Abstract
Photobiomodulation (PBM) of deep brain structures through transcranial infrared irradiation might be an effective treatment for Parkinson’s disease (PD). However, the mechanisms underlying this intervention should be elucidated to optimize the therapeutic outcome and maximize therapeutic efficacy. The present study aimed at investigating the oxidative stress-related parameters of malondialdehyde (MDA), nitric oxide (NO), and reduced glutathione (GSH) and the enzymatic activities of sodium–potassium-ATPase (Na+, K+-ATPase), Acetylcholinesterase (AChE), and monoamine oxidase (MAO) and monoamine levels (dopamine (DA), norepinephrine (NE) and serotonin (5-HT) in the midbrain and striatum of reserpine-induced PD in an animal model treated with PBM. Furthermore, the locomotor behavior of the animals has been determined by the open field test. Animals were divided into three groups; the control group, the PD-induced model group, and the PD-induced model treated with the PBM group. Non-invasive treatment of animals for 14 days with 100 mW, 830 nm laser has demonstrated successful attainment in the recovery of oxidative stress, and enzymatic activities impairments induced by reserpine (0.2 mg/kg) in both midbrain and striatum of adult male Wistar rats. PBM also improved the decrease in DA, NE, and 5-HT in the investigated brain regions. On a behavioral level, animals showed improvement in their locomotion activity. These findings have shed more light on some mechanisms underlying the treatment potential of PBM and displayed the safety, easiness, and efficacy of PBM treatment as an alternative to pharmacological treatment for PD.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Parkinson's disease (PD) is a neurodegenerative disorder that is characterized by the degeneration of dopaminergic neurons in the substantia nigra region of the brain [1]. Although the exact cause of PD is not fully understood, there is evidence to suggest that both enzymatic activity and oxidative stress play a role in the development and progression of the disease [2]. Enzymes play a pivotal role in neuronal conduction and information processing and their impairment might be linked to the etiology of the disease. In PD, it is suggested that enzymes such as monoamine oxidase (MAO), sodium–potassium-ATPase (Na+, K+-ATPase), and acetylcholinesterase (AchE) may be involved in the degeneration of dopaminergic neurons [3, 4].
MAO is an enzyme that breaks down dopamine (DA), a neurotransmitter that is essential for the proper functioning of the brain. However, when DA is broken down by MAO, it produces hydrogen peroxide and other reactive oxygen species (ROS) that can cause oxidative stress and damage to cells [5]. Acetylcholinesterase (AchE) is an enzyme that breaks down the neurotransmitter acetylcholine (Ach) in the synaptic cleft, terminating its action. Although PD is primarily characterized by the degeneration of dopaminergic neurons, there is evidence to suggest that cholinergic neurons, which release Ach, are also affected by the disease [6]. In PD, the loss of dopaminergic neurons in the substantia nigra (SN) results in a reduction of DA levels in the striatum, which can lead to a compensatory increase in ACh release from cholinergic neurons [7]. This increase in ACh release may contribute to the motor symptoms of PD, such as tremors, rigidity, and bradykinesia, by disrupting the balance between DA and ACh in the striatum [8]. Furthermore, it was reported that AChE activity is decreased in the brains of individuals with PD [9]. The decreased AchE activity in the basal ganglia may deteriorate the balance between dopamine and Ach [10].
Oxidative stress refers to the imbalance between the production of ROS and the ability of the body to detoxify them. In PD, it is documented that oxidative stress plays a significant role in the degeneration of dopaminergic neurons [5]. ROS can damage cells by oxidizing lipids, proteins, and DNA, which can lead to cell death [11]. This damage can also trigger inflammation and immune responses, which can further contribute to the progression of the disease [12]. Nitric oxide (NO) is a signaling molecule that plays a critical role in a variety of physiological processes, including the regulation of blood pressure, immune function, and neurotransmission [13]. In the context of PD, NO has been implicated in the pathogenesis of the disease [14]. There is evidence that NO production is increased in the brains of animal models of PD [15]. This increase in NO may contribute to the degeneration of dopaminergic neurons by promoting oxidative stress, inflammation, and apoptosis (programmed cell death) [15, 16]. NO can also react with superoxide to form peroxynitrite, a highly reactive molecule that can cause further damage to cells [17]. In addition, it is reported that NO may play a role in the regulation of DA release in the brain [18]. NO can modulate the release of DA by interacting with dopaminergic neurons and the cells that surround them, known as glial cells [19, 20]. This modulation of DA release may contribute to the motor symptoms of PD, such as tremors and rigidity [8].
Ample evidence suggests that mitochondrial dysfunction contributes to oxidative stress, which can lead to cellular damage and death [21]. Mitochondrial dysfunction can lead to a reduction in the production of ATP, the main source of energy for cells, which can lead to increased ROS production [22]. ROS can then cause oxidative damage to cellular components, such as lipids, proteins, and DNA, which can further impair mitochondrial function and lead to cell death [12]. In addition to mitochondrial dysfunction, there is evidence to suggest that enzymatic impairments may also contribute to the development and progression of PD. Enzymes such as monoamine oxidase (MAO) and catechol-O-methyltransferase (COMT) are involved in the metabolism of DA, a neurotransmitter that is depleted in PD [23]. The breakdown of DA by these enzymes can produce ROS and other reactive species, which can contribute to oxidative stress and cellular damage [24]. The interplay between mitochondrial dysfunction, oxidative stress, and enzymatic impairment in PD is complex and multifactorial. While each of these factors may contribute independently to the development and progression of the disease, they are also interrelated and can influence each other. Further research is needed to fully understand the mechanisms underlying PD and to develop effective treatments for the disease.
In recent years photobiomodulation (PBM) has emerged as an effective, non-invasive treatment of neurological disorders with minimal side effects [25, 26]. Evidence suggests that PBM can reduce oxidative stress by increasing the activity of antioxidant enzymes, such as superoxide dismutase (SOD) and catalase, which can protect cells from damage caused by ROS. Thereby, PBM can reduce lipid peroxidation, a process that can lead to cell damage and death [27]. PBM has also been shown to increase the activity of enzymes involved in the synthesis of neurotransmitters, such as tyrosine hydroxylase, which can improve dopaminergic function in the brain [28]. While the exact mechanisms underlying the effects of PBM on oxidative stress and enzymatic activity in PD are not fully understood, it is thought that PBM may work by increasing mitochondrial function and reducing inflammation in the brain [29, 30]. Therefore, PBM has shown promise as a potential treatment for reducing oxidative stress and modulating enzymatic activity in various neurological disorders [31]. However, further research is needed to fully understand the mechanisms underlying these effects and to determine the optimal parameters for PBM treatment in PD.
The present work aimed at inducing PD in an animal model by the monoamine-depleting drug reserpine and investigating the potential treatment efficacy of PBM in terms of oxidative stress parameters, monoamine neurotransmitters, and enzymatic activities in two related brain regions midbrain and striatum. Furthermore, the assessment of animal locomotion behavior has been carried out to determine the overall outcome of this treatment modality.
