Abstract
Glass samples with the composition 40 Bi2O3-60 GeO2-x Er2O3 are prepared using the melt quenching procedure. Fourier-transform infrared spectroscopy (FTIR), differential thermal analysis (DTA), Ultraviolet/Visible/Near-infrared (UV/Vis–NIR) absorption, photoluminescence spectroscopy (PL), and X-ray diffraction (XRD) are used to characterize the glass and glass–ceramic materials. The glass transition and crystallization temperatures are determined. Crystallization kinetics was studied in non-isothermal conditions. The oscillator strengths and Judd–Ofelt parameters (Ω2, Ω4, Ω6) for reported Er3+ absorption transitions are estimated, and they follow the trend Ω2 > Ω6 > Ω4 for observed Er3+ absorption transitions. The iconicity of glass samples was unaffected by an increase in Er3+ ions. XRD and FTIR confirmed the formation of the Bi4Ge3O12 phase after heat treatment of glass samples at the crystallization temperature. The CIE chromaticity diagram computes the CIE chromatic coordinates. The values for all glass and crystal samples are close to bright blue and green. Glass and glass ceramic samples are suitable for green and blue optoelectronics device applications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The glass crystal system offers two distinct advantages. It functions as a glass composition with various rare-earth or transition metal compositions. Glass samples containing rare-earth ions are of great interest due to their many practical and potential applications.
Due to the high conversion process of converting infrared rays into visible light and flat screens, which uses as glass lasers, optical fiber amplifiers, phosphors, electroluminescent devices, and memory devices [1, 2].
The application of bismuth and germanium in glass is significant because it uses as a layer for optical and optoelectronic devices, thermal and mechanical sensors, infrared transmitting windows, and active media for Raman optical fiber amplifiers. Due to its outstanding structural characteristics, this glass system uses in a wide range of fields [1], temperature sensors, 1.55 m amplifications, and planar waveguides [3]. The other method converts glass into a crystal phase through heat treatment. The crystal phase that results from this procedure has the same properties as a single crystal. It also has the advantages of being inexpensive and simple to make. Bismuth germinate oxide is a component of the glass–ceramic system.
The crystalline phase generates by heat treatment and is controlled by many parameters, such as the ratio of bismuth to germanium oxide, the melting temperature, and cooling rates [4].
Many researchers directed their attention to the preparation of bismuth germinate glasses and the Bi4Ge3O12 phase. According to their physical properties, they can use optical waveguides in the IR (infrared) region, as well as active media for Raman optical fiber, scintillation counters, thermal and mechanical sensors, as well as other optical devices, such as optical fibers, optical switching, and optical memory [1, 5].
Chen et al. and Sanghi et al. [6, 7] have studied and compared the optical properties of different glass formations, such as bismuth glass, borate glass, and commercial laser glass doped with Er3+ ions. From this study, the researchers concluded that the spectral parameters of bismuth borate glass are better than those of other glasses.
Polosan et al. [8] investigated the melt-quenched glass-controlled crystallization of bismuth–germinate glass (40Bi2O3–60 GeO2 mol %). And found that the formed phase affected by the annealing of the glass sample above the crystallization temperature (558 °C) formed the Bi4Ge3O12 nano and traces of Bi2GeO5 crystallites. The number of nanocrystals grows as the annealing temperature rises, while their size remains constant in the 50 nm range.
Polosan et al. [9] investigated the production of Bi4Ge3O12 (BGO) from a combination of oxides at 1323 K. The optical characteristics and dielectric functions of a sample made using BGO single crystals were compared.
In this article, we direct our efforts toward studying glass samples prepared in two steps: firstly, study the glass structure and determine the physical, thermal, optical, and emission properties, and determine the effect of adding Er2O3. Secondly, investigate the production and characteristics of the Bi4Ge3O12 phase.
2 Experimental work
Glass samples having a composition of 40 Bi2O3-60 GeO2-x Er2O3 mol%, created with x=0, 0.5, 1.0, 1.5, and 2 mol%. The samples were placed in an oven at 1100 °C for one h., a molten poured between two copper plates in the air. XRD system from Philips Analytical (type PW3710) used a Cu tube anode with wavelengths of Kα1 =1.54060 and Kα2 =1.54439 to examine the nature of all samples. The starting angle (2θ) was 10°, and the ending was 70°. The FTIR JASCO, 6300 infrared spectrometer using to measure the absorption spectra in the range of 400–4000 cm-1 of all samples using the KBr pellets technique. The prepared samples were analyzed using differential thermal analysis (DTA) at temperatures ranging from 30 to 1200 °C, with heating rates of 5, 10, 20, and 30 °C/min, and Al2O3 powder as a reference material (Shimadzu DTA-50 analyzer) measured. A computerized recording spectrophotometer uses to measure the optical transmission spectra in the region of 190 to 2500 nm (JASCO, V-570). The emission measurements were calculated by using the (JASCO–FP-6300) Spectrofluorometer in the (200–800 nm) wavelength range.
