Abstract
Background
Women with polycystic ovary syndrome (PCOS) are characterized by increased cardiometabolic risk. The aim of the current study was to compare the impact of atorvastatin on plasma levels of cardiometabolic risk factors between men whose sisters had either PCOS or were unaffected.
Methods
The study population consisted of two age-, fat-free mass index-, blood pressure- and plasma lipid-matched groups of men with elevated total and LDL cholesterol levels: 20 brothers of PCOS probands (group 1) and 20 brothers of healthy women (group 2). Both groups were then treated with atorvastatin (40 mg daily) for the following 6 months. At the beginning and at the end of the study, we assessed plasma lipid levels, glucose homeostasis markers and levels of dehydroepiandrosterone sulfate, testosterone, bioavailable testosterone, uric acid, high-sensitivity C-reactive protein (hsCRP), homocysteine, fibrinogen and 25-hydroxyvitamin D.
Results
At the beginning of the study, both treatment arms differed in the degree of insulin resistance, calculated bioavailable testosterone, as well as in plasma levels of dehydroepiandrosterone sulfate, uric acid, hsCRP and 25-hydroxyvitamin D. Although atorvastatin reduced total and LDL cholesterol levels, this effect was stronger in group 2 than group 1. In group 2, atorvastatin exerted also a more potent impact on hsCRP, fibrinogen and homocysteine. An unfavorable impact on insulin sensitivity was observed only in group 1; while, statistically significant changes in uric acid and 25-hydroxyvitamin D levels were found only in group 2.
Conclusion
The obtained results suggest that cardiometabolic effects of atorvastatin are less pronounced in male siblings of PCOS probands than in brothers of unaffected women.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Polycystic ovary syndrome (PCOS) affects 6–20% of premenopausal women and seems to be the most common endocrine disorder in women of reproductive age [1,2]. In addition to chronic anovulation, hyperandrogenism and polycystic ovaries, the disorder is characterized by an increased prevalence of abdominal obesity, insulin resistance, deterioration of glucose metabolism, dyslipidemia and low-grade systemic inflammation [1,2]. PCOS also carries significant risk for the development of metabolic and cardiovascular complications, including diabetes, metabolic syndrome and cardiovascular disease [3,4].
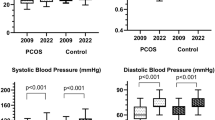
The results of several studies are in favor of increased cardiometabolic risk also in male relatives of PCOS probands. Brothers of women with PCOS were more obese and more prone to central adiposity than unrelated healthy men [5]. Systolic, but not diastolic blood, pressure was significantly higher in male siblings of PCOS probands than in control men [5,6]. Men whose sisters were diagnosed with PCOS had increased levels of 2-h postchallenge glucose, were more insulin-resistant, more frequently met the criteria of metabolic syndrome [5,7,8,9,10], as well as were characterized by a higher prevalence of type 2 diabetes and impaired glucose tolerance than brothers of unaffected women [11]. They were also characterized by impaired brachial artery flow-mediated dilatation and this impairment was particularly pronounced in subjects with a family history of diabetes mellitus [9,12]. Circulating levels of adiponectin, but not of resistin and homocysteine, were lower in male siblings of PCOS probands than unrelated healthy control subjects [13]. Finally, brothers of women with PCOS had increased levels of plasminogen activator inhibitor-1 and factor VII [7]. Interestingly, compared with matched controls, male siblings of women with PCOS were found to have significantly elevated dehydroepiandrosterone-sulfate (DHEA-S) levels [9,10,14], as well as lower concentrations of bioavailable testosterone [5]. Recently, we have observed that exogenous testosterone potentiated lipid-lowering and extra-lipid effects of hypolipidemic agents in subjects with low testosterone levels [15,16]. Therefore, the aim of the current study was to compare the impact of atorvastatin on plasma levels of cardiometabolic risk factors between men whose sisters had either PCOS or were unaffected.