2 Materials and methods
2.1 Animals
Twenty-one adult male Wistar rats weighing 150–200 g were obtained from a local breeding facility and maintained in 7-animal cages for 1 week prior to studies as part of the laboratory acclimatization period. The animals were kept in a standard light/dark cycle (12/12) and at a constant temperature (25 ± 2 °C). Animals had unlimited access to standard food pellets and tap water throughout the research period. All experimental procedures adhered to international standards for animal ethics and care and were authorized by the local animal care and use committee under permission number (F-46-19).
2.2 Experimental design
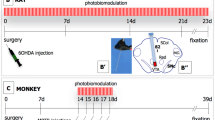
Animals were divided into three groups (n = 7). The first group, the control group, was injected with the drug vehicle for 14 days followed by the drug vehicle and sham exposure to laser (laser off) for another 14 days. The second group is the rat model of PD that was injected daily with reserpine (0.2 mg/kg, i.p.) for 2 weeks (from 1st to 14th day, model establishment period) and (0.1 mg/kg, i.p.) for another 2 weeks to sustain the Parkinsonism state [32], and the third group is the rat model of PD induced by reserpine (0.2 mg/kg) for the first 14 days followed by reserpine treatment (0.1 mg/kg/day) and daily PBM via transcranial low-laser irradiation (830 nm, 100 mW and 127.4 mW/cm2 irradiance) for 2 weeks (from 15 to 28th day). The heads of all animal groups were shaved whether they were exposed or not to the laser irradiation. Transcranial laser irradiation was carried out, while the animals were gently fixed in the hand of the experimenter, on six different points (three on each side of the longitudinal commissure and between bregma and lambda) avoiding any uncomfortable conditions for the animals. At the end of the experiment, the open field test (OFT) was conducted on the different animal groups. The OFT is a commonly used behavioral test in animal models of PD that measures locomotor activity, exploratory behavior, and anxiety-like behavior in rodents. The test is often used to assess the effects of experimental treatments on motor function and behavior in PD animal models.
2.3 Preparation of samples
Animals were decapitated following OFT. Then, the brain of each rat was dissected out on an ice-cold plate and divided into two halves; right and left. Each half was dissected to get the midbrain and striatum. The left half of each midbrain and striatum was homogenized in an ice-cold solution of acidified n-butanol and used for monoamine determination. The right half of each area was homogenized in Tris–HCl buffer (pH 7.4) and used for oxidative stress and enzymatic activity measurements. All tissues were frozen at − 80 °C until measurements.
2.4 Laser irradiation procedures
A diode laser (GaAlAs, Lasotronic Inc., Zug, Switzerland) with an output of 100 mW was employed as a laser source in this work. Before each irradiation session, the wavelength and power of the laser probe were measured using a handheld laser power meter (BWT-840000361, Metrohm, UK). During the irradiation session, the animal's shaved and cleaned head was carefully positioned to the laser probe. To expose the entire cortex, the laser probe was put above and in touch with the animal's head in six different places for two minutes each. Laser parameters and places of the laser irradiation on the animal’s head are illustrated in Table 1 and Fig. 1.
2.5 Open field test
The open-field apparatus was constructed of white plywood and measured 72 cm × 72 cm with 36 cm walls. One of the walls was clear plexiglass, so rats could be visible in the apparatus. Blue lines were drawn on the floor with a marker and were visible through the clear plexiglass floor. The lines divided the floor into sixteen 18 cm × 18 cm squares. A central square (18 cm × 18 cm) was drawn in the middle of the open field [33]. Rats were placed individually in the center of the open-field and behavioral parameters were assessed manually for 10 min. Four motor parameters were quantified throughout this test: the number of squares crossed (the number of times the rats crossed one of the squares with all four paws), time spent in the central area (the duration of time the rats spent in the central square), immobilization (freezing) time (duration in which the rat was completely immobilized) and number of rearing (the number of times the rats stood on their hind legs in the maze). The open-field apparatus was cleaned after each session using 70% ethanol and permitted to dry between tests.
2.6 Determination of lipid peroxidation
Lipid peroxidation was assayed by measuring malondialdehyde (MDA) levels in the midbrain and striatum using the method described by Ruiz-Larrea et al. [34]. MDA reacts with thiobarbituric acid producing a pink-colored complex whose absorbance is read at 532 nm.
2.7 Determination of nitric oxide
Nitric oxide (NO) was estimated spectrophotometrically in the midbrain and striatum according to the method described by Montgomery and Dymock [35]. This method depends on the measurement of endogenous nitrite concentration as an indicator of nitric oxide production in biological samples. On the addition of the Griess reagent, the nitrite is converted into a bright reddish–purple color whose absorbance is measured at 540 nm.
2.8 Determination of reduced glutathione
The estimation of reduced glutathione (GSH) was carried out spectrophotometrically in the midbrain and striatum using the method described by Beutler et al. [36]. This method depends on the reaction GSH reacts with 5,5′ dithiobis-2-nitrobenzoic acid (DTNB) yielding a yellow-colored chromogen whose absorbance can be measured at 412 nm.
2.9 Determination of Na+/ K+-ATPase activity
The estimation of Na+/K+-ATPase activity was carried out spectrophotometrically in the midbrain and striatum using the method described by Tsakiris et al. [37]. The activity of Na+, K+-ATPase was calculated as the difference between the total ATPase activity (Na+, K+-ATPase, and Mg-ATPase activity) and Mg-ATPase activity. Total ATPase activity was assayed by adding 50 μL of the brain tissue homogenate to 2.5 mL of the incubation medium consisting of 50 mM Tris–HCl (pH 7.4), 120-mM NaCl, 20-mM KCl, 4-mM MgCl2, 240-mM sucrose, 1-mM ethylenediamine tetra-acetic acid, and 3-mM disodium ATP (substrate). After an incubation period of 10 min at 37°C, the reaction was stopped by the addition of 50-μL ice-cold trichloroacetic acid (30%). Then the mixture was centrifuged at 3000 rpm for 15 min. 1mL of the supernatant was added to 500 μL of 10% trichloroacetic acid, 250-μL of 1% ammonium molybdate, and 250-μL of 20% ascorbic acid and used for the determination of the liberated inorganic phosphate (Pi). After 20 min at room temperature, the developed color was read at 640 nm against the blank, which contained 1.5 mL of 10% trichloroacetic acid, 250-μL of 1% ammonium molybdate, and 250-μL of 20% ascorbic acid. The amount of liberated inorganic phosphate was quantified using KH2PO4 as a reference standard. The previous steps were repeated in the presence of 1 mM of ouabain (specific Na+, K+-ATPase inhibitor) in the incubation medium.
