Abstract
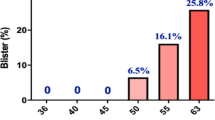
It is crucial to discover biomarkers for non-invasive evaluations of the dosages of UV exposures to a person during post-UV exposure period, and for non-invasive prediction of UV-induced skin damage. Our current study has obtained findings: UVB exposures produced dose-dependent increases in skin’s green autofluorescence (AF) intensity of mice, which were significantly associated with the UVB dosages. The UVC-induced green AF increases were dose dependent, which were highly associated with the UVC dosages. Moreover, both previous reports and our current study have collectively shown significant association between UVB/UVC dosages and UVB/UVC-induced skin damage. Collectively, our study has indicated that the UVB/UVC-induced skin’s AF are first biomarkers for both non-invasive evaluations of the dosages of UV exposures to a person during post-UV exposure period and non-invasive and label-free prediction of UVB/UVC-induced skin damage.
Graphical abstract







Similar content being viewed by others
References
Chen, H., Weng, Q. Y., & Fisher, D. E. (2014). UV signaling pathways within the skin. The Journal of Investigative Dermatology, 134(8), 2080–2085. https://doi.org/10.1038/jid.2014.161
D’Orazio, J., et al. (2013). UV radiation and the skin. International Journal of Molecular Sciences, 14(6), 12222–12248. https://doi.org/10.3390/ijms140612222
Elwood, J. M., & Jopson, J. (1997). Melanoma and sun exposure: An overview of published studies. International Journal of Cancer., 73(2), 198–203.
de Jager, T. L., Cockrell, A. E., & Du Plessis, S. S. (2017). Ultraviolet light induced generation of reactive oxygen species. Advances in Experimental Medicine and Biology, 996, 15–23. https://doi.org/10.1007/978-3-319-56017-5_2
Mullenders, L. H. F. (2018). Solar UV damage to cellular DNA: From mechanisms to biological effects. Photochemical & Photobiological Sciences, 17(12), 1842–1852. https://doi.org/10.1039/c8pp00182k
Burke, K. E. (2018). Mechanisms of aging and development-A new understanding of environmental damage to the skin and prevention with topical antioxidants. Mechanisms of Ageing and Development, 172, 123–130. https://doi.org/10.1016/j.mad.2017.12.003
Elwood, J. M., & Jopson, J. (1997). Melanoma and sun exposure: An overview of published studies. International Journal of Cancer, 73(2), 198–203. https://doi.org/10.1002/(sici)1097-0215(19971009)73:2%3c198::aid-ijc6%3e3.0.co;2-r
Mohania, D., et al. (2017). Ultraviolet radiations: Skin defense-damage mechanism. Advances in Experimental Medicine and Biology, 996, 71–87. https://doi.org/10.1007/978-3-319-56017-5_7
Roy, S. (2017). Impact of UV radiation on genome stability and human health. Advances in Experimental Medicine and Biology, 996, 207–219. https://doi.org/10.1007/978-3-319-56017-5_17
Guerra, K. C., Zafar, N., & Crane, J. S. (2021). Skin cancer prevention. StatPearls. Treasure Island.
Sproul, C. D., et al. (2014). Cyclobutane pyrimidine dimer density as a predictive biomarker of the biological effects of ultraviolet radiation in normal human fibroblast. Photochemistry and Photobiology, 90(1), 145–154. https://doi.org/10.1111/php.12194
Ikehata, H., et al. (2018). Quantitative analysis of UV photolesions suggests that cyclobutane pyrimidine dimers produced in mouse skin by UVB are more mutagenic than those produced by UVC. Photochemical & Photobiological Sciences, 17(4), 404–413. https://doi.org/10.1039/c7pp00348j
Khalil, C., & Shebaby, W. (2017). UVB damage onset and progression 24 h post exposure in human-derived skin cells. Toxicology Reports, 4, 441–449. https://doi.org/10.1016/j.toxrep.2017.07.008
ten Berge, O., et al. (2011). Assessment of cyclobutane pyrimidine dimers by digital photography in human skin. Journal of Immunological Methods, 373(1–2), 240–246. https://doi.org/10.1016/j.jim.2011.07.014
Moran, C., et al. (2015). Type 2 diabetes, skin autofluorescence, and brain atrophy. Diabetes, 64(1), 279–283. https://doi.org/10.2337/db14-0506
Varikasuvu, S. R., Aloori, S., & Bhongir, A. V. (2021). Higher skin autofluorescence detection using AGE-Reader technology as a measure of increased tissue accumulation of advanced glycation end products in dialysis patients with diabetes: A meta-analysis. Journal of Artificial Organs, 24(1), 44–57. https://doi.org/10.1007/s10047-020-01189-6
Bos, D. C., de Ranitz-Greven, W. L., & de Valk, H. W. (2011). Advanced glycation end products, measured as skin autofluorescence and diabetes complications: A systematic review. Diabetes Technology & Therapeutics, 13(7), 773–779. https://doi.org/10.1089/dia.2011.0034
Pena, A., et al. (2005). Spectroscopic analysis of keratin endogenous signal for skin multiphoton microscopy. Optics Express, 13(16), 6268–6274.