3 Results and discussion
3.1 XRD
The state of the samples is amorphous or crystalline examined using XRD equipment. Fig. 1 reveals that the XRD pattern of the materials under analysis is devoid of identifiable peaks.
Figure 1 indicates that the samples are amorphous.
3.2 FTIR
FTIR is a guide instrument that detects any structural changes formed in the vibration groups of the glass network. Fig. 2 shows the glass free of Er, an obvious band for Bi and Ge beginning at 475 cm−1, representing Bi-O vibration in BiO6 [10]. The band at 540 cm−1 represents the distorted BiO6 [1, 11]. The bands around 560–580 cm−1 and 1045 cm−1 represent the stretching vibration of GeO4 overlapped with the BiO6 vibration band [1, 10]. The two bands at 730 cm−1 and 770 cm−1 represent the stretching of GeO4 [1]. The band at 860 cm−1 represents BiO6 [1] and the asymmetric stretching of GeO4 [10]. The band at 1035 cm−1 indicates the stretching band of Ge–O in GeO4 [1]. The band at 1100 cm−1 represents the Bi-O-Bi bond or Bi-O-Ge [1]. The first addition of Er2O3 begins to appear in several bands as the band at 495 cm−1 represents the BiO6 octahedral units [7, 12] or the bending vibration of BiO3 units [1]. A little band at 600 Cm−1 represents the Bi-O- stretching vibration in BiO6 [1]. The band at 443 cm−1 was attributed to symmetry stretching of bridging oxygen (BO) in 6-membered GeO4 rings Ge–O–Ge [13], and the continuous addition of Er broadened the band and could include bands. The appearance of a new band at 520 cm−1 represents the defect mode of GeO4 [13], while the band at 630 Cm−1 represents the stretching of BiO6. The bands at 710–750 cm−1 represent GeO4 [1]. The band at 845 cm−1 corresponds to the symmetric stretching of BiO3 [1, 7], while the band ranging from 750 to 900 cm−1 corresponds to the Bi-O stretching vibration in BiO3 units [11] and the asymmetric stretching of bridging oxygen in Ge–O-Ge [13].
As for undoped and Er-doped Bi2O3-GeO2 glass samples, there are broadband located between 850 cm−1 and 1200 cm−1, which should be corresponding to the vibrations of 850 to 930 cm−1, 1016 to 1031 cm−1 and 1090 to 1110 cm−1, respectively. However, the line shape of the vibrations becomes broad compared to that of the 2Bi2O3-3GeO2 glass sample, which suggests that the structures of 2Bi2O3-3GeO2 and Er-doped Bi2O3-GeO2 are slightly different. With the deconvolution the broad bands consist of three bands at of 850 to 930 cm−1, 1016 to 1031 cm−1 and 1090 to 1110 cm−1. Fig. 3 shows the effect of Er2O3 addition on these bands. From Fig. 3, observed the Bi–O–Ge linkage (1090 to 1110 cm−1) decreases and the vibration of Bi–O bonds in BiO6 units and Ge–O–Ge asymmetric stretching of GeO4 increases as Er2O3 equal and greater than 1 mol %, while GeO4 vibration groups (1016 to 1031) constant for all samples. It is clear the structure variation between samples.
3.3 Optical absorption
Figure 4 depicts the absorption spectra of all glass samples. The absorption spectrum of the glass sample free from Er3+ ions doesn’t have any transition band.
The first addition of Er2O3 in bismuth germanium glasses causes a range of Er3+ absorption transitions, which translate from the 4I15/2 ground state to various excited states due to the f—f transition. The resolved bands in the 400–2000 nm range arise from the 4I15/2 ground state to several excited states [7, 14,15,16,17] as 4F7/2, 2H11/2, 4S3/2, 4F9/2, 4I9/2, 4I11/2, and 4I13/2, with center wavelengths of 486, 522, 542, 650, 796, 980, and 1524 nm, respectively.
3.4 Judd–Ofelt parameters
The intensity of the optical absorption line of Ln3+ ions is used in the modified Judd–Ofelt theory to determine the laser and optical characteristics of ions in crystals and glass [18]. The Judd–Ofelt hypothesis aids in determining the oscillator strength empirically. According to the following relationship [19]:
ε (ν) is the molar absorbance of the peaks at a wave number (cm−1).
The experimental oscillator strength of an electronic transition contains the electric and magnetic dipole strengths.
The magnetic dipole's intensity is determined by the properties of the rare-earth ion, not by the host. Magnetic dipole transitions have a lower probability of occurring than electric dipole transitions. The experimental oscillator line strength determines using [2, 17, 20, 21]:
where λ is the wavelength (nm) of each transition from the ground state (J) to the excited state (J'), c is the velocity of light in a vacuum, m is an electron's rest mass, \({\boldsymbol{\Omega }}_{{\varvec{t}}}\) is the Judd–Ofelt parameters, n is the refractive index, h is plank's constant, and \({{\varvec{U}}}^{{\varvec{\lambda}}}\) is a doubly reduced matrix element.