2.10 Determination of acetylcholinesterase activity
Acetylcholinesterase (AchE) activity was measured in the midbrain and striatum using the method described by Gorun et al. [38]. The principle of the method is the measurement of the thiocholine produced as acetylthiocholine is hydrolyzed. The absorbance was measured at 412 nm. The results were expressed as μmol SH/min/g brain tissue.
2.11 Determination of monoamine oxidase activity
Monoamine oxidase (MAO) activity was estimated in the midbrain and striatum using the method described by Holt et al. [39]. The method depends on the conversion of benzylamine to benzaldehyde whose absorbance was measured at 250 nm.
2.12 Determination of monoamine concentrations
The determination of serotonin (5-HT), norepinephrine (NE), and dopamine (DA) levels in the midbrain and striatum tissue homogenates was carried out according to the method of Ciarlone [40]. The fluorescence was measured using a spectrofluorometer (Jasco FP-6500, JASCO Ltd., Tokyo, Japan) with a source of xenon arc lamp 150 W (excitation slit bandwidth of excitation monochromator: 5 nm, and emission slit bandwidth of emission monochromator: 5 nm).
2.13 Statistical analysis
All results were expressed as means ± S.E. All statistical calculations were carried out using Statistical Package for Social Sciences (SPSS) software (version 16). Statistical difference between the groups under investigation was tested by one-way analysis of variance (ANOVA) followed by Duncan as a post hoc test. The difference was considered significant at a p value ≤ 0.05.
3 Results
3.1 Open field test
As illustrated in Fig. 2, statistical analysis revealed a significant decrease in the number of squares crossed (74.62%) and number of rearing (79.08%) in the rat model of PD, as compared to control rats. This was associated with a significant increase in the time spent in the central area (184.74%) and freezing time (462.98%) as compared to control rats. However, treatment with transcranial IR laser restored these changes to control-like values except for the number of rearing which was still significantly decreased below the control values.
Open field test (OPF) results in control, PD model, and PD model treated with IR laser groups. A Number of squares crossed and rearing, B Time spent in the central zone and freezing. Data represented as mean values ± SD. Bars labeled with different numbers are significantly different at P < 0.05 and with same numbers are not significantly different at P < 0.05
3.2 Oxidative stress
Malondialdehyde (MDA), nitric oxide (NO), and glutathione (GSH) are molecules that are involved in oxidative stress and have been studied in relation to PD. Elevated levels of MDA, NO, and GSH indicate increased oxidative stress in the affected tissues. As shown in Fig. 3, the analysis revealed that there is a significant increase in MDA (111.11% & 83.33%), NO (80% & 111.11%), and GSH (26.13% & 25.32%) for both midbrain and striatum regions in the brain of animals representing the PD model with respect to the control group, respectively. Treatment of the reserpinized animals for 14 days with transcranial IR laser has recovered MDA, NO, and GSH in the midbrain and striatum to be nonsignificant with respect to the control group of animals.
Variation in A lipid peroxidation (MDA), B nitric oxide (NO), and C reduced glutathione (GSH) in the midbrain and striatum of different animal groups. Bars labeled with the same letters denote nonsignificant change, while bars labeled with different letters denote significant differences at P < 0.05
3.3 Enzymatic activities
Na+, K+-ATPase, AchE, and MAO are enzymes that play important roles in the pathophysiology of PD. Dysfunction of these enzymes may lead to impaired cellular function which may contribute to their degeneration. A significant attenuation in the activities of Na+, K+-ATPase (26.32% & 23.91%), and AchE (28.64 & 31.91%) have been observed in the midbrain and striatum of the PD model group of animals with respect to their control values, respectively. This was accompanied by a significant increase of MAO (34.05% & 30.07%) in both the midbrain and striatum, respectively as compared with the control group (Fig. 4). Treatment with IR laser for 14 days has recovered the activities of all enzymes to be non-significantly different from control values in both the midbrain and striatum except the Na+, K+-ATPase activity in the midbrain which did not recover to its normal level.
3.4 Monoamines level
Monoamines, including dopamine (DA), norepinephrine (NE), and serotonin (5-HT), play important roles in the regulation of motor and non-motor functions in the brain. In animal models of PD, there are changes in the levels of monoamines in the midbrain and striatum. Compared to levels of monoamines in the control group of animals, a significant decrease in 5-HT (43.87% & 22.79%), NE (40.43% & 43.66%), and DA (32.56% & 37%) has occurred in the PD model group of animals in both midbrain and striatum, respectively (Fig. 5). In the midbrain and striatum, treatment with IR laser for 14 days restored the levels of 5-HT and NE to control-like values and improved the DA level to a level that was nonsignificant as compared to control and PD rats.
4 Discussion
Photobiomodulation (PBM) is a non-invasive therapy that uses low-level light mainly in infrared wavelengths to stimulate cellular function and promote tissue repair [41]. It can also improve motor function and reduce oxidative stress and neuronal damage in animal models of neurodegenerative disease [42, 43]. This effect may be mediated by its ability to increase the activity of antioxidant enzymes, reduce cerebral inflammation, and increase the expression of anti-apoptotic genes [31, 34, 44]. We sought in the present work to investigate the efficacy of PBM to alleviate motor deficits, oxidative stress, enzymatic inhibition/activation, and decreased monoamine levels induced by reserpine in two PD-related brain regions: midbrain and striatum.
Reserpine induces PD symptoms in animals via the disruption of monoamine transport into synaptic vesicles by blocking the vesicular monoamine transporter (VMAT), which is responsible for the transport of DA, 5-HT, and NE into synaptic vesicles [45], thus decreasing the availability of these neurotransmitters in the synaptic cleft and causing motor and non-motor symptoms of PD [46]. In addition, reserpine has been shown to decrease mitochondrial respiration and ATP production, as well as increase oxidative stress and mitochondrial DNA damage [47]. This can lead to mitochondrial dysfunction, which can contribute to the pathophysiology of PD. Furthermore, reserpine-induced mitochondrial dysfunction can lead to the activation of neuroinflammatory pathways, which can contribute to the neurodegeneration observed in PD [48].
The present decrease in the monoamine neurotransmitters in the midbrain and striatum induced by reserpine might occur as a result of the inhibition of VMAT which in turn, inhibits the vesicular re-uptake of monoamines and increases their presence in the cytosol and consequently their exposure to monoamine oxidase (MAO) which is the monoamines metabolizing enzyme. VMAT inhibition can lead to an increase in MAO activity, which can contribute to the degeneration of dopaminergic neurons in PD [49]. This mechanism could potentially explain the observed increase in MAO activity, as it is activated to break down the excess monoamines, ultimately leading to a decrease in monoamine levels. This decrease in monoamines could then contribute to the development of both motor and non-motor symptoms in PD [50, 51]. This condition may underlie the reduced motor activity of the rat model of PD observed in the present study. In the midbrain and striatum, monoamine neurotransmitters especially dopamine play a crucial role in controlling motor activity [52]. Furthermore, the increase in MAO activity has been linked to oxidative stress in the brain [53]. The oxidative deamination of monoamine neurotransmitters generates hydrogen peroxide (H2O2) and other ROS, which can lead to oxidative stress and damage to cellular components.