Bader, A. N., et al. (2011). Fast nonlinear spectral microscopy of in vivo human skin. Biomedical Optics Express, 2(2), 365–373. https://doi.org/10.1364/BOE.2.000365
Sheng, C., et al. (2012). NAD+ administration significantly attenuates synchrotron radiation X-ray-induced DNA damage and structural alterations of rodent testes. International Journal of Physiology, Pathophysiology and Pharmacology, 4(1), 1–9.
Hennings, L., et al. (2009). Dead or alive? Autofluorescence distinguishes heat-fixed from viable cells. International Journal of Hyperthermia., 25(5), 355–363.
Kozlova, A. A., et al. (2020). Changes in autofluorescence level of live and dead cells for mouse cell lines. Journal of Fluorescence., 30(6), 1–7.
Kang, S. W., et al. (2000). Antisense oligonucleotide of clusterin mRNA induces apoptotic cell death and prevents adhesion of rat ASC-17D Sertoli cells. Molecules and Cells, 10(2), 193–198. https://doi.org/10.1007/s10059-000-0193-3
Narayanan, D. L., Saladi, R. N., & Fox, J. L. (2010). Ultraviolet radiation and skin cancer. International Journal of Dermatology, 49(9), 978–986. https://doi.org/10.1111/j.1365-4632.2010.04474.x
Zhou, Y., et al. (2021). Photoprotective effect of artemisia sieversiana ehrhart essential oil against UVB-induced photoaging in mice. Photochemistry and Photobiology. https://doi.org/10.1111/php.13561
Park, H. S., et al. (2014). Toll-like receptor 2 mediates a cutaneous reaction induced by repetitive ultraviolet B irradiation in C57/BL6 mice in vivo. Experimental Dermatology., 23, 8.
Dimitrios, et al. (2014). In-vivo imaging of psoriatic lesions with polarization multispectral dermoscopy and multiphoton microscopy. Biomedical Optics Express., 2, 2.
Beermann, F., et al. (1990). Rescue of the albino phenotype by introduction of a functional tyrosinase gene into mice. EMBO Journal, 9(9), 2819–2826.
Patterson, G. H., et al. (2000). Separation of the glucose-stimulated cytoplasmic and mitochondrial NAD(P)H responses in pancreatic islet beta cells. Proceedings of the National Academy of Sciences of the United States of America, 97(10), 5203–5207. https://doi.org/10.1073/pnas.090098797
Georgakoudi, I., et al. (2002). NAD(P)H and collagen as in vivo quantitative fluorescent biomarkers of epithelial precancerous changes. Cancer Research., 62(3), 682–687.
Nogueira, M.S. and C. Kurachi. Assessing the photoaging process at sun exposed and non-exposed skin using fluorescence lifetime spectroscopy. in Optical Biopsy XIV: Toward Real-Time Spectroscopic Imaging and Diagnosis. 2016.
Andrei, et al. (2008). Imaging of zinc oxide nanoparticle penetration in human skin in vitro and in vivo. Journal of Biomedical Optics., 13(6), 064031.
Acknowledgements
The authors would like to acknowledge the financial support by two research grants from a Major Special Program Grant of Shanghai Municipality (Grant # 2017SHZDZX01) (to W.Y.).
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, M., Ying, W. UV-induced skin’s green autofluorescence is a biomarker for both non-invasive evaluations of the dosages of UV exposures of the skin and non-invasive prediction of UV-induced skin damage. Photochem Photobiol Sci 22, 159–168 (2023). https://doi.org/10.1007/s43630-022-00306-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43630-022-00306-z