The fit quality between the experimental and estimated oscillator strengths is determined by the root mean square deviation (δrms). The following relationship is used to calculate δrms:
The total number of energy levels is N. In Table 1, fexp, fcalc, and δrms are calculated and tabulated. The δrms value indicates the fitting between experimental fexp and calculated fcalc strength. Furthermore, using Judd–Ofelt’s theory, intensity parameters JO Ωλ (2, 4, and 6) for all samples are calculated and presented in Table 1.
For all glass samples, the values of Judd Ofelt's parameters have a Ω2 > Ω6 > Ω4 trend. The higher the value of the Ω2 intensity parameter, the more asymmetric and covalent the rare-earth ion sites [22]. The value of Ωλ showed the glass composition containing 1 mol% Er2O3 has the largest Ω2, Ω4, and Ω6. The spectroscopy quality factor (χ) is connected to rigidity, laser determination, and amplifier application [16] and is equivalent to the ratio between Ω4 and Ω6 [22].
The resulting value was less than 1, indicating a hard glass [7, 23] used in the application of a fiber amplifier with high emission intensity (2H11/2 → 4I15/2) [12, 15, 24] in comparison to the literature [12, 25,26,27,28]. The glass composition doped with rare earth causes a wavelength shift, named the nephelauxetic effect. The nephelauxetic effect parameter was determined using the relation [23]:
where νc is the wave number of the Er3+ ion in the host and νa is the wave number of the Er3+ ion in aqua ions.
The bonding parameter is calculated using the formula δ = (1-β')/β' *100 where β' is the average of nephelauxetic. The bonding parameter determines the type of bonding between rare-earth ions and their environment. νa, νc, β, β', and δ are calculated and tabulated in Table 2.
The band position appears in the same place in Fig. 3, and the negative sign of the value denotes the ionic link between Er2O3 ions and the surrounding host.
The value of band gap energy Eopt (energy excites the electron from the top of the valance band to the bottom of the conduction band) indicates the bridging and non-bridging oxygen formed in the glass structure.
The optical band gap determines by drawing the relationship between the absorption coefficient and (αhv)2 [Tauc plots] with hv, as shown in Fig. 5. Table 3 summarizes and tabulates the results gathered.
Table 3 shows that the sample without Er has a higher Eopt value. The value of Eopt decreases with the increase in Er3+, which indicates an increase in NBOs (non-bridging oxygen) [23, 30].
As shown in Fig. 2, the glass cuts off the UV and a small strip of the visible region, indicating the possibility of using glass samples as a filter [31].
3.5 Density and molar volume
The glass system’s density ρ and molar volume Vm are vital in determining a deep sight at any structural alterations in the glass network formation, density, and molar volume are estimated using the relationships [32].
where Wa signifies the sample's weight in the air, Wb denotes the sample's weight in the immersion fluid, and 0.865 is the immersion fluid's density (toluene).
where \({X}_{{\varvec{i}}}\) and \({M}_{{\varvec{i}}}\) are the concentration and molecular weight of each oxide.
Figure 6 depicts the connection between density and molar volume as Er concentration rises. It detected a reversal of the relationship, the density values decreasing up to 1 mol percent Er2O3 and then increasing in the other manner with MV values. The density changes attributed to the addition of Er3+ is due to the breakdown of bonds, and the creation of NBOs, attributed to increased volume as Er ions enter the interstitial site.
Another option is that the RE enters the interstitial site generated by the polymerization of glass [20, 33], which is responsible for the compactness of glass, increasing the molecular weight (MW) of Er2O3.
3.6 Excitation and emission of glass
Figure 7 shows the excitation spectra of Er-doped glass samples at 550 nm emission. Peaks at 317, 368, 381, 410, 453, and 490 nm describe the transition [34, 35] from the ground state 4I15/2 to the excited states 2P3/2, 4G9/2, 4G11/2, 2G9/2, 4F5/2, and 4F7/2. The high intensity of two-level transitions at 4G9/2 and 4F7/2 choose to be the emission energy of the title glass shown in Fig. 8.
It observed the difference in emission energy resulting from different energy peak transitions. The resulting emission spectra showed three peaks at 530, 550, and 639 nm, corresponding to Er3+ [12, 23, 25] transitions 2H11/2 → 4I15/2 (green emission), 4S3/2 → 4I15/2 (green emission), and 4F9/2 → 4I15/2 (red emission).
As seen in Fig. 8, emission peaks have two physical qualities. The splitting of the peak results from the stark splitting. The intensity of peak emission increases up to 1 mol% Er2O3 and then decreases. This decreases due to the presence of the quenching process caused by non-radiative resonant cross-relaxation at the 4S3/2 level [12, 23, 36]. The elimination of red emissions is due to this change [25]. The peak is responsible for the blue emission in the 474–490 region assigned to the 4F3/2, 5/2 → 4I15/2 transition in another exciting energy at 380 nm [34, 37].
