Abstract
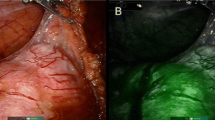
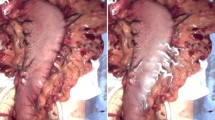
Accurate intraoperative identification of gastric cancer lesions and determination of the extent of resection are important for curability and function preservation. This study aimed to investigate the potential of the near-infrared fluorescence (NIRF) imaging agent ASP5354 for in vivo fluorescence imaging of gastric cancer. The capability of ASP5354 was evaluated using an MKN-45 human gastric cancer xenograft mouse model. A single dose of ASP5354 was intravenously administered to the mice at a concentration of 120 nmol (0.37 mg)/kg body weight. In vivo NIRF images of the mouse backs were obtained using an NIRF camera system. Moreover, the cancer tissues were dissected, and the NIRF intensity in the tissue sections was measured using the NIRF camera system. ASP5354 uptake in MKN-45 cells was assessed in vitro using the NIRF microscope. The NIRF signal of ASP5354 was selectively detected in gastric cancer tissues immediately after the intravenous administration of ASP5354. The cancer tissues emitted stronger NIRF signals than adjacent normal tissues. The difference in the NIRF intensity between the normal and cancer tissues was clearly observed at the boundary between them in the macrolevel NIRF images. Cancer tissues can be distinguished from normal tissues based on the measurement of the NIRF of ASP5354, using an NIRF camera system. ASP5354 is a promising agent for NIRF imaging of gastric cancer tissues.
Graphical abstract









Similar content being viewed by others
References
GLOBOCAN 2020. https://www.uicc.org/news/globocan-2020-new-global-cancer-data. Accessed 31 July 2022.
Japanese Gastric Cancer Association. (2021). Japanese gastric cancer treatment guidelines 2018 (5th edition). Gastric Cancer, 24, 1–21. https://doi.org/10.1007/s10120-020-01042-y
Folkman, J., & Klagsbrun, M. (1987). Angiogenetic factors. Science, 235, 442–447.
Iseki, K., Tatsuta, M., Iishi, H., Sakai, N., Yano, H., & Ishiguro, S. (2000). Effectiveness of the near-infrared electronic endoscope for diagnosis of the depth of involvement of gastric cancers. Gastrointestinal Endoscopy, 52, 755–762. https://doi.org/10.1067/mge.2000.110455
Reinhart, M. B., Huntington, C. R., Blair, L. J., Heniford, B. T., & Augenstein, V. A. (2016). Indocyanine green: historical context, current applications, and future considerations. Surgical Innovation, 23, 166–175. https://doi.org/10.1177/1553350615604053
Kimura, T., Muguruma, N., Ito, S., Okamura, S., Imoto, Y., Miyamoto, H., Kaji, M., & Kudo, E. (2007). Infrared fluorescence endoscopy for the diagnosis of superficial gastric tumors. Gastrointestinal Endoscopy, 66, 37–43. https://doi.org/10.1016/j.gie.2007.01.009
Weissleder, R. (2001). A clearer vision for in vivo imaging. Nature Biotechnology, 19, 316–317. https://doi.org/10.1038/86684
Cheng, H., Chi, C., Shang, W., Rengaowa, S., Cui, J., Ye, J., Jiang, S., Mao, Y., Zeng, C., Huo, H., Chen, L., & Tian, J. (2017). Precise integrin-targeting near-infrared imaging-guided surgical method increases surgical qualification of peritoneal carcinomatosis from gastric cancer in mice. Oncotarget, 8, 6258–6272. https://doi.org/10.18632/oncotarget.14058
Shao, J., Zheng, X., Feng, L., Lan, T., Ding, D., Cai, Z., Zhu, X., Liang, R., & Wei, B. (2020). Targeting fluorescence imaging of RGD-modified indocyanine green micelles on gastric cancer. Frontiers in Bioengineering and Biotechnology, 8, 575365. https://doi.org/10.3389/fbioe.2020.575365
Shao, J., Liang, R., Ding, D., Zheng, X., Zhu, X., Hu, S., Wei, H., & Wei, B. (2021). A smart multifunctional nanoparticle for enhanced near-infrared image-guided photothermal therapy against gastric cancer. International Journal of Nanomedicine, 16, 2897–2915. https://doi.org/10.2147/IJN.S289310
Zhao, N., Zhang, C., Zhao, Y., Bai, B., An, J., Zhang, H., Wu, J. B., & Shi, C. (2016). Optical imaging of gastric cancer with near-infrared heptamethine carbocyanine fluorescence dyes. Oncotarget, 7, 57277–57289. https://doi.org/10.18632/oncotarget.10031
Teranishi, K. (2020). A Near-infrared fluorescent probe coated with β-cyclodextrin molecules for real-time imaging-guided intraoperative ureteral identification and diagnosis. Molecular Pharmaceutics, 17, 2672–2681. https://doi.org/10.1021/acs.molpharmaceut.0c00364
