Abstract
Background
Non-deleterious episodes of seizure preconditioning can efficiently increase the brain’s resistance to the consequent severe status epilepticus (SE). In the present investigation, we intended to elucidate further (i) the effects of preconditioning with pentylenetetrazole (PTZ) in the lithium-pilocarpine model of SE in male rats, along with (ii) the possible contribution of opioid, N-Methyl-D-aspartate (NMDA) receptors, and nitric oxide (NO) signaling transduction.
Methods
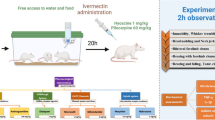
In male Wistar rats, the SE was incited by lithium administration (127 mg/kg, ip) 20 h before pilocarpine (60 mg/kg, ip). PTZ preconditioning was induced via a low-dose injection of PTZ (25 mg/kg) for 5 repeated days. To investigate the underlying signaling pathway, naltrexone (NTX; a non-specific opioid receptor antagonist), MK-801 (NMDA antagonist), L-NAME (a non-specific nitric oxide synthase (NOS) inhibitor), aminoguanidine (AG; a specific inducible NOS inhibitor), and 7-Nitroindazole (7-NI; a specific neuronal NOS inhibitor) were administered 15 min before PTZ injection.
Results
Preconditioning with PTZ successfully ameliorates the increased SE scores due to lithium-pilocarpine-induced SE (p < 0.05). None of the drugs given without PTZ preconditioning had an impact on SE outcomes. The observed anti-convulsant effect of PTZ preconditioning is reversed by the opioid receptor antagonists and NOS inhibitors. Conversely, the NMDA receptor antagonist enhanced the anti-convulsion activity caused by PTZ preconditioning. Quantifying nitrite level in the hippocampus showed a significant NO level decline in the PTZ-preconditioned animals.
Conclusions
Therefore, PTZ preconditioning generates endogenous protection against SE, possibly through targeting opioid/NMDA receptors and NO signaling transduction in the animal model of lithium-pilocarpine-induced SE.





Similar content being viewed by others
Data availability
All data were generated in-house, and no paper mill or other ways of manipulating research materials were used. All data generated or analyzed during this study are included in this published article and its supplementary material S1 (SE scores and biochemical measurements) and S2 (mortality rate). The Prism files, including the data analysis and figures, are available in supplementary material S3–S5.
Abbreviations
- AG:
-
Aminoguanidine
- ANOVA:
-
Analysis of variance
- Ca2 + :
-
Calcium
- EEG:
-
Electroencephalographic
- eNOS:
-
Endothelial nitric oxide synthase
- FSH:
-
Follicle-stimulating hormone
- GABA:
-
Gamma-aminobutyric acid
- iNOS:
-
Inducible nitric oxide synthase
- ip:
-
Intraperitoneal
- KA:
-
Kainic acid
- LH:
-
Luteinizing hormone
- L-NAME:
-
L-NG-Nitro-Larginine methyl ester hydrochloride
- NIH:
-
National Institutes of Health
- NMDA:
-
N-Methyl-D-aspartate
- nNOS:
-
Neuronal nitric oxide synthase
- NO:
-
Nitric oxide
- NOS:
-
Nitric oxide synthase
- NTX:
-
Naltrexone
- OD:
-
Optical density
- PCOS:
-
Polycystic ovary syndrome
- PTZ:
-
Pentylenetetrazole
- SD:
-
Standard deviation
- SE:
-
Status epilepticus
- 7-NI:
-
7-Nitroindazole
References
Dirnagl U, Becker K, Meisel A. Preconditioning and tolerance against cerebral ischaemia: from experimental strategies to clinical use. Lancet Neurol. 2009;8:398–412.
Dirnagl U, Meisel A. Endogenous neuroprotection: mitochondria as gateways to cerebral preconditioning? Neuropharmacology. 2008;55:334–44.
McDonough A, Weinstein JR. Neuroimmune response in ischemic preconditioning. Neurotherapeutics. 2016;13:748–61.
Stevens SL, Vartanian KB, Stenzel-Poore MP. Reprogramming the response to stroke by preconditioning. Stroke. 2014;45:2527–31.
Eslami F, Rahimi N, Ostovaneh A, Ghasemi M, Dejban P, Abbasi A, et al. Sumatriptan reduces severity of status epilepticus induced by lithium-pilocarpine through nitrergic transmission and 5-HT(1B/D) receptors in rats: a pharmacological-based evidence. Fundam Clin Pharmacol. 2021;35:131–40. https://doi.org/10.1111/fcp.12590.