The color coordinates of the CIE chromaticity diagram use to check the variation in light color according to the emission spectra.
As shown in Fig. 9, the CIE chromaticity diagram could be affected in prepared glass mainly by two parameters, the change of Er concentration and the change of exciting wavelength. The values of chromaticity coordinate listed in Table 4 indicate the samples excited with λ = 489 nm, used in the emission of green laser [36]. And the emission ranged from white to green light when excited with high energy at 380 nm.
Pandey et al. [37] reported that white light forms from the combination of three colors: blue, green, and red. The sample contains 0.5 mol% close to the expected value of the white light coordinate (0.33, 0.33).
3.7 Thermal properties
DTA curve plays a vital role in the two-point explanation. It explains the glass structure through the Tg value, which is affected by the coordination number, the density of the crosslink, and the bond. strength [11, 38, 39]. On the other hand, it could explain thermal stability. DTA curve characterizes by an endothermic appearance related to annealing, softening, nucleation, and reversible phase transformations determined through Tg and Tm. The exothermic peak represents crystallization and phase change, as defined by the crystallization temperature [40, 41]. The obtained values tabulated in Table 3 show higher values of Tg compared to glass formed as bismuth boro-tellurite [42], TeO2-Ga2O3-GeO2 system [43], boron bismuth/fluorooxy glass [44], calcium bismuth silicate glass–ceramics [45], Ga-Ge-Sb–Se glass composition [46]. The Tg values increase with the increase of Er concentration as the increase in the compactness of the glass is formed by the presence of Bi in two coordination numbers, BiO3 and BiO6 [47], which agrees with the molar volume and FTIR result. Fig. 10 indicates two peaks of different intensities, representing the presence of two crystalline phases, and with the addition of Er2O3, the second peak disappears. It interprets as the formation of a single homogenous phase. The thermal stability characterizes by three parameters Tg, the onset of crystallization Tx, and ΔT = Tp-Tg. The higher Tg value could indicate glass has a higher damage threshold due to the transmitted high-power pump, and the higher thermal stability (ΔT is larger than 100 °C) [42, 48] indicate a wide range of working temperatures for the benefit of fiber drawing [49, 50].
Another parameter is the Hrupy parameter HR = (Tp-Tg)/(Tm-Tp) [38], which comes from a relationship that shows the glass's stability and anti-crystallization ability to form [51, 52].
Relatively such a high value of activation energy for the crystallization, resistance of nucleation and high thermal stability for glass samples under study indicates the glass promises for high-powered optoelectronic device applications [53].
The crystallization mechanism of glass is studied using the kinetic parameters obtained from DTA data. Fig. 11 shows the DTA curve for the glass samples at different rates at 5, 10, 20, and 30 °C/min. Fig. 11 shows that the peak of crystallization temperature becomes sharp and shifts to a higher temperature with the increase in the heating rate [54]. Figs. 12, 13.
The activation energy of crystallization can calculate using various approaches [52, 54,55,56]. The activation energy of crystallization glass samples under investigation is determined using the Augis and Bennett method.
where α is the heating rate, Tp is the crystallization temperature, R is the gas constant, and ko is a constant.
The relationship between ln (α /Tp) and 1000/Tp (Fig. 12) gives a straight line, and from the slope, the value of the activation energy is determined and tabulated in Table 3.
Ozawa equation used to calculate Avrami coefficient (r) according to [54]:
X is the crystallization fraction calculated using the equation X = (AT / A), and AT is the partial area at a given temperature. A is the total area of exothermic crystallization [57].
r value determines from the slope of the straight line of the relation between ln[-ln(1-x)] and lnα (Fig.13). The r values of the prepared glass samples are 1.9, 1.56, 1.56, 1.89, and 1.54. This value indicates that surface crystallization occurred during one-dimensional growth [54, 58].
3.8 XRD of heat-treated samples
Young [59] investigated the heat-treated glass sample (40Bi2O3-60GeO2 mol %) at 521 and 584 °C. The formation of two phases, Bi2GeO5 and Bi4Ge3O12, can be observed. They also formed glass samples with different concentrations of Bi2O3 and GeO2 containing B2O3 or Sm2O3 mol %, and by thermally treating them at 630 °C, the crystalline phase of the Bi4Ge3O12 cube formed.
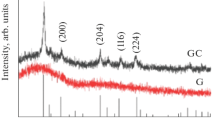
The current study comprises adding different amounts of Er2O3 mol % to a 40 Bi2O3-60 GeO2 mol % glass sample manufactured and heat-treated at 650 °C. The development of crystals causes bulk samples to become opaque. According to the XRD study, the only Bi4Ge3O12 phase developed is isolated tetrahedral GeO4, and distorted BiO6 units make up the BGO crystal structure [59, 60]. Fig. 14 shows the XRD patterns at room temperature for materials generated by heat treatments at 650 °C for 5 h. The Bi4Ge3O12 crystalline phase (ICDD: 01–074-6243) ascribed to the XRD peaks seen in samples free of Er3+ ions and containing Er2O3.