In the present study, repeated reserpine administration in animals led to oxidative stress in both midbrain and striatum tissues. This manifests itself in the significant increase obtained in MDA, NO, and GSH levels compared to control values. Reserpine as a VMAT inhibitor resulted in an increase in cytoplasmic monoamine levels, particularly DA [51], which can increase the likelihood of DA oxidation and production of free radicals and cellular oxidative stress [54]. Monoamines are involved in the regulation of mitochondrial function, and they play a key role in the electron transport chain (ETC), which generates ATP in mitochondria [55, 56]. Inhibition of VMAT can lead to a decrease in monoamine availability for the ETC, which can impair mitochondrial function. This causes an imbalance between ROS production and antioxidant defenses, leading to an accumulation of ROS and oxidative stress [57]. Moreover, impaired ETC function resulted in electrons leaking out of the chain and reacting with oxygen to form ROS [58, 59].
NO is a signaling molecule that plays a role in many physiological processes, including the regulation of blood flow and neurotransmitter release [14]. However, the overproduction of NO can lead to mitochondrial dysfunction, which can contribute to the pathogenesis of various diseases including neurodegenerative diseases such as PD [15, 60]. In the context of PD, an increase in NO levels has been observed in the substantia nigra, a brain region that is particularly vulnerable to oxidative stress and dopaminergic cell death in PD [61]. The increase in NO levels is thought to be due to the activation of inducible nitric oxide synthase (iNOS), an enzyme that produces NO in response to inflammation and oxidative stress [62]. NO can impair mitochondrial function by inhibiting complex I of the ETC [63]. Inhibition of complex I leads to a decrease in ATP production and an increase in ROS production [64]. NO can also react with other molecules to form reactive nitrogen species (RNS), which can further contribute to mitochondrial dysfunction. For example, peroxynitrite, a highly reactive RNS formed from the reaction of NO with superoxide radicals, can damage mitochondrial components such as DNA, proteins, and lipids, leading to further impairment of mitochondrial function [19].
An increase in GSH levels is often considered a compensatory mechanism to counteract oxidative stress and mitochondrial dysfunction. GSH is a tripeptide antioxidant that plays a crucial role in protecting cells from oxidative damage by scavenging free radicals and ROS [65]. When cells are exposed to oxidative stress or mitochondrial dysfunction, they can increase the production of GSH to neutralize ROS and prevent further damage. GSH can interact directly with ROS, neutralizing them and converting them to less harmful molecules [66]. However, in some cases, an increase in GSH levels may not be sufficient to prevent oxidative damage, especially if there are other factors contributing to oxidative stress, such as inflammation or mitochondrial dysfunction [67].
One main finding of the present work is the significant alleviating and treating effect of PBM on oxidative stress induced by reserpine in the midbrain and striatum of the animal model of PD. This is suggested to be intimately related to the known effect of PBM on mitochondrial function and its complex enzymes such as cytochrome c oxidase [68, 69]. Several studies have shown that PBM can improve mitochondrial function by increasing ATP production, reducing ROS production, and improving mitochondrial respiration and membrane potential [68, 70]. PBM has been shown to increase the activity of antioxidant enzymes such as superoxide dismutase (SOD) and catalase, which can scavenge reactive oxygen species (ROS) and reduce oxidative stress [31]. Returning the GSH level observed in the PBM-treated group to its normal level may indicate the enhancement in the cellular antioxidant capacity through increasing antioxidant enzymes induced by PBM. Additionally, PBM has been shown to inhibit the activity of the inducible nitric oxide synthase (iNOS) enzyme, which produces NO in response to inflammation and oxidative stress [71]. By reducing NO production, PBM can prevent NO-mediated mitochondrial dysfunction and oxidative stress.
Neuronal enzymatic activity is crucial to neuronal function because enzymes play a role in maintaining cellular homeostasis, regulating signaling pathways, and producing and degrading neurotransmitters. Na+, K+-ATPase is a transmembrane enzyme that plays a role in maintaining the ion gradient across the cell membrane [72]. AchE is an enzyme that breaks down the neurotransmitter Ach. MAO is a mitochondrial outer membrane enzyme that breaks down monoamine neurotransmitters such as DA, 5-HT, and NE.
In the PD animal model group, a significant decrease in Na+, K+-ATPase has been observed in the midbrain and striatum which can lead to ion imbalance and neuronal dysfunction. It has been reported that the progressive attenuation in the Na+, K+-ATPase activity may exacerbate neurodegeneration [73]. Clinically, studies found that Na+, K+-ATPase activity is reduced in PD patients [74]. Na+, K+-ATPase is highly sensitive to oxidative stress and reacts promptly to energy deficiency induced by mitochondrial dysfunction in the brain [75]. This is also evident in other animal models of PD induced by 6-hydroxydopamine [76] and rotenone [4] which are known as neurotoxins that induce mitochondrial dysfunction.
On the other hand, PBM has been shown to restore the activity of Na+, K+-ATPase. PBM can increase Na+, K+-ATPase activity by promoting the release of NO from cytochrome c oxidase and activating the PI3K/Akt signaling pathway [77]. A study has demonstrated the beneficial effects of PBM on Na+, K+-ATPase activity in neuronal injury [78]. PBM has also been shown to increase Na+, K+-ATPase activity and improve mitochondrial function in animal models of neurodegenerative diseases such as AD [33].
The balance between Ach and DA in the striatum is critical for normal motor function. In PD, the loss of dopaminergic neurons and subsequent decrease in DA levels can lead to an imbalance between Ach and DA, with an increase in cholinergic tone relative to dopaminergic tone. This imbalance can contribute to the motor symptoms of PD [79]. In the present study, a significant inhibition in the AchE activity has been determined in both the midbrain and striatum of PD model animals. This inhibition in AchE activity contributes to the enhancement of cholinergic levels in the midbrain and striatum regions of the brain which may be associated with some physiological and behavioral effects of reserpine on animals. Mitochondrial dysfunction can affect the activity of AchE and its expression in the brain [80].
Additionally, the increase in MAO activity can also contribute to mitochondrial dysfunction [63], as ROS generated by MAO can damage mitochondrial DNA, proteins, and lipids, leading to impaired mitochondrial function and energy production. Conversely, mitochondrial dysfunction can also contribute to an increase in MAO activity. Mitochondrial dysfunction can lead to an increase in oxidative stress and ROS production, which can activate MAO and further exacerbate oxidative stress in neurons [63, 81].
