngas is due to the increase in CO2 yield and presence of H2O in the product gas.
It can be concluded that the hydrogen yield from MSW steam gasification is relatively high which consequently makes the energy generation from gasification product gas relatively cheap albeit lower when compared with the processes like incineration. However, steam gasification still has the advantage of producing multiuse gaseous products like methane (CH4). High moisture contents present in MSW feedstock lower the steam gasification efficiency but increase the yield of hydrogen (H2) and lower the carbon monoxide (CO) yield. To make a final decision about the steam gasification plant feasibility for hydrogen production it is advisable to carry out the economic analysis as well as the techno-feasible study. The results obtained from this model combined with the system performance can act as guidelines for the process optimization soon.
Dat availability
All data gathered, deduced and analyzed during this study is sited in this published article. No additional information is required.
Abbreviations
- GHG:
-
Green-house gases
- HHV:
-
Higher heating value
- LHV:
-
Lower heating value
- LWMC:
-
Lahore waste management company
- MSW:
-
Municipal solid waste
References
Ivanova NH. Waste to energy (WTE) for the survival and the development. Eur Sci J 2015 Feb 1.
Furlan C, Mortarino C. Forecasting the impact of renewable energies in competition with non-renewable sources. Renew Sustain Energy Rev. 2018;81:1879–86.
Basu P. Biomass gasification, pyrolysis and torrefaction: practical design and theory. Academic press; 2018.
Acharya B, Dutta A, Basu P. An investigation into steam gasification of biomass for hydrogen enriched gas production in presence of CaO. Int J Hydrogen Energy. 2010;35(4):1582–9.
Shehzad A, Bashir MJ, Sethupathi S. System analysis for synthesis gas (syngas) production in Pakistan from municipal solid waste gasification using a circulating fluidized bed gasifier. Renew Sustain Energy Rev. 2016;60:1302–11.
Hoornweg D, Bhada-Tata P. What a waste : a global review of solid waste management. Urban development series; knowledge papers no. 15. World Bank, Washington, DC. © World Bank (2012). https://worldbank.org/urban.
Panwar N, Kothari R, Tyagi V. Thermo chemical conversion of biomass–Eco friendly energy routes. Renew Sustain Energy Rev. 2012;16(4):1801–16.
Donskoy I et al. Investigation of the features of polyolefins and wood biomass thermochemical conversion for their energy utilization. In: Journal of physics: conference series. IOP Publishing; 2019.
Bhaskar T, Pandey A. Advances in thermochemical conversion of biomass—introduction. In: Recent advances in thermo-chemical conversion of biomass. Elsevier; 2015. p. 3–30.
Hooshmand P, KhakRah H, Balootaki HK, Jamalabadi MY. Recycling municipal solid waste utilizing gasification technology: a case study. J Thermal Anal Calorim. 2020;139(4):2705–18.
Yang Y, Liew RK, Tamothran AM, Foong SY, Yek PN, Chia PW, Van Tran T, Peng W, Lam SS. Gasification of refusederived fuel from municipal solid waste for energy production: a review. Environ Chem Lett. 2021;13:1–4.
Arafat HA, et al. Influence of socio-economic factors on street litter generation in the Middle East: effects of education level, age, and type of residence. Waste Manage Res. 2007;25(4):363–70.
Wei J, et al. Synergy mechanism analysis of petroleum coke and municipal solid waste (MSW)-derived hydrochar co-gasification. Appl Energy. 2017;206:1354–63.
Kumar A, Jones DD, Hanna MA. Thermochemical biomass gasification: a review of the current status of the technology. Energies. 2009;2(3):556–81.
Abu El-Rub Z, Bramer EA, Brem G. Review of catalysts for tar elimination in biomass gasification processes. Ind Eng Chem Res. 2004;43(22):6911–9.
Narvaez I, et al. Biomass gasification with air in an atmospheric bubbling fluidized bed. Effect of six operational variables on the quality of the produced raw gas. Ind Eng Chem Res. 1996;35(7):2110–20.
Fredriksson HO, et al. Olivine as tar removal catalyst in biomass gasification: catalyst dynamics under model conditions. Appl Catal B. 2013;130:168–77.
Tamai Y, Watanabe H, Tomita A. Catalytic gasification of carbon with steam, carbon dioxide and hydrogen. Carbon. 1977;15(2):103–6.
Walker Jr PJ, Shelef M, Anderson RA. Catalysis of carbon gasification. Chem Phys, Carbon;(United States). 1968;4.
Maa PS, Gorbaty ML. Hydropyrolysis-gasification of carbonaceous material. Google Patents. 1991
Mudge L, et al. Catalytic steam gasification of biomass for methanol and methane production. J SolEnergy Eng. 1985;107(1):88–92.