The size of particles computed using the Scherrer equation [9] was between 55 and 73 nm. Another approach considers all diffraction peaks, although the findings are relatively similar.
3.9 FTIR of heat-treated samples
FTIR measurements demonstrate any structural changes when the glass samples are converted to glass–ceramic using heat treatment (Fig. 15). As well as compare the results of heat treatment on the glass structure.
The range of 490–540 cm−1 indicates a significant quantity of crystallization (Bi4Ge3O12 phase). [60], which is consistent with the XRD results.
The band at 540 to 830 cm−1 becomes wide and more intense than observed in the glass samples, representing the overlapping structure of the BiO6 and GeO4 compositions.
Heat treatment separates the band created in glass samples ranging from 830 to 1100 cm−1 into two bands, broadening and intensifying it. An increase in BiO6 groups causes a sharp band at 870 cm−1. Bi-O-Bi bonds or the interaction between Bi-O-Ge [1] cause the band at 1110 cm−1.
The formation of symmetric vibrations of hydrogen bond (OH) groups is due to the addition of a modifier or change of the bismuth from the former to the modifier in the glass network, which breaks the bridging oxygen and leads to the formation of NBO atoms. The formation of NBO leads to give vacant oxygen atoms and these oxygen atoms will take part in the formation of OH groups. And the high concentration of OH groups in glass can increase the crack growth, impurities and trapping and leads to a decrease in the overall strength and luminous intensity of the glass. The OH content present in a glass can be evaluated using the following formula [61, 62]:
Here, \({\tau }_{o}\) is the maximum transmission, \({\tau }_{D}\) is the transmission at 3000 cm−1 and L is the thickness of the glass sample. The OH content can be calculated according to the following equation:
The estimated OH content for the glass samples under study is found between 94 to 102 ppm, which is relatively low when compared with the other glasses such as LBTAF (120 ppm), LHG-8 (773 ppm), GeS2 (175.5 ppm) and KBS (133 ppm) [63,64,65,66].
Hence present systems of glasses are quite suitable for visible photonic applications.
3.10 Excitation and emission of heat-treated sample
Due to the importance of Bi4Ge3O12 phase formation in glass ceramics, it uses in laser host crystal and scintillation detector applications [67, 68].
This phase could exhibit green emission [69]. Fig. 16 displays the excitation curve for the glass–ceramic sample doped with 2 mol % Er.
Figure 16 shows the same exciting peaks in the glass sample curve (Fig. 7) in addition to the band at 522 nm. The intense emission obtains by exciting the sample with the energy corresponding to the prominent excitation band. Furthermore, glass–ceramic excited at 380 nm and 522 nm emit green light, whereas glass–ceramic excited at 380 nm emit blue light as shown in Fig. 17A and 17B.
The CIE chromaticity program shows the emission color. The x–y color parameters calculate and included in Table 4. From Table 4, the emission difference is due to the difference in the emitted wavelengths. Excitation at 522 nm coordinates the green emission chromaticity. The apparent excitation at 380 nm coordinates the blue-green emission chromaticity. Figs. 18A and18B.
3.11 lifetime decay
The lifetime is an important factor for potential laser materials. The fluorescence decay curve of Er3+ ion excited at 380 nm and monitored at 522 nm at room temperature of the present samples was measured and is shown in Fig. 19. The decay curves have deviated from single exponential, the decay profile is found to be biexponential for all samples except the sample containing 1.5 mol% Er2O3 follow single exponential decay. The bi-exponential nature may be because of the reason that the energy transfer from excited donor ion to unexcited acceptor ion transpires. The intensity of luminescence can be articulated as follows [66]:
where, I0 is the intensity at t = 0, and I is intensity at time t, A1 and A2. are the amplitudes of decay constants and τ1 and τ2 are the luminescence lifetimes for fast and slow channels of decay, respectively. The average values of decay time are calculated by using the formula given as [66]:
The average lifetime values for Er3+ ion-doped glasses are inset in Table 4 and are shown in Fig. 18. It can be seen the average lifetime decreases with an increase in Er3+ ion concentration signifying the energy transfer between Er3+— Er3+ ions [71–73].
4 Conclusion
Bismuth germanium glass systems doped with Er3+ ions were studied using different techniques and concluded that:
-
1.
The pyramidal [BiO3] octahedral [BiO6] units and GeO4 tetrahedral units are the glass network of samples under study.
-
2.
The Ω2 and Ω4/Ω6 values were higher for the sample containing one mol% Er3+, which was attributed to the covalent nature and suggested it as a better glass for laser material applications.
-
3.
The XRD spectra show the Bi4Ge3O12 crystalline phase (50–73 nm size nano-crystals) participating in the glass samples.
-
4.
The values of the chromaticity coordinates refer to the samples excited at 489 nm used for green laser emission, while excited at 380 nm used for emission ranging from white light to green emission.