Materials and methods
Patients
The study population consisted of 40 adult men (aged 30–70 years) with elevated levels of total cholesterol (above 200 mg/dL) and low-density lipoprotein (LDL) cholesterol (more than 130 mg/dL), who had been following the lifestyle modification intervention for at least 12 weeks before entering the study. These subjects were screened at the outpatient clinic of our department for the presence of lipid abnormalities. Group 1 included 20 male siblings of women with PCOS (diagnosed based on the Rotterdam 2003 criteria). Group 2, serving as a control group, enrolled 20 male siblings of healthy adult women. These subjects were selected among hypercholesterolemic men by a computer algorithm, aimed at obtaining two groups matched for age, body mass index, blood pressure and plasma lipid levels. All participants were enrolled either in December or January (n = 21), or in June or July (n = 19) to limit the impact of seasonal fluctuations in the measured parameters.
Potential participants were excluded if they met any of the following criteria: cardiovascular disease (with the exception of mild arterial hypertension), diabetes, thyroid disorders, acute and chronic inflammatory processes, kidney or liver failure, malabsorption syndromes, as well as any pharmacotherapy.
All patients gave written informed consent before participating in the research after the nature of the study had been explained and each participant had the opportunity to ask questions. The study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the local ethics committee.
Study design
Over the entire study period (6 months), all men were treated with atorvastatin (40 mg daily), which was administered once daily at bedtime. No changes in treatment regimen were allowed. Throughout the study, all participants were also required to restrict intakes of total fat to less than 30% of energy intake, saturated fat to less than 7% of energy intake, and cholesterol to less than 200 mg per day, as well as were encouraged to increase fiber intake to 15 g per 1000 kcal. They were also recommended to do at least 150 min of moderate-intensity aerobic physical activity per week. Moderate-intensity activity was defined based on the rate of energy expenditure during the activity (between 3 and 6 metabolic equivalents) and on a person’s level of effort (5 or 6 on a scale of 0–10, where sitting is 0 and the highest level of effort is 10). The recommended activities included brisk walking (at least 4 km/h), water aerobics, slow dancing (ballroom or social), tennis (doubles), biking slower than 16 km/h, hiking, washing windows, sweeping the floor, vacuuming or pushing a lawn mower. The participants were also asked to do muscle-strengthening activities that were moderate intensity and involved all major muscle groups on two or more days a week. Compliance was assessed every 8 weeks by tablet counts and analysis of individual dietary questionnaires and diaries in which they continuously recorded all their activities.
Anthropometric measurements
Body mass index was calculated as weight (in kg) divided by height (in m) squared. Fat-free mass was measured using bioelectrical impedance analysis (model BF-511 B, Omron Healthcare Europe, Hoofddorp, the Netherlands), which is based on different conductance and impedance of fat and fat-free tissue. Fat-free mass index was calculated with the following formula: fat-free mass/ height squared.
Laboratory assays
Laboratory assays were performed in the first and in the last days of the study. All venous blood samples were collected between 7.30 and 8.30 a.m., after an overnight 12-h fasting and assayed in duplicate. Plasma concentrations of total cholesterol, LDL cholesterol, HDL cholesterol, triglycerides, glucose, creatinine and uric acid were measured with standard methods (Roche Diagnostics, Basel, Switzerland). Plasma levels of insulin, DHEA-S, total testosterone, sex hormone-binding globulin, high-sensitivity C-reactive protein (hsCRP), homocysteine and 25-hydroxyvitamin D were assayed using an electrochemiluminescent analyzer (Cobas e411, Roche Diagnostics, Mannheim, Germany). Fibrinogen was measured by the Clauss technique using an automated BCS XP analyzer (Siemens Healthcare, Warsaw, Poland). The homeostatic model assessment 1 of insulin resistance (HOMA1-IR) was calculated as fasting blood glucose (mg/dL)/x fasting insulin (mIU/L)/405. Bioavailable testosterone was calculated based on testosterone and sex hormone-binding globulin levels using the online calculator (https://www.issam.ch/freetesto.htm). The estimated glomerular filtration rate was calculated as follows: 175 × [plasma creatinine (µmol/L) × 0.0113]−1.154 × age (years)0.203 (the Modification Diet in Renal Disease Study equation).