Bangala DN, Abatzoglou N, Chornet E. Steam reforming of naphthalene on Ni–Cr/Al2O3 catalysts doped with MgO, TiO2, and La2O3. AIChE J. 1998;44(4):927–36.
Kapteijn F, Porre H, Moulijn J. CO2 gasification of activated carbon catalyzed by earth alkaline elements. AIChE J. 1986;32(4):691–5.
Xiong R, et al. Fundamentals of coal topping gasification: characterization of pyrolysis topping in a fluidized bed reactor. Fuel Process Technol. 2010;91(8):810–7.
Umeki K, et al. High temperature steam-only gasification of woody biomass. Appl Energy. 2010;87(3):791–8.
Kadiyala A, Kommalapati R, Huque Z. Evaluation of the life cycle greenhouse gas emissions from different biomass feedstock electricity generation systems. Sustainability. 2016;8(11):1181.
Parthasarathy P, Narayanan KS. Hydrogen production from steam gasification of biomass: influence of process parameters on hydrogen yield–a review. Renew Energy. 2014;66:570–9.
Patra TK, Sheth PN. Biomass gasification models for downdraft gasifier: a state-of-the-art review. Renew Sustain Energy Rev. 2015;50:583–93.
Farzad S, Mandegari MA, Görgens JF. A critical review on biomass gasification, co-gasification, and their environmental assessments. Biofuel Res J. 2016;3(4):483–95.
Janajreh I, AlShrah M. Numerical and experimental investigation of downdraft gasification of wood chips. Energy Convers Manage. 2013;65:783–92.
Bruchmüller J, et al. Modeling the thermochemical degradation of biomass inside a fast pyrolysis fluidized bed reactor. AIChE J. 2012;58(10):3030–42.
Rehman A, et al. Towards environmental Sustainability: devolving the influence of carbon dioxide emission to population growth, climate change, Forestry, livestock and crops production in Pakistan. Ecol Indic. 2021;125:107460.
Azam M, et al. Status, characterization, and potential utilization of municipal solid waste as renewable energy source: Lahore case study in Pakistan. Environ Int. 2020;134:105291.
Farooq MK, Kumar S. An assessment of renewable energy potential for electricity generation in Pakistan. Renew Sustain Energy Rev. 2013;20:240–54.
Doherty W, Reynolds A, Kennedy D. Aspen plus simulation of biomass gasification in a steam blown dual fluidised bed. In: Méndez-Vilas A (ed) Materials and processes for energy: communicating current research and technological developments, Formatex Research Centre (2013)
Chui E, et al. Simulation of entrained flow coal gasification. Energy Procedia. 2009;1(1):503–9.
Shen L, Gao Y, Xiao J. Simulation of hydrogen production from biomass gasification in interconnected fluidized beds. Biomass Bioenerg. 2008;32(2):120–7.
Emun F, et al. Integrated gasification combined cycle (IGCC) process simulation and optimization. Comput Chem Eng. 2010;34(3):331–8.
Anukam A, et al. Computer simulation of the mass and energy balance during gasification of sugarcane bagasse. J Energy. 2014;2014:1–9.
Ramzan N, et al. Simulation of hybrid biomass gasification using Aspen plus: a comparative performance analysis for food, municipal solid and poultry waste. Biomass Bioenerg. 2011;35(9):3962–9.
Nikoo MB, Mahinpey N. Simulation of biomass gasification in fluidized bed reactor using ASPEN PLUS. Biomass Bioenerg. 2008;32(12):1245–54.
Fernandez-Lopez M, et al. Simulation of the gasification of animal wastes in a dual gasifier using Aspen Plus®. Energy Convers Manage. 2017;140:211–7.
Gartner L, et al. Kinetic entrained flow gasifier modeling in Aspen Plus—a simulation study on fuel blends. In: DBFZ Workshop zur Flieβbildsimulation in der Energietechnik, Leipzig. 2012.
Lee J, et al. Effects of burner type on a bench-scale entrained flow gasifier and conceptual modeling of the system with Aspen Plus. Korean J Chem Eng. 2012;29(5):574–82.
Al-Khatib IA, et al. Solid waste characterization, quantification and management practices in developing countries. A case study: Nablus district–Palestine. J Environ Manage. 2010;91(5):1131–8.
Batool SA, Ch MN. Municipal solid waste management in Lahore city district, Pakistan. Waste Manage. 2009;29(6):1971–81.
Consonni S, Giugliano M, Grosso M. Alternative strategies for energy recovery from municipal solid waste: part A: mass and energy balances. Waste Manage. 2005;25(2):123–35.