The activity of AchE and MAO has been recovered in animals treated with PBM for 14 days. This important finding shows the potential implication of PBM as a treatment modality of PD. The alteration in both these enzymes is interrelated to the symptoms displayed by PD in animal models and human patients. This alteration in enzyme activity has been suggested to be associated with mitochondrial dysfunction and oxidative stress. Due to the ability of PBM to enhance mitochondrial function and reduce oxidative stress in dopaminergic neurons, it offers a promising approach for the treatment of neurological disorders such as PD. Recent studies have provided compelling evidence supporting the efficacy of PBM in penetrating tissues and effectively reaching its intended targets to initiate therapeutic effects, especially when utilizing IR wavelengths and administering an appropriate dosage [82, 83]. The exact mechanisms underlying the interaction between PBM and enzymatic activity, specifically MAO and AChE, are not fully understood. However, one proposed mechanism is that PBM may modulate enzymatic activity by altering the redox state of the cell [31]. PBM has been shown to increase the activity of enzymes involved in cellular energy production, such as cytochrome c oxidase, which can improve cellular function and reduce oxidative stress. By altering the redox state of the cell, PBM may indirectly influence the activity of enzymes such as AchE, which are involved in neurotransmitter metabolism. The other suggested mechanism is the direct interaction between the laser radiation and the enzymes via a photochemical reaction that may alter the activity of the enzyme [84].
On the behavioral level, PBM improved locomotor activity and reduced anxiety-like behavior in the animal model of PD treated with transcranial laser irradiation. This behavioral outcome can be explained by the positive effects PBM has on oxidative stress and modulation of enzymatic activities which may arise from the direct effect of PBM on mitochondrial function in the areas of the brain related to movement and anxiety. This finding is in agreement with Reinhart et al. [85] who found an improvement in the locomotion of an animal model of PD induced by MPTP after treatment with 810 nm near-infrared light. They attributed this outcome to the stimulation of dopaminergic cell transmission through the striatum or the activity of non-dopaminergic cells in the brain that are involved in movement generation.
Based on the present study findings, we can conclude that PBM has a promising efficacy in reducing oxidative stress, alteration of neuronal enzymatic activity, and neurotransmission which has been reflected in the improvement of the behavioral outcomes in an animal model of PD. The potential of this modality as a non-invasive and safe therapeutic approach might be obtained by the direct interaction of the infrared laser radiation with the specific targets via photochemical interaction or indirectly through the enhancement of mitochondria function. Further research is needed to fully understand the mechanisms underlying the effects of PBM and to optimize the parameters of the treatment.
References
Collins, L. M., Toulouse, A., Connor, T. J., & Nolan, Y. M. (2012). Contributions of central and systemic inflammation to the pathophysiology of Parkinson’s disease. Neuropharmacology, 62(7), 2154–2168.
Dias, V., Junn, E., & Mouradian, M. M. (2013). The role of oxidative stress in Parkinson’s disease. Journal of Parkinson’s Disease, 3(4), 461–491.
Blum, D., Torch, S., Lambeng, N., Nissou, M., Benabid, A. L., Sadoul, R., & Verna, J. M. (2001). Molecular pathways involved in the neurotoxicity of 6-OHDA, dopamine and MPTP: Contribution to the apoptotic theory in Parkinson’s disease. Progress in Neurobiology, 65(2), 135–172.
Khadrawy, Y. A., Salem, A. M., El-Shamy, K. A., Ahmed, E. K., Fadl, N. N., & Hosny, E. N. (2017). Neuroprotective and therapeutic effect of caffeine on the rat model of Parkinson’s disease induced by rotenone. Journal of Dietary Supplements, 14, 553–572.
Meiser, J., Weindl, D., & Hiller, K. (2013). Complexity of dopamine metabolism. Cell Communication and Signaling: CCS, 11(1), 34. https://doi.org/10.1186/1478-811X-11-34
Müller, M. L., & Bohnen, N. I. (2013). Cholinergic dysfunction in Parkinson’s disease. Current Neurology and Neuroscience Reports, 13(9), 377. https://doi.org/10.1007/s11910-013-0377-9
Swathi, G., Bhuvaneswar, C., & Rajendra, W. (2013). Alterations of cholinergic neurotransmission in rotenone induced parkinson’s disease: Protective role of bacopa monnieri. International Journal of Pharmacy and Biological Science, 3, 286–292.
Lester, D. B., Rogers, T. D., & Blaha, C. D. (2010). Acetylcholine-dopamine interactions in the pathophysiology and treatment of CNS disorders. CNS Neuroscience & Therapeutics, 16(3), 137–162.
Bohnen, N. I., Müller, M. L., Kotagal, V., Koeppe, R. A., Kilbourn, M. R., Gilman, S., Albin, R. L., & Frey, K. A. (2012). Heterogeneity of cholinergic denervation in Parkinson’s disease without dementia. Journal of Cerebral Blood Flow and Metabolism, 32(8), 1609–1617.
Bohnen, N. I., Kaufer, D. I., Ivanco, L. S., Lopresti, B., Koeppe, R. A., Davis, J. G., Mathis, C. A., Moore, R. Y., & DeKosky, S. T. (2003). Cortical cholinergic function is more severely affected in parkinsonian dementia than in Alzheimer disease: An in vivo positron emission tomographic study. Archives of Neurology, 60, 1745–1748.
Sanders, L. H., & Greenamyre, T. J. (2013). Oxidative damage to macromolecules in human Parkinson disease and the rotenone model. Free Radical Biology & Medicine, 62, 111–120.
Pajares, M., Rojo, A. I., Manda, G., Boscá, L., & Cuadrado, A. (2020). Inflammation in Parkinson’s disease: mechanisms and therapeutic implications. Cells, 9(7), 1687. https://doi.org/10.3390/cells9071687
Tuteja, N., Chandra, M., Tuteja, R., & Misra, M. K. (2004). Nitric oxide as a unique bioactive signaling messenger in physiology and pathophysiology. Journal of Biomedicine & Biotechnology, 2004(4), 227–237.
Hirsch, E. C., & Hunot, S. (2000). Nitric oxide, glial cells and neuronal degeneration in parkinsonism. Trends in Pharmacological Sciences, 21(5), 163–165.
Xiong, Z. K., Lang, J., Xu, G., Li, H. Y., Zhang, Y., Wang, L., Su, Y., & Sun, A. J. (2015). Excessive levels of nitric oxide in rat model of Parkinson’s disease induced by rotenone. Experimental and Therapeutic Medicine, 9(2), 553–558.
Wei, T., Chen, C., Hou, J., Xin, W., & Mori, A. (2000). Nitric oxide induces oxidative stress and apoptosis in neuronal cells. Biochimica et Biophysica Acta, 1498(1), 72–79.
Huie, R. E., & Padmaja, S. (1993). The reaction of no with superoxide. Free Radical Research Communications, 18(4), 195–199.