Statistical analysis
Before statistical analysis, all data were subjected to log-transformation to meet the criteria of normality and homogeneity. The study arms, as well as differences between percent changes from baseline after adjustment for baseline values (reflecting atorvastatin action), were compared using Student’s t test for independent samples after consideration of age, fat-free mass index, smoking and blood pressure as potential confounders. The differences between baseline and post-treatment values within the same study arm were analyzed with Student’s paired t test. Qualitative data were compared with χ2 tests. Associations between variables were tested using Pearson's correlation coefficient. Two-tailed p values below 0.05 were considered statistically significant.
Results
At baseline, there were no statistically significant differences between groups 1 and 2 in age, body smoking, mass index, fat-free mass, fat-free mass index, blood pressure, as well as in plasma levels of total cholesterol, LDL cholesterol, HDL cholesterol, triglycerides, glucose, total testosterone, fibrinogen, homocysteine and the glomerular filtration rate. HOMA1-IR, DHEA-S, uric acid and hsCRP were higher, while calculated bioavailable testosterone and 25-hydroxyvitamin D were lower in group 1 than group 2 (Table 1).
Because no serious adverse effects were reported, all patients completed the follow-up and were included in the final analysis. Atorvastatin did not affect body mass index, fat-free mass and fat-free mass index. There were no differences between both groups in physical activity.
In both groups, atorvastatin reduced total and LDL cholesterol levels, as well as decreased hsCRP, homocysteine and fibrinogen. Moreover, in group 1, the drug increased HOMA1-IR; while in group 2, atorvastatin reduced uric acid and increased 25-hydroxyvitamin D levels. In both groups, atorvastatin exerted a neutral effect on plasma levels of HDL cholesterol, triglycerides, glucose, DHEA-S, testosterone and calculated bioavailable testosterone, as well as on the estimated glomerular filtration rate. The effect of atorvastatin on total cholesterol, LDL cholesterol, uric acid, hsCRP, homocysteine, fibrinogen and 25-hydroxyvitamin D was stronger; while, the impact on HOMA1-IR was less expressed in group 2 than group 1. At the end of the study period, there were differences between the treatment arms in total cholesterol, LDL cholesterol, HOMA1-IR, DHEA-S, calculated bioavailable testosterone, uric acid, hsCRP, homocysteine, fibrinogen and 25-hydroxyvitamin (Table 2).
At baseline, plasma concentrations of uric acid, hsCRP, homocysteine and fibrinogen correlated with plasma levels of total cholesterol [group 1: r values between 0.25 (p = 0.0493) and 0.37 (p = 0.0074); group 2: r values between 0.32 (p = 0.0231) and 0.47 (p = 0.0002)] and LDL cholesterol (group 1: r values between 0.26 (p = 0.043) and 0.41 (p = 0.0004)] group 2: r values between 0.34 (p = 0.0180) and 0.49 (p = 0.0001)]. There were also correlations between total and LDL cholesterol and 25-hydroxyvitamin D [group 1: r = − 0.35 (p = 0.0110) and r = − 0.38 (p = 0.0095); group 2: r = − 0.40 (p = 0.0008) and r = − 0.43 (p = 0.0002)], as well as between baseline HOMA1-IR and baseline values of uric acid [group 1: r = 0.40 (p = 0.0005); group 2: r = 0.49 (p < 0.0001)], CRP [group 1: r = 0.37 (p = 0.0068); group 2: r = 0.39 (p = 0.0001)] and 25-hydroxyvitamin D [group 1: r = − 0.32 (p = 0.0325); group 2: r = − 0.34 (p = 0.0285)]. The impact of atorvastatin on uric acid, hsCRP, fibrinogen, homocysteine and 25-hydroxyvitamin D correlated with baseline calculated bioavailable testosterone levels [group 1: r values between − 0.26 (p = 0.0415) and − 0.35 (p = 0.0092); group 2: r values between − 0.34 (p = 0.0012) and − 0.49 (p < 0.0001)] but did not correlate with its action on plasma lipids. Treatment-induced changes in uric acid, hsCRP, fibrinogen and homocysteine inversely correlated with baseline 25-hydroxyvitamin D levels [group 1: r values between − 0.24 (p = 0.046) and − 0.34 (p = 0.0355); group 2: r values between − 0.31 (p = 0.0261) and − 0.42 (p = 0.0002)], and in group 2 with treatment-induced increase in 25-hydroxyvitamin D [r values between 0.28 (p = 0.0295) and 0.38 (p = 0.0025)]. In group 1, atorvastatin-induced increase in HOMA1-IR correlated with its baseline value [r = − 0.47 (p = 0.0001)]. No other correlations were significant.