Kangas P, Koukkari P, Hupa M. Modeling biomass conversion during char gasification, pyrolysis, and torrefaction by applying constrained local thermodynamic equilibrium. Energy Fuels. 2014;28(10):6361–70.
Finlayson BA. Introduction to chemical engineering computing. Wiley; 2012.
Inayat A, et al. Biomass steam gasification with in-situ CO2 capture for enriched hydrogen gas production: a reaction kinetics modelling approach. Energies. 2010;3(8):1472–84.
Wang C, et al. Thermodynamics and LCA analysis of biomass supercritical water gasification system using external recycle of liquid residual. Renewable Energy. 2019;141:1117–26.
Nipattummakul N, et al. Hydrogen and syngas yield from residual branches of oil palm tree using steam gasification. Int J Hydrogen Energy. 2011;36(6):3835–43.
He M, et al. Hydrogen-rich gas from catalytic steam gasification of municipal solid waste (MSW): Influence of catalyst and temperature on yield and product composition. Int J Hydrogen Energy. 2009;34(1):195–203.
He M, et al. Hydrogen-rich gas from catalytic steam gasification of municipal solid waste (MSW): Influence of steam to MSW ratios and weight hourly space velocity on gas production and composition. Int J Hydrogen Energy. 2009;34(5):2174–83.
Tinaut FV, et al. Method for predicting the performance of an internal combustion engine fuelled by producer gas and other low heating value gases. Fuel Process Technol. 2006;87(2):135–42.
Hoque M, Rashid F, Aziz M. Gasification and power generation characteristics of rice husk, sawdust, and coconut shell using a fixed-bed downdraft gasifier. Sustainability. 2021;13(4):2027.
Moghadam RA, et al. Syngas production from palm kernel shell and polyethylene waste blend in fluidized bed catalytic steam co-gasification process. Energy. 2014;75:40–4.
Zhang Q, et al. Modeling of steam plasma gasification for municipal solid waste. Fuel Process Technol. 2013;106:546–54.
Kardani N, et al. Modelling of municipal solid waste gasification using an optimised ensemble soft computing model. Fuel. 2021;289:119903.
Khan Z, et al. Integrated catalytic adsorption (ICA) steam gasification system for enhanced hydrogen production using palm kernel shell. Int J Hydrog Energy. 2014;39(7):3286–93.
Shahbaz M, et al. The influence of catalysts in biomass steam gasification and catalytic potential of coal bottom ash in biomass steam gasification: a review. Renew Sustain Energy Rev. 2017;73:468–76.
Chin BLF, et al. Comparative studies on catalytic and non-catalytic co-gasification of rubber seed shell and high density polyethylene mixtures. J Clean Prod. 2014;70:303–14.
Filitz R, et al. Highly efficient CO2 sorbents: development of synthetic, calcium-rich dolomites. Environ Sci Technol. 2011;46(1):559–65.
Han L, et al. Hydrogen production via CaO sorption enhanced anaerobic gasification of sawdust in a bubbling fluidized bed. Int J Hydrogen Energy. 2011;36(8):4820–5482.
Acknowledgements
Author would like to acknowledge National University of Sciences & Technology for financial support.
Novelty statement
Despite the tariff compensation and incentives announced by the Government of Pakistan very few case studies have been reported related to the MSW usage for the hydrogen gas production. In this research indigenous MSW of Lahore, Pakistan has been considered as the feed for the dual fluidized bed gasification technology (Silvia gas technology) for the thermochemical treatment of MSW. Previously researchers have considered the circulating and bubbling fluidized bed reactors, but dual fluidized bed configuration has never been considered for the MSW gasification. Additionally, the systematic analysis and modeling of MSW dual bed gasification unit provides us with the differential analysis of effect of different operating parameters and therefore enabling us to identify the best suited parameters for maximum hydrogen production. Such results only augment the MSW usage as an energy source for sustainable future and provides us the foundation for the economic analysis and the scaleup of the gasification unit on an industrial level.
Funding
This research received no external funding.
Author information
Authors and Affiliations
Contributions
Conceptualization, SH and SH; methodology, SH; software, SH; validation, SH, UAS and HA; formal analysis, SH; data curation, SH; writing—original draft preparation, UAS; writing—review and editing, UAS; visualization, HA; supervision, HA; project administration, HA; funding acquisition, YY. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethical approval and consent to participate
Not applicable.
Consent to publish
Not applicable.
Competing interests
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Shafiq, H., Azam, S.U. & Hussain, A. Steam gasification of municipal solid waste for hydrogen production using Aspen Plus® simulation. Discov Chem Eng 1, 4 (2021). https://doi.org/10.1007/s43938-021-00004-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s43938-021-00004-9