Lv R-J, Wang Q, Cui T, Zhu F, Shao X-Q. Status epilepticus-related etiology, incidence and mortality: a meta-analysis. Epilepsy Res. 2017;136:12–7.
Faghir-Ghanesefat H, Keshavarz-Bahaghighat H, Rajai N, Mokhtari T, Bahramnejad E, Roodsari SK, et al. The possible role of nitric oxide pathway in pentylenetetrazole preconditioning against seizure in mice. J Mol Neurosci. 2019;67:477–83.
Andre V, Ferrandon A, Marescaux C, Nehlig A. The lesional and epileptogenic consequences of lithium–pilocarpine-induced status epilepticus are affected by previous exposure to isolated seizures: effects of amygdala kindling and maximal electroshocks. Neuroscience. 2000;99:469–81.
Najm IM, Hadam J, Ckakraverty D, Mikuni N, Penrod C, Sopa C, et al. A short episode of seizure activity protects from status epilepticus-induced neuronal damage in rat brain. Brain Res. 1998;810:72–5.
Zhang X, Cui S-S, Wallace AE, Hannesson DK, Schmued LC, Saucier DM, et al. Relations between brain pathology and temporal lobe epilepsy. J Neurosci. 2002;22:6052–61.
Jimenez-Mateos EM, Hatazaki S, Johnson MB, Bellver-Estelles C, Mouri G, Bonner C, et al. Hippocampal transcriptome after status epilepticus in mice rendered seizure damage-tolerant by epileptic preconditioning features suppressed calcium and neuronal excitability pathways. Neurobiol Dis. 2008;32:442–53.
Wasterlain CG, Baxter CF, Baldwin RA. GABA metabolism in the substantia nigra, cortex, and hippocampus during status epilepticus. Neurochem Res. 1993;18:527–32.
Hassanipour M, Shirzadian A, Boojar MM-A, Abkhoo A, Abkhoo A, Delazar S, et al. Possible involvement of nitrergic and opioidergic systems in the modulatory effect of acute chloroquine treatment on pentylenetetrazol induced convulsions in mice. Brain Res Bull. 2016;121:124–30.
Youssef FF, Addae JI, Stone TW. NMDA-induced preconditioning attenuates synaptic plasticity in the rat hippocampus. Brain Res. 2006;1073:183–9.
Deryagin OG, Gavrilova SA, Gainutdinov KL, Golubeva AV, Andrianov VV, Yafarova GG, et al. Molecular bases of brain preconditioning. Front Neurosci. 2017;11:427.
Shafaroodi H, Khosravani E, Fakhrzad A, Moezi L. The interaction between morphine and propranolol in chemical and electrical seizure models of mice. Neurol Res. 2016;38:166–76.
Saboory E, Gholami M, Zare S, Roshan-Milani S. The long-term effects of neonatal morphine administration on the pentylenetetrazol seizure model in rats: the role of hippocampal cholinergic receptors in adulthood. Dev Psychobiol. 2014;56:498–509.
Rubaj A, Gustaw K, Zgodziński W, Kleinrok Z, Sieklucka-Dziuba M. The role of opioid receptors in hypoxic preconditioning against seizures in brain. Pharmacol Biochem Behav. 2000;67:65–70.
Kohl B, Dannhardt G. The NMDA receptor complex: a promising target for novel antiepileptic strategies. Curr Med Chem. 2001;8:1275–89.
Förstermann U, Sessa WC. Nitric oxide synthases: regulation and function. Eur Heart J. 2012;33:829–37.
Marini A, Jiang X, Wu X, Pan H, Guo Z, Mattson M, et al. Preconditioning and neurotrophins: a model for brain adaptation to seizures, ischemia and other stressful stimuli. Amino Acids. 2007;32:299–304.
Corasaniti MT, Maiuolo J, Maida S, Fratto V, Navarra M, Russo R, et al. Cell signaling pathways in the mechanisms of neuroprotection afforded by bergamot essential oil against NMDA-induced cell death in vitro. Br J Pharmacol. 2007;151:518–29. https://doi.org/10.1038/sj.bjp.0707237.
Severino PC, Muller GdAS, Vandresen-Filho S, Tasca CI. Cell signaling in NMDA preconditioning and neuroprotection in convulsions induced by quinolinic acid. Life Sci. 2011;89:570–6.