Discussion
The current study has shown for the first time that male siblings of PCOS probands were characterized by abnormal levels of some of factors known to be involved in the pathogenesis of atherosclerosis and carbohydrate disorders and to predict the development of cardiovascular disease, diabetes and metabolic syndrome [17,18,19,20,21,22], as well as that hypolipidemic and pleiotropic effects of atorvastatin were less pronounced in brothers of PCOS probands than in unrelated matched control men. Although at baseline both groups differed in HOMA1-IR and in plasma levels of uric acid, hsCRP and 25-hydroxyvitamin D, the extent of these abnormalities was less pronounced than observed previously in women with PCOS [23]. Moreover, both groups differed in androgen levels. Owing to preselection resulting in similar baseline levels, the obtained results cannot be explained by differences in body mass index, fat-free mass, blood pressure or plasma lipids. Moreover, strict inclusion and exclusion criteria minimized the possible impact of other disorders or concomitant therapies.
Based on the obtained results, some clinical conclusions can be drawn. First, our findings are in line with the view that genetic factors play an important role in etiology of PCOS [24]. Second, male brothers of women with PCOS syndrome may be more prone to the development and progression of metabolic and cardiovascular disorders, especially if they have concomitant risk factors. However, relative cardiovascular risk in male siblings of women with PCOS seems to be lower than in probands. Third, male siblings of women with PCOS are probably less likely to benefit or benefit to a lesser degree from statin therapy than their unrelated peers. Finally, less expressed benefits of atorvastatin treatment in those men may be partially associated with abnormal testosterone production.
Male siblings of women with PCOS were characterized by higher DHEA-S levels, as well as lower values of calculated bioavailable testosterone than their counterparts without a family history of this disorder. Bioavailable testosterone calculated by Vermeulen’s formula, used in the present study, reflects the sex hormone-binding protein-unbound fraction of this hormone and correlates well with free testosterone assessed by equilibrium dialysis [25]. Therefore, small differences in testosterone production between both arms, as in the current study, may be reflected by differences in bioavailable testosterone despite similar levels of total hormone. The lack of fluctuations and long half-life cause that DHEA-S reflects well adrenal production of dehydroepiandrosterone [26], which is a precursor of sex steroid hormones and has the potential to be converted to testosterone [26]. Therefore, increased DHEA-S levels and decreased values of calculated bioavailable testosterone in brothers of PCOS probands suggest that they may be characterized by impaired conversion of dehydroepiandrosterone to testosterone. It seems that abnormal production of testosterone rather than of dehydroepiandrosterone contributes to increased cardiometabolic risk in male siblings of PCOS. In line with this conclusion, baseline and treatment-induced changes in levels of all measured risk factors correlated with calculated bioavailable testosterone but not with DHEA-S. Interestingly, between-group differences in testosterone were discrete and this finding may explain why attenuating effect on atorvastatin action was less pronounced in brothers of PCOS probands that in men with late-onset hypogonadism participating in the previous study [15]. In both treatment arms, atorvastatin exerted a neutral effect on total and calculated bioavailable testosterone, which is line with previous observations that only aggressive statin therapy, reducing cholesterol levels to subnormal values, impairs testicular steroidogenesis [27].
