Abstract
Objectives
Our objectives were to evaluate the effectiveness of humanoid robot-based distraction on reducing distress and pain in children undergoing intravenous insertion.
Methods
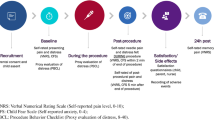
A two-arm, open-label randomized controlled trial was conducted April 2017–May 2018, in a pediatric emergency department (ED). A sample of 86 children aged 6–11 years who required intravenous insertion were recruited. Exclusion criteria included hearing/visual impairments, neurocognitive delay, sensory impairment to pain, previous enrollment, and ED clinical staff discretion. Outcome measures included the Observed Scale of Behavioral Distress-Revised (OSBD-R) (distress) and the Faces Pain Scale-Revised (FPS-R) (pain).
Results
Of the 86 children recruited (median age 9 years, IQR 7,10); 55% (47/86) were male, 9% (7/82) were premature, 82% (67/82) had a previous ED visit, 31% (25/82) had a previous hospitalization and 78% (64/82) had previous intravenous insertion. Ninety-six percent (78/81) received topical anesthetic prior to intravenous insertion. Total OSBD-R distress score was 1.49 ± 2.36 (standard care) versus 0.78 ± 1.32 (robot) (p < 0.05). FPS-R pain score was 4 (IQR 2,6) (standard care) versus 2 (IQR 0,4) (robot) (p = 0.13). Parental anxiety immediately after the procedure was 36.7 (11.1) (standard care) versus 31.3 (8.5) (robot) (p = 0.04). Parents were more satisfied with pain management in the robotic distraction group (95% vs 72% very satisfied) (p = 0.002).
Conclusions
Humanoid robot-based distraction therapy is associated with a modest positive impact on child distress for pediatric intravenous insertion, but not pain. It can be considered a potential tool in the ED toolkit for procedural pain-associated distress reduction.
Clinical trial registration
Clinicaltrials.gov Identifier: NCT02997631.
Résumé
Objectifs
Nos objectifs étaient d'évaluer l'efficacité de la distraction robotique humanoïde pour réduire la détresse et la douleur chez les enfants subissant une insertion intraveineuse.
Méthodes
Un essai contrôlé randomisé ouvert à deux bras a été mené d'avril 2017 à mai 2018, dans un service d'urgence pédiatrique. Un échantillon de 86 enfants âgés de 6 à 11 ans ayant besoin d'une insertion intraveineuse a été recruté. Les critères d'exclusion comprenaient des déficiences auditives / visuelles, un retard neurocognitif, une déficience sensorielle de la douleur, une inscription antérieure et la discrétion du personnel clinique des urgences. Les mesures des résultats comprenaient l’échelle d’hétéro-évaluation comportementale (OSBD-R: Observational Scale of Behavioral Distress – Revised) (détresse) et l’échelle de visages (FPS-R: Faces Pain Scale-Revised) (douleur).
Résultats
Sur les 86 enfants recrutés (âge médian 9 ans, IQR 7,10) ; 55 % (47/86) étaient de sexe masculin, 9 % (7/82) étaient prématurés, 82 % (67/82) avaient une visite antérieure aux urgences, 31 % (25/82) avaient déjà été hospitalisés et 78 % (64/82) avaient déjà été insérés par voie intraveineuse. Quatre-vingt-seize pour cent (78/81) ont reçu une anesthésie topique avant l'insertion intraveineuse. Le score total de détresse OSBD-R était de 1,49 ± 2,36 (soins standard) contre 0,78 ± 1,32 (robot) (p < 0,05). Le score de douleur FPS-R était de 4 (IQR 2,6) (soins standard) contre 2 (IQR 0, 4) (robot) (p=0,13). L’anxiété parentale immédiatement après l’intervention était de 36,7 (11,1) (soins standard) contre 31,3 (8,5) (robot) (p=0,04). Les parents étaient plus satisfaits de la gestion de la douleur dans le groupe de distraction robotique (95 % vs 72 % très satisfaits) (p = 0,002).
Conclusions
La thérapie de distraction à base de robot humanoïde est associée à un impact positif modeste sur la détresse de l’enfant pour l’insertion intraveineuse pédiatrique, mais pas la douleur. Il peut être considéré comme un outil potentiel dans la boîte à outils des Services d’Urgences pour la réduction de la détresse associée à la douleur procédurale.



