Abstract
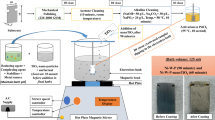
This research examines the impact of different NaCl concentrations on the corrosion performance of AZ91D nanocomposites enhanced with nano metal oxides such as Zinc oxide (ZnO), Manganese oxide (MnO), and Titanium oxide (TiO2) through an electrochemical test method. The proposed materials were fabricated using a stir-squeeze casting process paired with an ultrasonication setup. In an argon gas-protected system, T6 heat treatment conditions were applied to these casted composites. The electrochemical corrosion results revealed that the ATO nanocomposite at 2.5% of NaCl concentration achieved lower corrosion current density (Icorr) (1.456 × 10–7 A/cm2) and higher corrosion potential (Ecorr) (− 1.08 V) owing to the formation of Mg2TiO3 precipitates, which act as a corrosion protective layer as well as reduce the corrosion rate. Based on the nyquist plot, the alloy and nanocomposites electron transfer rate were varied in the following sequence of AZD < AMO < AZO < ATO. The corroded samples Scanning Electron Microscope (SEM) and 3D profile image show ATO composites have minimum amount of crevice corrosion and peaks formation than other materials. Results from EDAX and elemental mapping confirm the presence of Mg2TiO3 precipitates on the ATO nanocomposite.















Similar content being viewed by others
Data availability
Data will be made available on request.
References
Liao S, Yu B, Zhang B, Zhou P, Zhang T, Wang F. Chemically depleting the noble impurities from AZ91-T4 magnesium alloy: a new and efficient pretreatment method to improve the corrosion resistance of phosphate conversion coatings. Corros Sci. 2021;191:109725. https://doi.org/10.1016/j.corsci.2021.109725.
Shao Z, Nishimoto M, Muto I, Sugawara Y. Real-time in situ observation of the corrosion process of die-cast AZ91D magnesium alloy in NaCl solutions under galvanostatic polarization. Corros Sci. 2021;192:109834. https://doi.org/10.1016/j.corsci.2021.109834.
Yin Z, Chen Y, Yan H, Zhou G, Wu X, Hu Z. Effects of the second phases on corrosion resistance of AZ91-xGd alloys treated with ultrasonic vibration. J Alloys Compd. 2019;783:877–85. https://doi.org/10.1016/j.jallcom.2019.01.002.
Xie Q, Ma A, Jiang J, Liu H, Cheng Z, Gu Y. Tailoring the corrosion behavior and mechanism of AZ31 magnesium alloys by different Ca contents for marine application. Corros Sci. 2021;192:109842. https://doi.org/10.1016/j.corsci.2021.109842.
Huang SJ, Lin CC, Huang JY, Tenne R. Mechanical behavior enhancement of AZ31/WS2 and AZ61/WS2 magnesium metal matrix nanocomposites. Adv Mech Eng. 2018;10:1–14. https://doi.org/10.1177/1687814017753442.
Huang SJ, Abbas A. Effects of tungsten disulfide on microstructure and mechanical properties of AZ91 magnesium alloy manufactured by stir casting. J Alloys Compd. 2020. https://doi.org/10.1016/j.jallcom.2019.153321.
Thomas S, Medhekar NV, Frankel GS, Birbilis N. Corrosion mechanism and hydrogen evolution on Mg. Curr Opin Solid State Mater Sci. 2015;19:85–94. https://doi.org/10.1016/j.cossms.2014.09.005.
Lamaka SV, Höche D, Petrauskas RP, Blawert C, Zheludkevich ML. A new concept for corrosion inhibition of magnesium: suppression of iron re-deposition. Electrochem Commun. 2016;62:5–8. https://doi.org/10.1016/j.elecom.2015.10.023.
Banerjee S, Poria S, Sutradhar G, Sahoo P. Dry sliding tribological behavior of AZ31-WC nano-composites. J Magnes Alloy. 2019;7:315–27. https://doi.org/10.1016/j.jma.2018.11.005.
Zs Q, Ma J, Jj H. Enhancement the mechanical properties of aluminum casting alloys (A356) by adding nanorods structures from zinc oxide. J Mater Sci Eng. 2017. https://doi.org/10.4172/2169-0022.1000328.
Gu X, Yue J, Li L, Xue H, Yang J, Zhao X. General synthesis of MnOx (MnO2, Mn2O3, Mn3O4, MnO) hierarchical microspheres as lithium-ion battery anodes. Electrochim Acta. 2015;184:250–6. https://doi.org/10.1016/j.electacta.2015.10.037.
Pc E, Radhakrishnan G, Emarose S. Investigation into physical, microstructural and mechanical behaviour of titanium dioxide nanoparticulate reinforced magnesium composite. Mater Technol. 2021;36:575–84. https://doi.org/10.1080/10667857.2020.1782050.