Despite numerous cardiovascular benefits associated with statin therapy in subjects with impaired glucose homeostasis, 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors slightly increase the risk of type 2 diabetes, probably because exerting an unfavorable effect on insulin sensitivity and secretion [28]. Both study arms, which included only diabetes-free men, differed in baseline values of HOMA1-IR and this finding is in line with the previous observations indicating that male siblings of PCOS probands are characterized by impaired insulin sensitivity [5,7,8,9,10]. The presence of correlation between baseline HOMA1-IR and baseline values of uric acid, hsCRP and 25-hydroxyvitamin D indicates that the increased cardiometabolic risk in these subjects is partially associated with disturbed insulin action. Moreover, unlike control subjects, atorvastatin administered to brothers of women with PCOS worsened insulin sensitivity, which correlated with baseline HOMA1-IR. This finding suggests that the unfavorable effect of HMG-CoA reductase inhibitor therapy on glucose homeostasis in male siblings of PCOS probands may be secondary to their more atherogenic metabolic profile.
The impact of atorvastatin on uric acid, hsCRP, fibrinogen, homocysteine and 25-hydroxyvitamin D did not correlate with treatment-induced changes in total and LDL cholesterol, suggesting its pleiotropic nature. Cardiometabolic effects of atorvastatin may be associated with the inhibitory impact on protein prenylation, nuclear factor-κB pathway and leukocyte function-associated antigen-1-intercellular adhesion molecule-1 interaction [29,30]. Interestingly, PCOS is associated with the activation of intranuclear nuclear factor-κB [31], as well as with the increased expressions of leukocyte function-associated antigen-1 and intercellular adhesion molecule-1 [32]. Moreover, extra-lipid effects of statin therapy in women with PCOS, mediated probably by the inhibitory effects on farnesylation and geranylgeranylation, seem to be weak [33]. If these mechanisms are disturbed also in brothers of women with PCOS, they may be more resistant to statin therapy as it was observed in our study.
The impact of atorvastatin on uric acid, hsCRP, fibrinogen and homocysteine inversely correlated with baseline 25-hydroxyvitamin D levels, and in subjects with a negative family history of PCOS also with treatment-induced increase in 25-hydroxyvitamin D. Interestingly, cholecalciferol is produced from 7-dehydrocholesterol, which is also a precursor for cholesterol [34] and the enhanced production of vitamin D may exert an inhibitory effect on the mevalonate pathway. Alternatively, taking into account the inhibitory effect of vitamin D on the nuclear factor κB signaling pathway [35], the improvement in vitamin D status only in one treatment arm may partially explain between-group differences in the strength of cardiometabolic effects of atorvastatin in our study.
Several study limitations need to be acknowledged. Because of the small sample size and short-term nature, this research should be regarded as a preliminary report. The current study did not investigate hard clinical endpoints, such as morbidity and mortality rates. It remains to be elucidated whether the obtained results represent a class effect of HMG-CoA reductase inhibitors or are associated with unique properties of atorvastatin. Finally, the study did not compare lipid and pleiotropic effects of atorvastatin between brothers of women with various phenotypes of PCOS.
In summary, compared with brothers of unaffected women, male siblings of women with PCOS were more insulin resistant, as well as had higher levels of uric acid, hsCRP and lower levels of 25-hydroxyvitamin D. In addition to differences in hypolipidemic effects, the impact of atorvastatin on cardiometabolic risk factors was more pronounced in subjects not having sisters with PCOS. The obtained results suggest that cardiometabolic effects of atorvastatin are less expressed in male siblings of women with PCOS. Because of study limitations, this issue needs further and deeper investigation in future studies with larger sample sizes.
Change history
26 May 2021
A Correction to this paper has been published: https://doi.org/10.1007/s43440-021-00276-6
Abbreviations
- DHEA-S:
-
Dehydroepiandrosterone-sulfate
- HDL:
-
High-density lipoproteins
- HMG-CoA:
-
3-Hydroxy-3-methylglutaryl coenzyme A
- HOMA1-IR:
-
The homeostasis model assessment 1 of insulin resistance index
- hsCRP:
-
High-sensitivity C-reactive protein
- LDL:
-
Low-density lipoproteins
- PCOS:
-
Polycystic ovary syndrome
- SD:
-
Standard deviation
References
Escobar-Morreale HF. Polycystic ovary syndrome: definition, aetiology, diagnosis and treatment. Nat Rev Endocrinol. 2018;14:270–84.