Liu, Y. (1996). Nitric oxide influences dopaminergic processes. Advances in Neuroimmunology, 6(3), 259–264.
Cook, J. A., Wink, D. A., Blount, V., Krishna, M. C., & Hanbauer, I. (1996). Role of antioxidants in the nitric oxide-elicited inhibition of dopamine uptake in cultured mesencephalic neurons. Insights into potential mechanisms of nitric oxide-mediated neurotoxicity. Neurochemistry International, 28(5–6), 609–617.
Nolan, S. O., Zachry, J. E., Johnson, A. R., Brady, L. J., Siciliano, C. A., & Calipari, E. S. (2020). Direct dopamine terminal regulation by local striatal microcircuitry. Journal of Neurochemistry, 155(5), 475–493.
Rego, A. C., & Oliveira, C. R. (2003). Mitochondrial dysfunction and reactive oxygen species in Excitotoxicity and apoptosis: Implications for the pathogenesis of neurodegenerative diseases. Neurochemical Research, 28, 1563–1574.
Bhatti, J. S., Bhatti, G. K., & Reddy, P. H. (1863). (2017): Mitochondrial dysfunction and oxidative stress in metabolic disorders: A step towards mitochondria based therapeutic strategies. Biochimica et Biophysica Acta, Molecular Basis of Disease, 5, 1066–1077.
Dorszewska, J., Prendecki, M., Oczkowska, A., Rozycka, A., Lianeri, M., & Kozubski, W. (2013). Polymorphism of the COMT, MAO, DAT, NET and 5-HTT genes, and biogenic amines in Parkinson’s disease. Current Genomics, 14(8), 518–533.
Graves, S. M., Xie, Z., Stout, K. A., Zampese, E., Burbulla, L. F., Shih, J. C., Kondapalli, J., Patriarchi, T., Tian, L., Brichta, L., Greengard, P., Krainc, D., Schumacker, P. T., & Surmeier, D. J. (2020). Dopamine metabolism by a monoamine oxidase mitochondrial shuttle activates the electron transport chain. Nature Neuroscience, 23(1), 15–20.
Mohammed, H. S. (2016). Transcranial low-level infrared laser irradiation ameliorates depression induced by reserpine in rats. Lasers in Medical Science, 31(8), 1651–1656. https://doi.org/10.1007/s10103-016-2033-5
Hong, N. (2019). Photobiomodulation as a treatment for neurodegenerative disorders: Current and future trends. Biomedical Engineering Letters, 9(3), 359–366.
Salehpour, F., Farajdokht, F., Erfani, M., Sadigh-Eteghad, S., Shotorbani, S. S., Hamblin, M. R., Karimi, P., Rasta, S. H., & Mahmoudi, J. (2018). Transcranial near-infrared photobiomodulation attenuates memory impairment and hippocampal oxidative stress in sleep-deprived mice. Brain Research, 1682, 36–43.
Moro, C., El Massri, N., Darlot, F., Torres, N., Chabrol, C., Agay, D., Auboiroux, V., Johnstone, D. M., Stone, J., Mitrofanis, J., & Benabid, A. L. (2016). Effects of a higher dose of near-infrared light on clinical signs and neuroprotection in a monkey model of Parkinson’s disease. Brain Research, 1648(Pt A), 19–26.
Yang, L., Youngblood, H., Wu, C., & Zhang, Q. (2020). Mitochondria as a target for neuroprotection: Role of methylene blue and photobiomodulation. Translational Neurodegeneration, 9(1), 19. https://doi.org/10.1186/s40035-020-00197-z
Cardoso, F. D. S., Salehpour, F., Coimbra, N. C., Gonzalez-Lima, F., & Gomes da Silva, S. (2022). Photobiomodulation for the treatment of neuroinflammation: A systematic review of controlled laboratory animal studies. Frontiers in Neuroscience, 16, 1006031. https://doi.org/10.3389/fnins.2022.1006031
Mohammed, H. S., & Khadrawy, Y. A. (2022). Antidepressant and antioxidant effects of transcranial irradiation with 830-nm low-power laser in an animal model of depression. Lasers in Medical Science, 37(3), 1615–1623. https://doi.org/10.1007/s10103-021-03410-1
Fernandes, V. S., Santos, J. R., Leão, A. H., Medeiros, A. M., Melo, T. G., Izídio, G. S., Cabral, A., Ribeiro, R. A., Abílio, V. C., Ribeiro, A. M., & Silva, R. H. (2012). Repeated treatment with a low dose of reserpine as a progressive model of Parkinson’s disease. Behavioural Brain Research, 231(1), 154–163.
Brown, R. E., Corey, S. C., & Moore, A. K. (1999). Differences in measures of exploration and fear in MHC-congenic C57BL/6J and B6-H-2K mice. Behavior Genetics, 29(4), 263–271.
Ruiz-Larrea, M. B., Leal, A. M., Liza, M., Lacort, M., & de Groot, H. (1994). Antioxidant effects of estradiol and 2-hydroxyestradiol on iron-induced lipid peroxidation of rat liver microsomes. Steroids, 59, 383–388.
Montgomery, H. A. C., & Dymock, J. F. (1961). The determination of nitrite in water. The Analyst, 86, 414–416.
Beutler, E., Duron, O., & Kelly, B. M. (1963). Improved method for the determination of blood glutathione. Journal of Laboratory and Clinical Medicine, 61, 882–888.
Tsakiris, S., Angelogianni, P., Schulpis, K. H., & Behrakis, P. (2000). Protective effect of L-cysteine and glutathione on rat brain Na+, K+-ATPase inhibition induced by free radicals. Zeitschrift fuer Naturforschung, C: Journal of Biosciences, 55, 271–277.
Gorun, V., Proinov, I., Baltescu, V., Balaban, G., & Barzu, O. (1978). Modified Ellman procedure for assay of cholinesterase in crude-enzymatic preparations. Analytical Biochemistry, 86, 324–326.
Holt, A., Sharman, D. S., Baker, G. B., & Palcic, A. M. M. (1997). continuous spectrophotometric assay for monoamine oxidase and related enzymes in tissue homogenates. Analytical Biochemistry, 244, 384–392.
Ciarlone, A. E. (1978). Further modification of a fluorometric method for analyzing brain amines. Microchemical Journal, 23(1), 9–12.