Esmaeilzadeh O, Eivani AR, Mehdizade M, Boutorabi SMA, Masoudpanah SM. An investigation of microstructural background for improved corrosion resistance of WE43 magnesium-based composites with ZnO and Cu/ZnO additions. J Alloys Compd. 2022;908:164437. https://doi.org/10.1016/j.jallcom.2022.164437.
Hu J, Zhang C, Cui B, Bai K, Guan S, Wang L, Zhu S. In vitro degradation of AZ31 magnesium alloy coated with nano TiO2 film by sol-gel method. Appl Surf Sci. 2011;257:8772–7. https://doi.org/10.1016/j.apsusc.2011.03.148.
Fatile BO, Adewuyi BO, Owoyemi HT. Synthesis and characterization of ZA-27 alloy matrix composites reinforced with zinc oxide nanoparticles. Eng Sci Technol Int J. 2017;20:1147–54. https://doi.org/10.1016/j.jestch.2017.01.001.
Aravindan S, Rao PV, Ponappa K. Evaluation of physical and mechanical properties of AZ91D/SiC composites by two step stir casting process. J Magnes Alloy. 2015;3:52–62. https://doi.org/10.1016/j.jma.2014.12.008.
Seenuvasaperumal P, Elayaperumal A, Jayavel R. Influence of calcium hexaboride reinforced magnesium composite for the mechanical and tribological behviour. Tribol Int. 2017;111:18–25. https://doi.org/10.1016/j.triboint.2017.02.042.
Sunu Surendran KT, Gnanavelbabu A. Tribological behaviour of AZ91D/ultra-high-temperature ceramic composites at room and elevated temperatures, Proc. Inst. Mech Eng Part J J Eng Tribol. 2022;236:1855–70. https://doi.org/10.1177/13506501211010035.
Chen J, Chen X, Luo Z. Effect of mechanical vibration on microstructure and properties of cast AZ91D alloy. Results Phys. 2018;11:1022–7. https://doi.org/10.1016/j.rinp.2018.10.047.
Chen G, Yang M, Jin Y, Zhang H, Han F, Chen Q, Zhao Z. Ultrasonic assisted squeeze casting of a wrought aluminum alloy. J Mater Process Technol. 2019;266:19–25. https://doi.org/10.1016/j.jmatprotec.2018.10.032.
Zhang C, Li Z, Ye Y, Yuan Y, Fang D, Wu H, Li W. Interaction of nanoparticles and dislocations with Mg17Al12 precipitates in n-SiCp/AZ91D magnesium matrix nanocomposites. J Alloys Compd. 2020;815:152416. https://doi.org/10.1016/j.jallcom.2019.152416.
Luong DD, Shunmugasamy VC, Cox J, Gupta N, Rohatgi PK. Heat treatment of AZ91D Mg-Al-Zn alloy: microstructural evolution and dynamic response. Jom. 2014;66:312–21. https://doi.org/10.1007/s11837-013-0800-3.
Ma Y, Xiong H, Chen B. Effect of heat treatment on microstructure and corrosion behavior of Mg-5Al-1Zn-1Sn magnesium alloy. Corros Sci. 2021. https://doi.org/10.1016/j.corsci.2021.109759.
Bansal P, Singh G, Sidhu HS. Investigation of surface properties and corrosion behavior of plasma sprayed HA/ZnO coatings prepared on AZ31 Mg alloy. Surf Coatings Technol. 2020;401:126241. https://doi.org/10.1016/j.surfcoat.2020.126241.
Lei T, Tang W, Cai SH, Feng FF, Li NF. On the corrosion behaviour of newly developed biodegradable Mg-based metal matrix composites produced by in situ reaction. Corros Sci. 2012;54:270–7. https://doi.org/10.1016/j.corsci.2011.09.027.
Li M, Chen Q, Zhang W, Hu W, Su Y. Corrosion behavior in SBF for titania coatings on Mg-Ca alloy. J Mater Sci. 2011;46:2365–9. https://doi.org/10.1007/s10853-010-5083-2.
García-Rodríguez S, Torres B, Maroto A, López AJ, Otero E, Rams J. Dry sliding wear behavior of globular AZ91 magnesium alloy and AZ91/SiCp composites. Wear. 2017;390–391:1–10. https://doi.org/10.1016/j.wear.2017.06.010.
Gnanavelbabu A, Surendran KTS, Loganathan P, Vinothkumar E. Effect of ageing temperature on the corrosion behaviour of UHTC particulates reinforced magnesium composites fabricated through ultrasonic assisted squeeze casting process. J Alloys Compd. 2021;856:158173. https://doi.org/10.1016/j.jallcom.2020.158173.
Talam S, Karumuri SR, Gunnam N. Synthesis characterization, and spectroscopic properties of ZnO nanoparticles. ISRN Nanotechnol. 2012;2012:1–6. https://doi.org/10.5402/2012/372505.