Similar content being viewed by others
References
Stevens BJ, Abbott LK, Yamada J, Harrison D, Stinson J, Taddio A, et al. Epidemiology and management of painful procedures in children in Canadian hospitals. CMAJ. 2011;183(7):e403–10. https://doi.org/10.1503/cmaj.101341.
Jacobson S. Common medical pains. Paediatr Child Health. 2007;12(2):105–9. https://doi.org/10.1093/pch/12.2.105.
McGrath PJ, McAlpine L. Psychologic perspectives on pediatric pain. J Pediatr. 1993;122(5):S2–8. https://doi.org/10.1016/s0022-3476(11)80002-8.
Curry SL, Russ SW. Identifying coping strategies in children. J Clin Child Psychol. 1985;14(1):61–8. https://doi.org/10.1207/s15374424jccp1401_10.
Maclaren JE, Cohen LL. Interventions for paediatric procedure-related pain in primary care. Paediatr Child Health. 2007;12(2):111–6 (PMID:19030349).
Uman LS, Birnie KA, Noel M, Parker JA, Chambers CT, McGrath PJ, et al. Psychological interventions for needle-related procedural pain and distress for children and adolescents. Cochrane Database Syst Rev. 2013;10:005179. https://doi.org/10.1002/14651858.cd005179.pub3.
Gates M, Hartling L, Shulhan-Kilroy J, MacGregor T, Guitard S, Wingert A, et al. Digital technology distraction for acute pain in children: a meta-analysis. Pediatrics. 2020;145(2):e20191139. https://doi.org/10.1542/peds.2019-1139.
Trottier ED, Doré-Bergeron MJ, Chauvin-Kimoff L, Baerg K, Ali S. Managing pain and distress in children undergoing brief diagnostic and therapeutic procedures. Paediatr Child Health. 2019;24(8):509–35. https://doi.org/10.1093/pch/pxz026.
Ali S, Sivakumar M, Beran T, Scott S, Vandermeer B, Curtis SJ, et al. Study protocol for a randomised controlled trial of humanoid robot-based distraction for venipuncture pain in children. BMJ Open. 2018;8(12):e023366. https://doi.org/10.1136/bmjopen-2018-023366.
Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–81. https://doi.org/10.1016/j.jbi.2008.08.010.
Elliott CH, Jay SM, Woody P. An observation scale for measuring children’s distress during medical procedures. J Pediatr Psychol. 1987;12(4):543–51. https://doi.org/10.1093/jpepsy/12.4.543.
Hicks CL, Von Baeyer CL, Spafford P, Van Korlaar I, Goodenough B. The Faces Pain Scale—Revised: toward a common metric in pediatric pain measurement. Pain. 2001;93(2):173–83. https://doi.org/10.1016/s0304-3959(01)00314-1.
Tucker TI, Slifer JJ, Dahlquist LM. Reliability and validity of the Brief Behavioral Distress Scale: a measure of children’s distress during invasive medical procedures. J Pediatr Psychol. 2001;26(8):513–23. https://doi.org/10.1093/jpepsy/26.8.513.
Spielberger CD, Sydeman SJ. State-trait anxiety inventory and state-trait anger expression inventory. In: Maruish ME, editor. The use of psychological testing for treatment planning and outcome assessment. Hillsdale: Lawrence Erlbaum Associates; 1994. p. 292–321.
Hartling L, Newton AS, Liang Y, Jou H, Hewson K, Klassen TP, et al. Music to reduce pain and distress in the pediatric emergency department: a randomized clinical trial. JAMA Pediatr. 2013;167(9):826–35. https://doi.org/10.1001/jamapediatrics.2013.200.
Tsze DS, Hirschfeld G, Von Baeyer CL, Bulloch B, Dayan PS. Clinically significant difference in acute pain measured on self-report pain scales in children. Acad Emerg Med. 2015;22(4):415–22. https://doi.org/10.1111/acem.12620.
Von Baeyer CL. Children’s self-reports of pain intensity: scale selection, limitations and interpretation. Pain Res Manag. 2006;11(3):157–62. https://doi.org/10.1155/2006/197616.
Bossart P, Fosnocht D, Swanson E. Changes in heart rate do not correlate with changes in pain intensity in emergency department patients. J Emerg Med. 2007;32(1):19–22. https://doi.org/10.1016/j.jemermed.2006.05.029.
Bartfield JM, Janikas JS, Lee RS. Heart rate response to intravenous catheter placement. Acad Emerg Med. 2003;10(9):1005–8. https://doi.org/10.1111/j.1553-2712.2003.tb00660.x.