Dompe, C., Moncrieff, L., Matys, J., Grzech-Leśniak, K., Kocherova, I., Bryja, A., Bruska, M., Dominiak, M., Mozdziak, P., Skiba, T. H. I., Shibli, J. A., Angelova Volponi, A., Kempisty, B., & Dyszkiewicz-Konwińska, M. (2020). Photobiomodulation-underlying mechanism and clinical applications. Journal of Clinical Medicine, 9(6), 1724. https://doi.org/10.3390/jcm9061724
Ying, R., Liang, H. L., Whelan, H. T., Eells, J. T., & Wong-Riley, M. T. (2008). Pretreatment with near-infrared light via light-emitting diode provides added benefit against rotenone- and MPP+-induced neurotoxicity. Brain Research, 1243, 167–173. https://doi.org/10.1016/j.brainres.2008.09.057
Oueslati, A., Lovisa, B., Perrin, J., Wagnières, G., van den Bergh, H., Tardy, Y., & Lashuel, H. A. (2015). Photobiomodulation suppresses alpha-synuclein-induced toxicity in an AAV-based rat genetic model of Parkinson’s disease. PLoS ONE, 10(10), e0140880. https://doi.org/10.1371/journal.pone.0140880
Zhang, H., Wu, S., & Xing, D. (2012). Inhibition of Aβ(25–35)-induced cell apoptosis by low-power-laser-irradiation (LPLI) through promoting Akt-dependent YAP cytoplasmic translocation. Cellular Signalling, 24(1), 224–232.
Nagakura, Y., Oe, T., Aoki, T., & Matsuoka, N. (2009). Biogenic amine depletion causes chronic muscular pain and tactile allodynia accompanied by depression: A putative animal model of fibromyalgia. Pain, 146(1–2), 26–33.
Li, Y., Yin, Q., Wang, B., Shen, T., Luo, W., & Liu, T. (2022). Preclinical reserpine models recapitulating motor and non-motor features of Parkinson’s disease: Roles of epigenetic upregulation of alpha-synuclein and autophagy impairment. Frontiers in Pharmacology, 13, 944376. https://doi.org/10.3389/fphar.2022.944376
Brum, E. D. S., Fialho, M. F. P., Fischer, S. P. M., Hartmann, D. D., Gonçalves, D. F., Scussel, R., Machado-de-Ávila, R. A., Dalla Corte, C. L., Soares, F. A. A., & Oliveira, S. M. (2020). Relevance of mitochondrial dysfunction in the reserpine-induced experimental fibromyalgia model. Molecular Neurobiology, 57(10), 4202–4217.
Cunha, D. M. G., Becegato, M., Meurer, Y. S. R., Lima, A. C., Gonçalves, N., Bioni, V. S., Engi, S. A., Bianchi, P. C., Cruz, F. C., Santos, J. R., & Silva, R. H. (2022). Neuroinflammation in early, late and recovery stages in a progressive parkinsonism model in rats. Frontiers in Neuroscience, 16, 923957. https://doi.org/10.3389/fnins.2022.923957
Sai, Y., Wu, Q., Le, W., Ye, F., Li, Y., & Dong, Z. (2008). Rotenone-induced PC12 cell toxicity is caused by oxidative stress resulting from altered dopamine metabolism. Toxicology in Vitro, 22(6), 1461–1468.
DeMaagd, G., & Philip, A. (2015). Parkinson’s disease and its management: Part 1: Disease entity, risk factors, pathophysiology, clinical presentation, and diagnosis. Pharmacy and Therapeutics, 40(8), 504–532.
Levin, J., Kurz, A., Arzberger, T., Giese, A., & Hoglinger, G. U. (2016). The differential diagnosis and treatment of atypical Parkinsonism. Deutsches Ärzteblatt International, 113(5), 61–69.
Ikenaka, K., Suzuki, M., Mochizuki, H., & Nagai, Y. (2019). Lipids as trans-acting effectors for α-synuclein in the pathogenesis of Parkinson’s disease. Frontiers in Neuroscience, 13, 693. https://doi.org/10.3389/fnins.2019.00693
Masato, A., Plotegher, N., Boassa, D., & Bubacco, L. (2019). Impaired dopamine metabolism in Parkinson’s disease pathogenesis. Molecular Neurodegeneration, 14(1), 35. https://doi.org/10.1186/s13024-019-0332-6
Goldstein, D. S., Kopin, I. J., & Sharabi, Y. (2014). Catecholamine autotoxicity Implications for pharmacology and therapeutics of Parkinson disease and related disorders. Pharmacology & Therapeutics, 144(3), 268–282.
Cohen, G., Farooqui, R., & Kesler, N. (1997). Parkinson disease: A new link between monoamine oxidase and mitochondrial electron flow. Proceedings of the National Academy of Sciences, 94(10), 4890–4894. https://doi.org/10.1073/pnas.94.10.4890. PMID: 9144160; PMCID: PMC24601.
Nolfi-Donegan, D., Braganza, A., & Shiva, S. (2020). Mitochondrial electron transport chain: Oxidative phosphorylation, oxidant production, and methods of measurement. Redox Biology, 37, 101674. https://doi.org/10.1016/j.redox.2020.101674
Guo, C., Sun, L., Chen, X., & Zhang, D. (2013). Oxidative stress, mitochondrial damage and neurodegenerative diseases. Neural Regeneration Research, 8(21), 2003–2014.
Zhao, R. Z., Jiang, S., Zhang, L., & Yu, Z. B. (2019). Mitochondrial electron transport chain, ROS generation and uncoupling (Review). International Journal of Molecular Medicine, 44(1), 3–15.
Bernardi, P., Gerle, C., Halestrap, A. P., Jonas, E. A., Karch, J., Mnatsakanyan, N., Pavlov, E., Sheu, S. S., & Soukas, A. A. (2023). Identity, structure, and function of the mitochondrial permeability transition pore: Controversies, consensus, recent advances, and future directions. Cell Death and Differentiation. https://doi.org/10.1038/s41418-023-01187-0
Brown, G. C. (1999). Nitric oxide and mitochondrial respiration. Biochimica et Biophysica Acta, 1411(2–3), 351–369.
He, Y., Imam, S. Z., Dong, Z., Jankovic, J., Ali, S. F., Appel, S. H., & Le, W. (2003). Role of nitric oxide in rotenone-induced nigro-striatal injury. Journal of Neurochemistry, 86(6), 1338–1345.
Aquilano, K., Baldelli, S., Rotilio, G., & Ciriolo, M. R. (2008). Role of nitric oxide synthases in Parkinson’s disease: A review on the antioxidant and anti-inflammatory activity of polyphenols. Neurochemical Research, 33(12), 2416–2426.
Tengan, C. H., & Moraes, C. T. (2017). NO control of mitochondrial function in normal and transformed cells. Biochimica et Biophysica Acta, Bioenergetics, 1858(8), 573–581.
Plaza Davila, M., Martin Muñoz, P., Tapia, J. A., Ortega Ferrusola, C., Balao da Silva, C. C., & Peña, F. J. (2015). Inhibition of mitochondrial complex I leads to decreased motility and membrane integrity related to increased hydrogen peroxide and reduced ATP production, while the inhibition of glycolysis has less impact on sperm motility. PLoS ONE, 10(9), e0138777. https://doi.org/10.1371/journal.pone.0138777
Narayanankutty, A., Job, J. T., & Narayanankutty, V. (2019). Glutathione, an antioxidant tripeptide: Dual roles in carcinogenesis and chemoprevention. Current Protein and Peptide Science, 20(9), 907–917. https://doi.org/10.2174/1389203720666190206130003. PMID: 30727890.