-
5.
The luminescence quenching of > 1 mol of Er3+ ion concentration occurs under both excitation in the prepared glass–ceramic samples due to the formation of a non-radiative cross-relaxation.
-
6.
Based on the structure study, OH content, thermal analysis, emission lifetime and CIE coordinates, it is concluded that the Er3 + ions doped Bismuth germanium glasses are suitable for green and blue optoelectronics device applications.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
P. Pascuta et al., The local structure of bismuth germanate glasses and glass ceramics doped with europium ions evidenced by FT-IR spectroscopy Vib. Spectrosc. 48, 281–284 (2008)
Q. Nie et al., Upconversion luminescence of Er3+/Yb3+-codoped natrium–germanium–bismuth glasses. J. Phys. Chem. Solids 67, 2345–2350 (2006)
S. Arunkumar, K. Marimuthu, Spectroscopic properties of Er3+ doped bismuth leadtelluroborate glasses for 1.53 μm optical amplifiers. J. Alloys Compd. 627, 54–68 (2015)
S. Polosan, Crystallisation of bismuth germanate glasses below their glass transition temperature. J. Non. Cryst. Solids 472, 55–60 (2017)
P. Beneventi et al., (1995) A Raman study of Bi4(GexSi1- x)3 O12 crystals Solid State Commun, 93, 143–146
Y. Chen et al., Spectroscopic properties of Er3+ ions in bismuth borate glasses Opt. Mater. (Amst) 25, 271–278 (2004)
S. Sanghi et al., Effect of Bi2O3 on spectroscopic and structural properties of Er3+ doped cadmium bismuth borate glasses spectrochim. Acta Part A Mol. Biomol. Spectrosc. 83, 94–99 (2011)
S. Polosan, F. Nastase, M. Secu, Structural changes during the crystallization of the Bi4Ge3O12 glasses. J. Non. Cryst. Solids 357, 1110–1113 (2011)
S. Polosan, A. Galca, M. Secu, Band-gap correlations in Bi4Ge3O12 amorphous and glass-ceramic materials. Solid State Sci. 13, 49–53 (2011)
X. He et al., Abnormal near-infrared luminescence property of bismuth doped calcium germanate glasses. J. Non. Cryst. Solids 402, 166–171 (2014)
M. Farouk et al., Optical absorption and structural studies of bismuth borate glasses containing Er3 + ions. J. Non. Cryst. Solids 371–372, 14–21 (2013)
J. Bhemarajam et al., Spectroscopic and optical investigations on Er3+/Yb3+co-doped bismuth–boroleadlithium glasses for solid state laser applications. Opt. Mater. 122, 111657 (2021)
R. Wang et al., Structure and luminescent property of Er3+- doped germanate glasses. J. Non. Cryst. Solids 383, 200–204 (2014)
M. Kumar et al., Luminescence and gain characteristics of 1.53 μm broadband of Er3+ in lead telluroborate glasses. J. Lumin. 142, 128–134 (2013)
A. Madhu et al., Er3+-ions doped lithium-bismuth-boro-phosphate glass for 1532 nm emission and efficient red emission up conversion for telecommunication and lasing applications. J. Non. Cryst. Solids 495, 35–46 (2018)
S. Nian et al., Optical properties of Er3+/Yb3+ co-doped bismuth calcium borate glass system for NIR lasers and fiber amplifiers. J. Lumin. 194, 440–445 (2018)
T. Wei et al., Structure and spectroscopic properties of Er3+ doped germanate glass for mid-infrared application. Solid state Sci. 31, 54–61 (2014)
A. Ghosh, R. Debnath, Judd-Ofelt analysis of Er+3 activated lead free fluoro–tellurite glass Opt. Mater. (Amst) 31, 604–608 (2009)
E. Mohamed et al., Crystallization kinetics and optical properties of titanium–lithium tetraborate glass containing europium oxide. Appl. Phys. A Mater. Sci. Process. 123, 479 (2017)
D. Ramteke, R. Kroon, H. Swart, Infrared emission spectroscopy and upconversion of ZnO-Li2O-Na2O-P2O5 glasses doped with Nd3 + ions. J. Non. Cryst. Solids 457, 157–163 (2017)
I. Khan et al., Photoluminescence and white light generation of Dy2O3 doped Li2O-BaO-Gd2O3-SiO2 for white light LED. J. Alloys Compd. 774, 244–254 (2019)
Sk. Mahamuda et al., Spectroscopic properties and luminescence behavior of Nd3+ doped zinc alumino bismuth borate glasses. J. Phys. Chem. Solids 74, 1308 (2013)
J. Bhemarajam et al., Spectroscopic studies on Er3+ ions incorporated bismuth borolead lithium glasses for solid state lasers and fiber amplifiers Opt. Mater. (Amst) 113, 110818 (2021)
N. Vijaya et al., Optical characterization of Er3+-doped zinc fluorophosphate glasses for optical temperature sensors. Sensors Actuators B Chem. 186, 156–164 (2013)