Vandresen-Filho S, Hoeller AA, Herculano BA, Duzzioni M, Duarte FS, Piermartiri TC, et al. NMDA preconditioning attenuates cortical and hippocampal seizures induced by intracerebroventricular quinolinic acid infusion. Neurotox Res. 2013;24:55–62.
Rejdak K, Rejdak R, Kleinrok Z, Sieklucka-Dziuba M. The influence of MK-801 on bicuculline evoked seizures in adult mice exposed to transient episode of brain ischemia. J Neural Transm (Vienna). 2000;107:947–57. https://doi.org/10.1007/s007020070044.
Zaitsev AV, Kim K, Vasilev DS, Lukomskaya NY, Lavrentyeva VV, Tumanova NL, et al. N-methyl-D-aspartate receptor channel blockers prevent pentylenetetrazole-induced convulsions and morphological changes in rat brain neurons. J Neurosci Res. 2015;93:454–65. https://doi.org/10.1002/jnr.23500.
Rejdak R, Rejdak K, Sieklucka-Dziuba M, Stelmasiak Z, Grieb P. Brain tolerance and preconditioning. Pol J Pharmacol. 2001;53:73–80.
Dejban P, Eslami F, Rahimi N, Takzare N, Jahansouz M, Dehpour AR. Involvement of nitric oxide pathway in the anti-inflammatory effect of modafinil on indomethacin, stress, and ethanol-induced gastric mucosal injury in rat. Eur J Pharmacol. 2020;887: 173579.
Kazemi Roodsari S, Bahramnejad E, Rahimi N, Aghaei I, Dehpour AR. Methadone’s effects on pentylenetetrazole-induced seizure threshold in mice: NMDA/opioid receptors and nitric oxide signaling. Ann N Y Acad Sci. 2019;1449:25–35.
Zamanian G, Shayan M, Rahimi N, Bahremand T, Shafaroodi H, Ejtemaei-Mehr S, et al. Interaction of morphine tolerance with pentylenetetrazole-induced seizure threshold in mice: the role of NMDA-receptor/no pathway. Epilepsy Behav. 2020;112: 107343. https://doi.org/10.1016/j.yebeh.2020.107343.
Centeno JM, Orti M, Salom JB, Sick TJ, Pérez-Pinzón MA. Nitric oxide is involved in anoxic preconditioning neuroprotection in rat hippocampal slices. Brain Res. 1999;836:62–9.
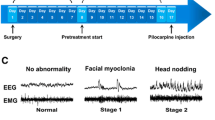
Racine RJ. Modification of seizure activity by electrical stimulation. II Motor seizure Electroencephalogr Clin Neurophysiol. 1972;32:281–94. https://doi.org/10.1016/0013-4694(72)90177-0.
Wang M, Deng X, Xie Y, Chen Y. Astaxanthin attenuates neuroinflammation in status epilepticus rats by regulating the ATP-P2X7R signal. Drug Des Devel Ther. 2020;14:1651.
Amanlou A, Eslami F, Shayan M, Mortazavi P, Dehpour AR. Anticonvulsive evaluation and histopathological survey of thalidomide synthetic analogs on lithium-pilocarpine-induced status epilepticus in rats. Res Pharm Sci. 2021;16:586–95. https://doi.org/10.4103/1735-5362.327505.
Do Val-da Silva RA, Peixoto-Santos JE, Scandiuzzi RC, Balista PA, Bassi M, Glass ML, et al. Decreased neuron loss and memory dysfunction in pilocarpine-treated rats pre-exposed to hypoxia. Neuroscience. 2016;332:88–100.
André V, Ferrandon A, Marescaux C, Nehlig A. The lesional and epileptogenic consequences of lithium-pilocarpine-induced status epilepticus are affected by previous exposure to isolated seizures: effects of amygdala kindling and maximal electroshocks. Neuroscience. 2000;99:469–81. https://doi.org/10.1016/s0306-4522(00)00209-8.
Leré C, El Bahh B, La Salle GLG, Rougier A. A model of ‘epileptic tolerance’for investigating neuroprotection, epileptic susceptibility and gene expression-related plastic changes. Brain Res Protoc. 2002;9:49–56. https://doi.org/10.1016/s1385-299x(01)00136-2.
Jimenez-Mateos EM, Henshall DC. Seizure preconditioning and epileptic tolerance: models and mechanisms. Int J Physiol Pathophysiol Pharmacol. 2009;1:180–91.