Pandey BK, Shahi AK, Gopal R. Optical and electrical transport properties of MnO nanoparticles, mater. Focus. 2013;2:221–6. https://doi.org/10.1166/mat.2013.1078.
He J, Du Y, Bai Y, An J, Cai X, Chen Y, Wang P, Yang X, Feng Q. Facile formation of anatase/rutile TiO2 nanocomposites with enhanced photocatalytic activity. Molecules. 2019;24:2996. https://doi.org/10.3390/molecules24162996.
Liu S, Wang B. Electrochemical corrosion behavior of a magnesium calcium alloy in simulated body fluids with different glucose concentrations. J Mater Res Technol. 2020;9:6612–9. https://doi.org/10.1016/j.jmrt.2020.04.052.
Medhashree H, Shetty AN. Electrochemical corrosion study of Mg-Al-Zn-Mn alloy in aqueous ethylene glycol containing chloride ions. J Mater Res Technol. 2017;6:40–9. https://doi.org/10.1016/j.jmrt.2016.04.003.
Liang J, Srinivasan PB, Blawert C, Dietzel W. Influence of pH on the deterioration of plasma electrolytic oxidation coated AM50 magnesium alloy in NaCl solutions. Corros Sci. 2010;52:540–7. https://doi.org/10.1016/j.corsci.2009.10.011.
Galicia G, Pébère N, Tribollet B, Vivier V. Local and global electrochemical impedances applied to the corrosion behaviour of an AZ91 magnesium alloy. Corros Sci. 2009;51:1789–94. https://doi.org/10.1016/j.corsci.2009.05.005.
Dias V, Maciel H, Fraga M, Lobo AO, Pessoa R, Marciano FR. Atomic layer deposited TiO2 and Al2 O3 thin films as coatings for aluminum food packaging application. Materials (Basel). 2019. https://doi.org/10.3390/ma12040682.
Gnanavelbabu A, Amul XJ, Surendran KTS. Investigation on the tribocorrosion and electrochemical corrosion behaviour of AA2014/Al2O3 nanocomposites fabricated through ultrasonication coupled stir-squeeze casting method. J Appl Electrochem. 2022;52:765–91. https://doi.org/10.1007/s10800-022-01666-1.
Bin Liu X, Shan DY, Song YW, Han EH. Effects of heat treatment on corrosion behaviors of Mg-3Zn magnesium alloy. Trans Nonferrous Met Soc China. 2010;20:1345–50. https://doi.org/10.1016/S1003-6326(09)60302-2.
Wang L, Shinohara T, Zhang BP. Influence of chloride, sulfate and bicarbonate anions on the corrosion behavior of AZ31 magnesium alloy. J Alloys Compd. 2010;496:500–7. https://doi.org/10.1016/j.jallcom.2010.02.088.
Wang C, Luo T, Liu Y, Huang Q, Yang Y. Residual stress and precipitation of Mg-5Zn-3.5Sn-1Mn-0.5Ea-0.5Cu alloy with different quenching rates. J Magnes Alloy. 2021;9:604–12. https://doi.org/10.1016/j.jma.2020.02.021.
Kubásek J, Vojtěch D, Lipov J, Ruml T. Structure, mechanical properties, corrosion behavior and cytotoxicity of biodegradable Mg–X (X=Sn, Ga, In) alloys. Mater Sci Eng C. 2013;33:2421–32. https://doi.org/10.1016/j.msec.2013.02.005.
Chen XB, Birbilis N, Abbott TB. Effect of [Ca 2+] and [PO43-] levels on the formation of calcium phosphate conversion coatings on die-cast magnesium alloy AZ91D. Corros Sci. 2012;55:226–32. https://doi.org/10.1016/j.corsci.2011.10.022.
Li Z, Peng Z, Qiu Y, Qi K, Chen Z, Guo X. Study on heat treatment to improve the microstructure and corrosion behavior of ZK60 magnesium alloy. J Mater Res Technol. 2020;9:11201–19. https://doi.org/10.1016/j.jmrt.2020.08.004.
Acknowledgements
The authors are thankful to Science and Engineering Research Board (SERB), Govt. of India for supporting this work through the research funding bearing the grant number EEQ/2017/000382.
Funding
Science and Engineering Research Board (SERB), EEQ/2017/000382, A. Gnanavelbabu.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The author declares that he has no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. The authors declare the following financial interests/personal relationships which may be considered as potential competing interests.
Ethical approval
The authors state that the research was conducted according to ethical standards.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gnanavelbabu, A., Vinothkumar, E., Ross, N.S. et al. Investigating the influence of NaCl concentration on the electrochemical corrosion behavior of metal oxide reinforced magnesium matrix composites. Archiv.Civ.Mech.Eng 23, 113 (2023). https://doi.org/10.1007/s43452-023-00658-y
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s43452-023-00658-y