K. Annapoorani et al., Influence of Er3+ ion concentration on spectroscopic properties and luminescence behavior in Er3+ doped Strontium telluroborate glasses. J. Lumin. 171, 19–26 (2016)
P. Babu et al., Optical spectroscopy, 1.5 μm emission, and upconversion properties of Er3+-doped metaphosphate laser glasses. J. Optical Society of America B 24, 2218–2228 (2007)
S. Saleem et al., Erbium-doped fluoroborate glasses for near infrared broadband amplifiers Int. J. Appl. Glas. Sci. 2, 215–221 (2011)
B. Jamalaiah et al., Visible and near infrared luminescence properties of Er3+-doped LBTAF glasses for optical amplifiers. Opt. Mater. (Amst) 34, 861–867 (2012)
M. Mariyappan et al., Effect Bi2O3 on the physical, structural and radiation shielding properties of Er3+ ions doped bismuth sodiumfluoroborate glasses. J. Non. Cryst. Solids 499, 75–85 (2018)
I. Kashif, A. Ratep, Effect of copper oxide on structure and physical properties of lithium lead borate glasses Appl. Phys. A 120, 1427–1434 (2015)
I. Kashif, A. Ratep, Effect of copper addition on BO4, H2O groups and optical properties of lithium lead borate glass. Opt. Quantum Electron. 49, 231 (2017)
I. Kashif, A. Ratep, Red and green emission from chromium metal or oxide on co-doped lithium tetraborate glass. Opt. Quantum Electron. 48, 516 (2016)
D. Ramteke, R. Gedam, H. Swart, Physical and optical properties of lithium borosilicate glasses doped with Dy3+ ions Phys. B Condens. Matter 535, 194–197 (2017)
G. Chandrashekaraiah et al., Correlation between non-linear optical parameter and structure of Li2B4O7 glasses doped with Er3+ ions. J. Non. Cryst. Solids 531, 119843 (2020)
L. Mukhopadhyay et al., 980 nm excited Er3+/Yb3+/Li+/Ba2+: NaZnPO4 upconverting phosphors in optical thermometry. J. Lumin. 187, 368–377 (2017)
C. Kesavulu et al., Influence of Er3+ ion concentration on optical and photoluminescence properties of Er3+-doped gadolinium-calcium silica borate glasses. J. Alloys Compd. 683, 590–598 (2016)
A. Pandey et al., Enhanced upconversion and temperature sensing study of Er3+–Yb3+ codoped tungsten-tellurite glass. Sensors Actuators B Chem. 202, 1305–1312 (2014)
I. Kashif, A. Ratep, S. El-Mahy, Structural and optical properties of lithium tetraborate glasses containing chromium and neodymium oxide Mater. Res. Bull. 89, 273–279 (2017)
A. Fayad et al., Influence of CuO on crystallization and electrical properties of B2O3-Bi2O3-GeO2-CaF2 glass system for thermoelectronic applications. J. Non. Cryst. Solids 544, 120185 (2020)
S. Abdel-Hameed, Y. Hamdy, H. Sadek, Characterization and Luminescence Properties of Mn-Doped Zinc Borosilicate Glasses and Glass-Ceramics Silicon. 11, 1185-1192 (2019)
X. Song et al., Broadband ~1.8 µm emission characteristics of Tm3+-doped bismuth germanate glass based on Ga2O3 modification. J. Non. Cryst. Solids 557, 120575 (2021)
N. Elkhoshkhany et al., Optical properties and crystallization of bismuth boro-tellurite glasses. J. Non. Cryst. Solids 476, 15–24 (2017)
A. Marczewska, M. Środa, Spectroscopic and thermal study of a new glass from TeO2–Ga2O3–GeO2 system. J. Mol. Struct. 1164, 100–108 (2018)
S. Li et al., Study on preparation and properties of boron bismuth/fluorooxy glass and glass-ceramics. J. Non. Cryst. Solids 561, 120749 (2021)
R. Guntu et al., Structure, and opto-dielectric investigations of Cu2+-doped calcium bismuth silicate glass ceramics. Opt. Mater. (Amst) 113, 110876 (2021)
E. Karaksina et al., Effect of Ge-rich Ga-Ge-Sb-Se glass composition on the optical and thermal properties Opt. Mater. (Amst) 104, 109943 (2020)
S. Abdel-Hameed, A. Fathi, M. Eltohamy, Structure, optical and electrical behaviour of x(2Bi2O3.MnO). (10–x)B2O3 glasses. J. Non. Cryst. Solids 510, 71–80 (2019)
M. Peng et al., Bismuth-doped zinc aluminosilicate glasses and glass-ceramics with ultra-broadband infrared luminescence. Opt. Mater. (Amst) 29, 556–561 (2007)
H. Sun et al., Effect of chloride ions’ introduction on structural, thermal stability, and spectroscopic properties in Yb3+Er3+-codoped germanate-bismuth-lead glasses. J. Opt. Soc. Am. B 22, 2601 (2005)
G. Zhao et al., Broadband near-IR emission and temperature dependence in Er/Tm co-doped Bi2O3-SiO2-Ga2O3 glasses. Phys. B Condens. Matter 407, 4622–4626 (2012)