Trikudanathan S. Polycystic ovarian syndrome. Med Clin N Am. 2015;99:221–35.
Bajuk Studen K, Pfeifer M. Cardiometabolic risk in polycystic ovary syndrome. Endocr Connect. 2018;7:R238–R251251.
Osibogun O, Ogunmoroti O, Michos ED. Polycystic ovary syndrome and cardiometabolic risk: opportunities for cardiovascular disease prevention. Trends Cardiovasc Med 2019;21:1–5.
Coviello AD, Sam S, Legro RS, Dunaif A. High prevalence of metabolic syndrome in first-degree male relatives of women with polycystic ovary syndrome is related to high rates of obesity. J Clin Endocrinol Metab. 2009;94:4361–6.
Yilmaz B, Vellanki P, Ata B, Yildiz BO. Metabolic syndrome, hypertension, and hyperlipidemia in mothers, fathers, sisters, and brothers of women with polycystic ovary syndrome: a systematic review and meta-analysis. Fertil Steril. 2018;109:356–64.
Baillargeon JP, Carpentier AC. Brothers of women with polycystic ovary syndrome are characterised by impaired glucose tolerance, reduced insulin sensitivity and related metabolic defects. Diabetologia. 2007;50:2424–32.
Sam S, Coviello AD, Sung YA, Legro RS, Dunaif A. Metabolic phenotype in the brothers of women with polycystic ovary syndrome. Diabetes Care. 2008;31:1237–41.
Karthik S, Vipin VP, Kapoor A, Tripathi A, Shukla M, Dabadghao P. Cardiovascular disease risk in the siblings of women with polycystic ovary syndrome. Hum Reprod. 2019;34:1559–666.
Subramaniam K, Tripathi A, Dabadghao P. Familial clustering of metabolic phenotype in brothers of women with polycystic ovary syndrome. Gynecol Endocrinol. 2019;35:601–3.
Yilmaz B, Vellanki P, Ata B, Yildiz BO. Diabetes mellitus and insulin resistance in mothers, fathers, sisters, and brothers of women with polycystic ovary syndrome: a systematic review and meta-analysis. Fertil Steril. 2018;110(523–33):e14.
Kaushal R, Parchure N, Bano G, Kaski JC, Nussey SS. Insulin resistance and endothelial dysfunction in the brothers of Indian subcontinent Asian women with polycystic ovaries. Clin Endocrinol (Oxf). 2004;60:322–8.
Yilmaz M, Bukan N, Ersoy R, Karakoç A, Yetkin I, Ayvaz G, Cakir N, Arslan M. Glucose intolerance, insulin resistance and cardiovascular risk factors in first degree relatives of women with polycystic ovary syndrome. Hum Reprod. 2005;20:2414–20.
Legro RS, Kunselman AR, Demers L, Wang SC, Bentley-Lewis R, Dunaif A. Elevated dehydroepiandrosterone sulfate levels as the reproductive phenotype in the brothers of women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2002;87:2134–8.
Krysiak R, Gilowski W, Okopień B. The effect of testosterone on cardiometabolic risk factors in atorvastatin-treated men with late-onset hypogonadism. Pharmacol Rep. 2016;68:196–200.
Krysiak R, Gilowski W, Okopień B. The effect of testosterone and fenofibrate, administered alone or in combination, on cardiometabolic risk factors in men with late-onset hypogonadism and atherogenic dyslipidemia. Cardiovasc Ther. 2015;33:270–4.
Kinlay S, Egido J. Inflammatory biomarkers in stable atherosclerosis. Am J Cardiol. 2006;98:2P–8P.
Feig DI, Kang DH, Johnson RJ. Uric acid and cardiovascular risk. N Engl J Med. 2008;359:1811–21.