Amini E, Rezaei M, Ibrahim NM, Golpich M, Ghasemi R, Mohamed Z, et al. A molecular approach to epilepsy management: from current therapeutic methods to preconditioning efforts. Mol Neurobiol. 2015;52:492–513.
Trinka E, Cock H, Hesdorffer D, Rossetti AO, Scheffer IE, Shinnar S, et al. A definition and classification of status epilepticus—Report of the ILAE task force on classification of status epilepticus. Epilepsia. 2015;56:1515–23. https://doi.org/10.1111/epi.13121.
Acharya MM, Hattiangady B, Shetty AK. Progress in neuroprotective strategies for preventing epilepsy. Prog Neurobiol. 2008;84:363–404.
Jung K-Y, Kim J-M, Kim DW. Nonlinear dynamic characteristics of electroencephalography in a high-dose pilocarpine-induced status epilepticus model. Epilepsy Res. 2003;54:179–88.
Rubaj A, Zgodziński W, Sieklucka-Dziuba M. The epileptogenic effect of seizures induced by hypoxia: the role of NMDA and AMPA/KA antagonists. Pharmacol Biochem Behav. 2003;74:303–11.
Zhu X, Dong J, Han B, Huang R, Zhang A, Xia Z, et al. Neuronal nitric oxide synthase contributes to PTZ kindling epilepsy-induced hippocampal endoplasmic reticulum stress and oxidative damage. Front Cell Neurosci. 2017;11:377.
Gross A, Benninger F, Madar R, Illouz T, Griffioen K, Steiner I, et al. Toll-like receptor 3 deficiency decreases epileptogenesis in a pilocarpine model of SE-induced epilepsy in mice. Epilepsia. 2017;58:586–96. https://doi.org/10.1111/epi.13688.
Fu L, Liu K, Wake H, Teshigawara K, Yoshino T, Takahashi H, et al. Therapeutic effects of anti-HMGB1 monoclonal antibody on pilocarpine-induced status epilepticus in mice. Sci Rep. 2017;7:1179. https://doi.org/10.1038/s41598-017-01325-y.
Curia G, Longo D, Biagini G, Jones RS, Avoli M. The pilocarpine model of temporal lobe epilepsy. J Neurosci Methods. 2008;172:143–57.
Serra M, Dazzi L, Cagetti E, Chessa MF, Pisu MG, Sanna A, et al. Effect of pentylenetetrazole-induced kindling on acetylcholine release in the hippocampus of freely moving rats. J Neurochem. 1997;68:313–8.
Petroff OA, Rothman DL, Behar KL, Mattson RH. Low brain GABA level is associated with poor seizure control. Ann Neurol. 1996;40:908–11. https://doi.org/10.1002/ana.410400613.
Villalpando-Vargas F, Medina-Ceja L. Effect of sparteine on status epilepticus induced in rats by pentylenetetrazole, pilocarpine and kainic acid. Brain Res. 2015;1624:59–70.
Meskinimood S, Rahimi N, Faghir-Ghanesefat H, Gholami M, Sharifzadeh M, Dehpour AR. Modulatory effect of opioid ligands on status epilepticus and the role of nitric oxide pathway. Epilepsy Behav. 2019;101: 106563.
Stumm RK, Zhou C, Schulz S, Höllt V. Neuronal types expressing mu- and delta-opioid receptor mRNA in the rat hippocampal formation. J Comp Neurol. 2004;469:107–18. https://doi.org/10.1002/cne.10997.
Fathalizade F, Baghani M, Khakpai F, Fazli-Tabaei S, Zarrindast MR. GABA-ergic agents modulated the effects of histamine on the behaviour of male mice in the elevated plus maze test. Exp Physiol. 2022;107:233–42. https://doi.org/10.1113/ep090060.
Kim YS, Yoon BE. Altered GABAergic signaling in brain disease at various stages of life. Exp Neurobiol. 2017;26:122–31. https://doi.org/10.5607/en.2017.26.3.122.
Ormandy GC, Jope RS, Snead OC. Anticonvulsant actions of MK-801 on the lithium-pilocarpine model of status epilepticus in rats. Exp Neurol. 1989;106:172–80. https://doi.org/10.1016/0014-4886(89)90091-5.
Morris PJ, Burke RD, Sharma AK, Lynch DC, Lemke-Boutcher LE, Mathew S, et al. A comparison of the pharmacokinetics and NMDAR antagonism-associated neurotoxicity of ketamine, (2R,6R)-hydroxynorketamine and MK-801. Neurotoxicol Teratol. 2021;87: 106993. https://doi.org/10.1016/j.ntt.2021.106993.