Presnell, C. E., Bhatti, G., Numan, L. S., Lerche, M., Alkhateeb, S. K., Ghalib, M., Shammaa, M., & Kavdia, M. (2013). Computational insights into the role of glutathione in oxidative stress. Current Neurovascular Research, 10(2), 185–194.
Beal, M. F. (2003). Mitochondria, oxidative damage, and inflammation in Parkinson’s disease. Annals of the New York Academy of Sciences, 991, 120–131. https://doi.org/10.1111/j.1749-6632.2003.tb07470.x
Pan, L. C., Hang, N. L., Colley, M. M. S., Chang, J., Hsiao, Y. C., Lu, L. S., Li, B. S., Chang, C. J., & Yang, T. S. (2022). Single cell effects of photobiomodulation on mitochondrial membrane potential and reactive oxygen species production in human adipose mesenchymal stem cells. Cells, 11(6), 972. https://doi.org/10.3390/cells11060972
Mosilhy, E. A., Alshial, E. E., Eltaras, M. M., Rahman, M. M. A., Helmy, H. I., Elazoul, A. H., Hamdy, O., & Mohammed, H. S. (2022). Non-invasive transcranial brain modulation for neurological disorders treatment: A narrative review. Life Sciences, 307, 120869. https://doi.org/10.1016/j.lfs.2022.120869
Chang, S. Y., Lee, M. Y., Chung, P. S., Kim, S., Choi, B., Suh, M. W., Rhee, C. K., & Jung, J. Y. (2019). Enhanced mitochondrial membrane potential and ATP synthesis by photobiomodulation increases viability of the auditory cell line after gentamicin-induced intrinsic apoptosis. Science and Reports, 9(1), 19248. https://doi.org/10.1038/s41598-019-55711-9
Muili, K. A., Gopalakrishnan, S., Eells, J. T., & Lyons, J. A. (2013). Photobiomodulation induced by 670 nm light ameliorates MOG35-55 induced EAE in female C57BL/6 mice: A role for remediation of nitrosative stress. PLoS ONE, 8(6), e67358. https://doi.org/10.1371/journal.pone.0067358
Pivovarov, A. S., Calahorro, F., & Walker, R. J. (2018). Na+/K+-pump and neurotransmitter membrane receptors. Invertebrate Neuroscience, 19(1), 1. https://doi.org/10.1007/s10158-018-0221-7.PMID:30488358;PMCID:PMC6267510
Scavone, C., Glezer, I., DemarchiMunhoz, C., de SenaBernardes, C., & Pekelmann, M. R. (2000). Influence of age on nitric oxide modulatory action on Na+, K+-ATPase activity through cyclic GMP pathway in proximal rat trachea. European Journal of Pharmacology, 388, 1–7.
Kumar, A. R., & Kurup, P. A. (2002). Inhibition of membrane Na+-K+ ATPase activity: A common pathway in central nervous system disorders. Journal of the Association of Physicians of India, 50, 400–406.
Zhu, M., Sun, H., Cao, L., Wu, Z., Leng, B., & Bian, J. (2022). Role of Na(+ )/K(+ )-ATPase in ischemic stroke: In-depth perspectives from physiology to pharmacology. Journal of Molecular Medicine, 100, 395–410.
Antunes, M. S., Ladd, F. V. L., Ladd, A., Moreira, A. L., Boeira, S. P., & Cattelan Souza, L. (2021). Hesperidin protects against behavioral alterations and loss of dopaminergic neurons in 6-OHDA-lesioned mice: The role of mitochondrial dysfunction and apoptosis. Metabolic Brain Disease, 36, 153–167.
Leyane, T. S., Jere, S. W., & Houreld, N. N. (2021). Cellular signalling and photobiomodulation in chronic wound repair. International Journal of Molecular Sciences, 22(20), 11223. https://doi.org/10.3390/ijms222011223
Rhee, Y. H., Moon, J. H., Jung, J. Y., Oh, C., Ahn, J. C., & Chung, P. S. (2019). Effect of photobiomodulation therapy on neuronal injuries by ouabain: The regulation of Na, K-ATPase; Src; and mitogen-activated protein kinase signaling pathway. BMC Neuroscience, 20(1), 19. https://doi.org/10.1186/s12868-019-0499-3
Rizzi, G., & Tan, K. R. (2017). Dopamine and acetylcholine, a circuit point of view in Parkinson’s disease. Frontiers in Neural Circuits, 22(11), 110. https://doi.org/10.3389/fncir.2017.00110
Wong, K. Y., Roy, J., Fung, M. L., Heng, B. C., Zhang, C., & Lim, L. W. (2020). Relationships between mitochondrial dysfunction and neurotransmission failure in Alzheimer’s disease. Aging & Disease, 11(5), 1291–1316.
Tong, J., Rathitharan, G., Meyer, J. H., Furukawa, Y., Ang, L. C., Boileau, I., Guttman, M., Hornykiewicz, O., & Kish, S. J. (2017). Brain monoamine oxidase B and A in human parkinsonian dopamine deficiency disorders. Brain: A Journal of Neurology, 140(9), 2460–2474. https://doi.org/10.1093/brain/awx172
Hamdy, O., & Mohammed, H. S. (2021). Investigating the transmission profiles of 808 nm laser through different regions of the rat’s head. Lasers in Medical Science, 36(4), 803–810. https://doi.org/10.1007/s10103-020-03098-9
Hamdy, O., & Mohammed, H. S. (2022). Variations in tissue optical parameters with the incident power of an infrared laser. PLoS ONE, 17(1), e0263164. https://doi.org/10.1371/journal.pone.0263164
Pope, N. J., & Denton, M. L. (2023). Differential effects of 808-nm light on electron transport chain enzymes in isolated mitochondria: Implications for photobiomodulation initiation. Mitochondrion, 68, 15–24. https://doi.org/10.1016/j.mito.2022.11.002
Reinhart, F., Massri, N. E., Darlot, F., Torres, N., Johnstone, D. M., Chabrol, C., Costecalde, T., Stone, J., Mitrofanis, J., Benabid, A. L., & Moro, C. (2015). 810nm near-infrared light offers neuroprotection and improves locomotor activity in MPTP-treated mice. Neuroscience Research, 92, 86–90.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The experimental research procedures were approved by the animal ethics committee.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mohammed, H.S., Hosny, E.N., Sawie, H.G. et al. Transcranial photobiomodulation ameliorates midbrain and striatum neurochemical impairments and behavioral deficits in reserpine-induced parkinsonism in rats. Photochem Photobiol Sci 22, 2891–2904 (2023). https://doi.org/10.1007/s43630-023-00497-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43630-023-00497-z