M. Mhareb et al., Effect of Dy2O3 impurities on the physical, optical and thermoluminescence properties of lithium borate glass. J. Lumin. 177, 366–372 (2016)
M. Abdel-Rahim et al., Kinetic study of non-isothermal crystallization of BixSe100- x chalcogenide glasses. Phys. B Condens. Matter 403, 2956–2962 (2008)
A.S. Ravia, Rao, Effective sensitization of Eu3+ visible red emission by Sm3+ in thermally stable potassium zinc alumino borosilicate glasses for photonic device applications. J. Lumin. 244, 118689 (2022)
S. Panyata et al., Non-isothermal crystallization kinetics of bismuth germanate glass-ceramics Ceram. Int. 43, S407–S411 (2017)
K. Matusita, T. Komatsu, R. Yokota, Kinetics of non-isothermal crystallization process and activation energy for crystal growth in amorphous materials. J. Mater. Sci. 19, 291–296 (1984)
J. Augis, J. Bennett, Calculation of the Avrami parameters for heterogeneous solid state reactions using a modification of the Kissinger method. J. Therm. Anal. 13, 283–292 (1978)
I. Kashif, A. Soliman, A. Sanad, Effect of grain size on the crystallization kinetics of copper vanadate glasses Physics and Chemistry of Glasses. 39, 195-199 (1998)
N. Afify et al., Kinetics study of non-isothermal crystallization in Se0.7-Ge0.2 Sb0.1 chalcogenide glass. J. Non. Cryst. Solids 128, 269–278 (1991)
C. Yang et al., Nano-crystallization and highly oriented crystal line patterning of Sm3+-doped Bi2GeO5 and Bi4Ge3O12 in bismuth germanate-based glasses. J. Non. Cryst. Solids 459, 116–122 (2017)
S. Rojas et al., Influence of ceria addition on thermal properties and local structure of bismuth germanate glasses. J. Non. Cryst. Solids 356, 2942–2946 (2010)
J. Zhang et al., 2.8 μm emission and OH quenching analysis in Ho3+ doped fuorotellurite-germanate glasses sensitized byYb3+ and Er3+. Sci. Rep. 7, 16794 (2017)
X. Fan et al., Spectroscopic properties of 2.7μm emission in Er3+ doped telluride glasses and fibers. J. Alloys Compd. 615, 475–481 (2014)
T. Suhasini et al., An investigation on visible fluorescence characteristics of Ho3+ activated LBTAF glasses. Phys. B 407, 523–527 (2012)
T. Som, B. Karmakar, Nephelauxetic effect of low phonon antimony oxide glass in absorption and photoluminescence of rare-earth ions, Spectrochim. Acta - Part A Mol. Biomol. Spectrosc. 79, 1766–1782 (2011)
B.B. Kale et al., Removal of OH impurities from GeS2 by reactive atmosphere and its glass preparation. Mater. Chem. Phys. 78, 330–336 (2003). https://doi.org/10.1016/S0254-0584(01)00551-X
P.R. Rani et al., Structural, absorption and photoluminescence studies of Sm3+ ions doped barium lead alumino fluoro borate glasses for optoelectronic device applications. Mat. Res. Bull. 110, 159–168 (2019)
S. Kaczmarek et al., Temperature dependence of Bi4Ge3O12 photoluminescence spectra mater. Sci. Pol. 32, 7–11 (2014)
L. Dimesso et al., The crystallization behaviour of bismuth germanate glasses. J. Mater. Sci. 26, 4215–4219 (1991)
F. Rooemond et al., Crystals under laser excitation : excited state. J. Lumin. 33, 455–486 (1985)
S. Kaur et al., Spectroscopic studies of Dy3+ doped borate glasses for cool white light generation. Mat. Res. Bull. 104, 77–82 (2019)
Acknowledgements
I.Kashif thanks Prof. Dr. A. M. Sanad for his support throughout my scientific career during his stay with us.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). No.
Author information
Authors and Affiliations
Contributions
All authors have seen and approved the final version of the manuscript being submitted, Equal share: A.R: Investigation, Writing—original draft, Methodology, Formal analysis. I.K: Writing—review and editing, administration, Formal analysis, Investigation.
Corresponding author
Ethics declarations
Conflict of interest
No conflict of interest.
Ethical approval
This paper meets the ethical standards of this journal.
Consent to participate
All authors agree with the review of this paper in this journal.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kashif, I., Ratep, A. Luminescence in Er3+ co-doped bismuth germinate glass–ceramics for blue and green emitting applications. J. Korean Ceram. Soc. 60, 511–526 (2023). https://doi.org/10.1007/s43207-022-00281-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43207-022-00281-2