Jibb LA, Birnie KA, Nathan PC, Beran TN, Hum V, Victor JC, et al. Using the MEDiPORT humanoid robot to reduce procedural pain and distress in children with cancer: a pilot randomized controlled trial. Pediatr Blood Cancer. 2018;65(9):27242. https://doi.org/10.1002/pbc.27242.
Beran TN, Ramirez-Serrano A, Vanderkooi OG, Kuhn S. Reducing children’s pain and distress towards flu vaccinations: a novel and effective application of humanoid robotics. Vaccine. 2013;31(25):2772–7. https://doi.org/10.1016/j.vaccine.2013.03.056.
Stevens BJ, Yamada J, Estabrooks CA, Stinson J, Campbell F, Scott SD, et al. Pain in hospitalized children: effect of a multidimensional knowledge translation strategy on pain process and clinical outcomes. Pain. 2014;155(1):60–8. https://doi.org/10.1016/j.pain.2013.09.007.
McCarthy AM, Kleiber C, Hanrahan K, Zimmerman MB, Westhus N, Allen S. Factors explaining children’s responses to intravenous needle insertions. Nurs Res. 2010;59(6):407–16. https://doi.org/10.1097/nnr.0b013e3181f80ed5.
Gagliese L, Katz J. Age differences in postoperative pain are scale dependent: a comparison of measures of pain intensity and quality in younger and older surgical patients. Pain. 2003;103(1–2):11–20. https://doi.org/10.1016/s0304-3959(02)00327-5.
Tak JH, Van Bon WJ. Pain- and distress- reducing interventions for venepuncture in children. Child Care Health Dev. 2006;32(3):257–68. https://doi.org/10.1111/j.1365-2214.2006.00578.x.
Rudolph KD, Dennig MD, Weisz JR. Determinants and consequences of children’s coping in the medical setting: conceptualization, review, and critique. Psychol Bull. 1995;118(3):328–57. https://doi.org/10.1037/0033-2909.118.3.328.
Walker LK, Baber KF, Garber J, Smith CA. A typology of pain coping strategies in pediatric patients with chronic abdominal pain. Pain. 2008;137(2):266–75. https://doi.org/10.1016/j.pain.2007.08.038.
Noel M, Chambers CT, McGrath PJ, Klein RM, Stewart SH. The influence of children’s pain memories on subsequent pain experience. Pain. 2012;153(8):1563–72. https://doi.org/10.1016/j.pain.2012.02.020.
Poonai N, Alawi K, Rieder M, Lynch T, Lim R. A comparison of amethocaine and liposomal lidocaine cream as a pain reliever before venipuncture in children: a randomized control trial. Pediatr Emerg Care. 2012;28(2):104–8. https://doi.org/10.1097/pec.0b013e3182442c3b.
Taddio A, Soin HK, Schuh S, Koren G, Scolnik D. Liposomal lidocaine to improve procedural success rates and reduce procedural pain among children: a randomized controlled trial. CMAJ. 2005;172(13):1691–5. https://doi.org/10.1503/cmaj.045316.
Acknowledgements
This study was funded through a Support Platforms for Integrated Research (SPIR) Grant from the Women & Children’s Health Research Institute (WCHRI, Edmonton, Alberta, Canada), for which Dr. Samina Ali and Dr. Sarah Curtis are co-principal investigators (2014–2018). Dr. Hartling is supported by a Canada Research Chair in Knowledge Synthesis and Translation, and is a Distinguished Researcher with the Stollery Science Lab. Dr. Scott is supported by a Canada Research Chair in Knowledge Translation in Child Health, and is a Distinguished Researcher with the Stollery Science Lab.
Funding
Dr. Tanya Beran is the founder of RxRobots, a company that is the maker of the robot’s software used in this study. Our team purchased the robot at full price, with a Stollery Children’s Hospital Foundation Grant. Dr. Tanya Beran has not participated in data collection or statistical analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Dr. Tanya Beran is the founder of RxRobots, a company that is the maker of the robot’s software used in this study. Dr. Tanya Beran has not participated in data collection or statistical analysis.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ali, S., Manaloor, R., Ma, K. et al. A randomized trial of robot-based distraction to reduce children’s distress and pain during intravenous insertion in the emergency department. Can J Emerg Med 23, 85–93 (2021). https://doi.org/10.1007/s43678-020-00023-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43678-020-00023-5