Fushiki, H., Yoshikawa, T., Matsuda, T., Sato, T., & Suwa, A. (2021). Preclinical development and validation of ASP5354: A near-infrared fluorescent agent for intraoperative ureter visualization. Molecular Imaging and Biology. https://doi.org/10.1007/s11307-021-01613-0
Kurahashi, T., Iwatsuki, K., Onishi, T., Arai, T., Teranishi, K., & Hirata, H. (2016). Near-infrared indocyanine dye permits real-time characterization of both venous and lymphatic circulation. Journal of Biomedial Optics, 21, 86009. https://doi.org/10.1117/1.jbo.21.8.086009
Murase, T., Takizawa, M., Galitz, L., Flach, S., Murray, V., Gufford, B., & Suwa, A. (2021). Randomized, double-blind, controlled study to evaluate safety and pharmacokinetics of single ascending doses of ASP5354, an investigational imaging product, in healthy adult volunteers. Clinical Pharmacology in Drug Development, 10, 1460–1468. https://doi.org/10.1002/cpdd.1013
Gao, M., Yu, F., Lv, C., Choo, J., & Chen, L. (2017). Fluorescence chemical probes for accurate tumor diagnosis and targeting therapy. Chemical Society Reviews, 46, 2237–2271. https://doi.org/10.1039/c6cs00908e
Nagaya, T., Nakamura, Y. A., Choyke, P. L., & Kobayashi, H. (2017). Fluorescence-guided surgery. Frontiers in Oncology. https://doi.org/10.3389/fonc.2017.00314
Wang, C., Wang, Z., Zhao, T., Li, Y., Huang, G., Sumer, B. D., & Gao, J. (2018). Optical molecular imaging for tumor detection and image-guided surgery. Biomaterials, 157, 62–75. https://doi.org/10.1016/j.biomaterials.2017.12.002
Perumal, V., Sivakumar, P. M., Zarrabi, A., Muthupandian, S., Vijayaraghavalu, S., Sahoo, K., Das, A., Das, S., Payyappilly, S. S., & Das, S. (2019). Near infra-red polymeric nanoparticle based optical imaging in cancer diagnosis. Journal of Photochemistry and Photobiology B: Biology, 199, 111630. https://doi.org/10.1016/j.jphotobiol.2019.111630
Namikawa, T., Iwabu, J., Munekage, M., Uemura, S., Maeda, H., Kitagawa, H., Nakayama, T., Inoue, K., Sato, T., Kobayashi, M., & Hanazaki, K. (2020). Evolution of photodynamic medicine based on fluorescence image-guided diagnosis using indocyanine green and 5-aminolevulinic acid. Surgery Today, 50, 821–831. https://doi.org/10.1007/s00595-019-01851-4
Teranishi, K. (2022). Near-infrared fluorescence imaging of renal cell carcinoma with ASP5354 in a mouse model for intraoperative guidance. International Journal of Molecular Sciences, 23, 7228. https://doi.org/10.3390/ijms23137228
Teranishi, K. (2023). In vivo optical imaging of bladder cancer tissues in an MB49 bladder cancer orthotopic mouse model using the intravesical or intravenous administration of near-infrared fluorescence probe. International Journal of Molecular Sciences, 24, 2349. https://doi.org/10.3390/ijms24032349
Boger, C., Warneke, V. S., Behrens, H. M., Kalthoff, H., Goodman, S. L., Becker, T., & Rocken, C. (2015). Integrins αvβ3 and αvβ5 as prognostic, diagnostic, and therapeutic targets in gastric cancer. Gastric Cancer, 18, 784–795. https://doi.org/10.1007/s10120-014-0435-2
Matsumura, Y., & Maeda, H. (1986). A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Research, 46, 6387–6392.
Usama, S. M., Park, G. K., Nomura, S., Baek, Y., Choi, H. S., & Burgess, K. (2020). Role of albumin in accumulation and persistence of tumor-seeking cyanine dyes. Bioconjugate Chemistry, 31, 248–259. https://doi.org/10.1021/acs.bioconjchem.9b00771
Acknowledgements
I thank J. Ito, M. Matsuura, and M. Itoda (ITECHLAB Co., Ltd., Gifu, Japan) for their help with the animal experiments and histopathological analysis. I would like to thank Editage for English-language editing.
Author information
Authors and Affiliations
Contributions
KT: conceptualization, methodology, investigation, data curation, formal analysis, funding acquisition, writing—original draft, writing—review and editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. This research received no external funding.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Teranishi, K. In vivo near-infrared fluorescence imaging of gastric cancer in an MKN-45 gastric cancer xenograft mouse model using intraoperative ureteral identification agent ASP5354. Photochem Photobiol Sci 22, 1721–1729 (2023). https://doi.org/10.1007/s43630-023-00410-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43630-023-00410-8