Krysiak R, Okopień B, Herman Z. Effects of HMG-CoA reductase inhibitors on coagulation and fibrinolysis processes. Drugs. 2003;63:1821–54.
McCully KS. Homocysteine, vitamins, and vascular disease prevention. Am J Clin Nutr. 1563S;86:1563S–S15681568.
Wang L, Song Y, Manson JE, Pilz S, März W, Michaëlsson K. Circulating 25-hydroxyvitamin D and risk of cardiovascular disease: a meta-analysis of prospective studies. Circ Cardiovasc Qual Outcomes. 2012;5:819–29.
Yazıcı D, Sezer H. Insulin resistance, obesity and lipotoxicity. Adv Exp Med Biol. 2017;960:277–304.
Krysiak R, Gilowska M, Okopień B. The effect of oral contraception on cardiometabolic risk factors in women with elevated androgen levels. Pharmacol Rep. 2017;69:45–9.
Crespo RP, Bachega TASS, Mendonça BB, Gomes LG. An update of genetic basis of PCOS pathogenesis. Arch Endocrinol Metab. 2018;62:352–61.
Keevil BG, Adaway J. Assessment of free testosterone concentration. J Steroid Biochem Mol Biol. 2019;190:207–11.
Klinge CM, Clark BJ, Prough RA. Dehydroepiandrosterone research: past, current, and future. Vitam Horm. 2018;108:1–28.
Krysiak R, Okopień B. The effect of aggressive rosuvastatin treatment on steroid hormone production in men with coronary artery disease. Basic Clin Pharmacol Toxicol. 2014;114:330–5.
Laakso M, Kuusisto J. Diabetes secondary to treatment with statins. Curr Diab Rep. 2017;17:10.
Zhou Q, Liao JK. Statins and cardiovascular diseases: from cholesterol lowering to pleiotropy. Curr Pharm Des. 2009;15:467–78.
Mathur N, Ramasubbu K, Mann DL. Spectrum of pleiotropic effects of statins in heart failure. Heart Fail Clin. 2008;4:153–61.
González F, Rote NS, Minium J, Kirwan JP. Increased activation of nuclear factor kappaB triggers inflammation and insulin resistance in polycystic ovary syndrome. J Clin Endocrinol Metab. 2006;91:1508–12.
Solano ME, Sander VA, Ho H, Motta AB, Arck PC. Systemic inflammation, cellular influx and up-regulation of ovarian VCAM-1 expression in a mouse model of polycystic ovary syndrome (PCOS). J Reprod Immunol. 2011;92:33–44.
Banaszewska B, Pawelczyk L, Spaczynski R. Current and future aspects of several adjunctive treatment strategies in polycystic ovary syndrome. Reprod Biol. 2019;19:309–15.
Kulie T, Groff A, Redmer J, Hounshell J, Schrager S. Vitamin D: an evidence-based review. J Am Board Fam Med. 2009;22:698–706.
Chen Y, Zhang J, Ge X, Du J, Deb DK, Li YC. Vitamin D receptor inhibits nuclear factor κB activation by interacting with IκB kinase β protein. J Biol Chem. 2013;288:19450–8.
Acknowledgements
This work was not supported by any external source of funding. The experiments comply with the current law of Poland.
Funding
Open access publishing of this article was funded by the Ministry of Science and Higher Education under the agreement No. 879/P-DUN/2019.
Author information
Authors and Affiliations
Contributions
RK conceived of the study, participated in its design, performed the statistical analysis, as well as drafted and edited the manuscript. WS conducted the literature search, carried out the assays and performed the statistical analysis. BO participated in its design and coordination, and provided critical input during manuscript preparations. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Ethical approval
The study was approved by the local institutional review board.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Krysiak, R., Szkróbka, W. & Okopień, B. The impact of atorvastatin on cardiometabolic risk factors in brothers of women with polycystic ovary syndrome. Pharmacol. Rep 73, 261–268 (2021). https://doi.org/10.1007/s43440-020-00135-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43440-020-00135-w