Rahimi N, Modabberi S, Faghir-Ghanesefat H, Shayan M, Farzad Maroufi S, Asgari Dafe E, et al. The possible role of nitric oxide signaling and NMDA receptors in allopurinol effect on maximal electroshock- and pentylenetetrazol-induced seizures in mice. Neurosci Lett. 2022;778: 136620. https://doi.org/10.1016/j.neulet.2022.136620.
Hanada T. Ionotropic glutamate receptors in epilepsy: a review focusing on ampa and nmda receptors. Biomolecules. 2020. https://doi.org/10.3390/biom10030464.
Abdelsameea AA. Effect of verapamil, cinnarizine and memantine on maximal electroshock, picrotoxin, and pilocarpine-induced seizure models in albino mice. Egypt J Basic Clin Pharmacol. 2015;5:11–8. https://doi.org/10.11131/2015/101356.
Krug M, Becker A, Grecksch G, Pfeiffer A, Matthies R, Wagner M. Effects of anticonvulsive drugs on pentylenetetrazol kindling and long-term potentiation in freely moving rats. Eur J Pharmacol. 1998;356:179–87.
Boeck CR, Ganzella M, Lottermann A, Vendite D. NMDA preconditioning protects against seizures and hippocampal neurotoxicity induced by quinolinic acid in mice. Epilepsia. 2004;45:745–50.
Mark LP, Prost RW, Ulmer JL, Smith MM, Daniels DL, Strottmann JM, et al. Pictorial review of glutamate excitotoxicity: fundamental concepts for neuroimaging. Am J Neuroradiol. 2001;22:1813–24.
Milatovic D, Gupta RC, Dettbarn W-D. Involvement of nitric oxide in kainic acid-induced excitotoxicity in rat brain. Brain Res. 2002;957:330–7.
Liu J, Wang A, Li L, Huang Y, Xue P, Hao A. Oxidative stress mediates hippocampal neuron death in rats after lithium–pilocarpine-induced status epilepticus. Seizure. 2010;19:165–72.
Moradi F, Eslami F, Rahimi N, Koohfar A, Shayan M, Maadani M, et al. Modafinil exerts anticonvulsive effects against lithium-pilocarpine-induced status epilepticus in rats: a role for tumor necrosis factor-α and nitric oxide signaling. Epilepsy Behav. 2022;130: 108649. https://doi.org/10.1016/j.yebeh.2022.108649.
Noroozi N, Shayan M, Maleki A, Eslami F, Rahimi N, Zakeri R, et al. Protective effects of dapsone on scopolamine-induced memory impairment in mice: involvement of nitric oxide pathway. Dement Geriatr Cogn Dis Extra. 2022;12:43–50. https://doi.org/10.1159/000522163.
Han D, Yamada K, Senzaki K, Xiong H, Nawa H, Nabeshima T. Involvement of nitric oxide in pentylenetetrazole-induced kindling in rats. J Neurochem. 2000;74:792–8.
Acknowledgements
This study was supported by a grant from the Tehran University of Medical Sciences (TUMS) and Iran National Sciences Foundation (INSF) (Grant No. 96002757).
Funding
A grant supported this study from the Tehran University of Medical Sciences (TUMS) and Iran National Sciences Foundation (INSF) (Grant No. 96002757).
Author information
Authors and Affiliations
Contributions
ARD: conceptualization, resources, methodology, supervision, writing, review and editing, FE and MS: investigation, project administration, writing original draft, methodology, formal analysis, review and editing, AA: project administration, methodology, writing, review and editing, NR: review and editing, PD: review and editing.
Corresponding author
Ethics declarations
Conflict of interest
None.
Ethics approval
All animal experiments were performed in agreement with the Guidelines for the Care and Use of Laboratory Animal Ethics Committee of Tehran University of Medical Sciences (Ethical code: IR.TUMS.MEDICINE.REC.1399.579) as well as the National Institutes of Health (NIH publication NO. 85–23; revised 1985).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Eslami, F., Shayan, M., Amanlou, A. et al. Pentylenetetrazole preconditioning attenuates severity of status epilepticus induced by lithium-pilocarpine in male rats: evaluation of opioid/NMDA receptors and nitric oxide pathway. Pharmacol. Rep 74, 602–613 (2022). https://doi.org/10.1007/s43440-022-00387-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43440-022-00387-8