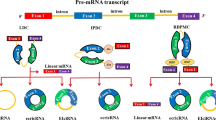
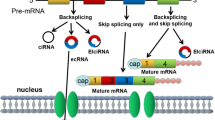
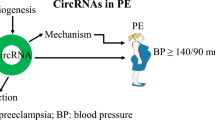
Abstract
Circular RNAs (circRNAs), produced by precursor mRNAs, are a type of covalently closed circular molecule without 5′ caps and 3′ polyadenylated tails. Recently, advances in high-throughput sequencing, transcriptomics and bioinformatics, have revealed that circRNAs with specific traits in tissue or cells play emerging roles in both physiological and panthological contexts instead of as simple by-products of transcription. However, bringing circRNAs to the forefront of clinical practice is still a long way off. In this review, we highlight the progress in the formation and function of circRNAs, and how circRNAs work in female reproductive–related diseases, such as recurrent spontaneous abortion, preeclampsia, and endometriosis. We also discussed the clinical potential of circRNAs as biomarkers, and therapeutic agents in female reproductive diseases as well as research controversies, technical issues, and biological knowledge gaps that need to be addressed. This review may instruct future basic research and clinical applications on circRNAs, especially in female reproduction.





Similar content being viewed by others
Data Availability
Not applicable.
Code Availability
Not applicable.
References
Dou C, Cao Z, Yang B, Ding N, Hou TY, Luo F, et al. Changing expression profiles of lncRNAs, mRNAs, circRNAs and miRNAs during osteoclastogenesis. Sci Rep-Uk. 2016;6, ARTN 21499. https://doi.org/10.1038/srep21499.
Wu P, Mo YZ, Peng M, Tang T, Zhong Y, Deng XY, et al. Emerging role of tumor-related functional peptides encoded by lncRNA and circRNA. Mol Cancer. 2020;19(1), ARTN 22. https://doi.org/10.1186/s12943-020-1147-3.
Cech TR, Steitz JA. The noncoding RNA revolution-trashing old rules to forge new ones. Cell. 2014;157(1):77–94. https://doi.org/10.1016/j.cell.2014.03.008.
Kolakofsky D. Isolation and characterization of Sendai virus DI-RNAs. Cell. 1976;8(4):547–55. https://doi.org/10.1016/0092-8674(76)90223-3.
Sanger HL, Klotz G, Riesner D, Gross HJ, Kleinschmidt AK. Viroids are single-stranded covalently closed circular Rna molecules existing as highly base-paired rod-like structures. P Natl Acad Sci USA. 1976;73(11):3852–6. https://doi.org/10.1073/pnas.73.11.3852.
Arnberg AC, Vanommen GJB, Grivell LA, Vanbruggen EFJ, Borst P. Some yeast mitochondrial Rnas are circular. Cell. 1980;19(2):313–9. https://doi.org/10.1016/0092-8674(80)90505-X.
Hsu MT, Cocaprados M. Electron-microscopic evidence for the circular form of RNA in the cytoplasm of eukaryotic cells. Nature. 1979;280(5720):339–40. https://doi.org/10.1038/280339a0.
Matsumoto Y, Fishel R, Wickner RB. Circular Single-stranded Rna replicon in Saccharomyces-Cerevisiae. P Natl Acad Sci USA. 1990;87(19):7628–32. https://doi.org/10.1073/pnas.87.19.7628.
Chen LL. The biogenesis and emerging roles of circular RNAs. Nat Rev Mol Cell Bio. 2016;17(4):205–11. https://doi.org/10.1038/nrm.2015.32.
Li X, Yang L, Chen LL. The Biogenesis, functions, and challenges of circular RNAs. Mol Cell. 2018;71(3):428–42. https://doi.org/10.1016/j.molcel.2018.06.034.
Cocquerelle C, Mascrez B, Hetuin D, Bailleul B. Mis-splicing yields circular RNA molecules. FASEB J. 1993;7(1):155–60. https://doi.org/10.1096/fasebj.7.1.7678559.
Jeck WR, Sorrentino JA, Wang K, Slevin MK, Burd CE, Liu JZ, et al. Circular RNAs are abundant, conserved, and associated with ALU repeats. RNA. 2013;19(3):141–57.
Rybak-Wolf A, Stottmeister C, Glazar P, Jens M, Pino N, Giusti S, et al. Circular RNAs in the mammalian brain are highly abundant, conserved, and dynamically expressed. Mol Cell. 2015;58(5):870–85. https://doi.org/10.1016/j.molcel.2015.03.027.
Ivanov A, Memczak S, Wyler E, Torti F, Porath HT, Orejuela MR, et al. Analysis of intron sequences reveals hallmarks of circular RNA biogenesis in animals. Cell Rep. 2015;10(2):170–7. https://doi.org/10.1016/j.celrep.2014.12.019.
Memczak S, Jens M, Elefsinioti A, Torti F, Krueger J, Rybak A, et al. Circular RNAs are a large class of animal RNAs with regulatory potency. Nature. 2013;495(7441):333–8. https://doi.org/10.1038/nature11928.
Ji P, Wu W, Chen S, Zheng Y, Zhou L, Zhang J, et al. Expanded expression landscape and prioritization of circular RNAs in mammals. Cell Rep. 2019;26(12):3444-60e5. https://doi.org/10.1016/j.celrep.2019.02.078.
Dong R, Ma XK, Li GW, Yang L. CIRCpedia v2: An updated database for comprehensive circular RNA annotation and expression comparison. Genom Proteom Bioinf. 2018;16(4):226–33. https://doi.org/10.1016/j.gpb.2018.08.001.
Dong R, Ma XK, Chen LL, Yang L. Increased complexity of circRNA expression during species evolution. RNA Biol. 2017;14(8):1064–74. https://doi.org/10.1080/15476286.2016.1269999.
Han B, Chao J, Yao H. Circular RNA and its mechanisms in disease: From the bench to the clinic. Pharmacol Ther. 2018;187:31–44. https://doi.org/10.1016/j.pharmthera.2018.01.010.
Li Y, Zheng QP, Bao CY, Li SY, Guo WJ, Zhao J, et al. Circular RNA is enriched and stable in exosomes: a promising biomarker for cancer diagnosis. Cell Res. 2015;25(8):981–4. https://doi.org/10.1038/cr.2015.82.
Seal RL, Chen LL, Griffiths-Jones S, Lowe TM, Mathews MB, O’Reilly D, et al. A guide to naming human non-coding RNA genes. Embo J. 2020;39(6), ARTN e10377 15252/embj.2019103777.
Zhang Y, Zhang XO, Chen T, Xiang JF, Yin QF, Xing YH, et al. Circular Intronic Long Noncoding RNAs. Mol Cell. 2013;51(6):792–806. https://doi.org/10.1016/j.molcel.2013.08.017.
Holdt LM, Kohlmaier A, Teupser D. Molecular roles and function of circular RNAs in eukaryotic cells. Cell Mol Life Sci. 2018;75(6):1071–98. https://doi.org/10.1007/s00018-017-2688-5.
Ashwal-Fluss R, Meyer M, Pamudurti NR, Ivanov A, Bartok O, Hanan M, et al. circRNA biogenesis competes with pre-mRNA splicing. Mol Cell. 2014;56(1):55–66. https://doi.org/10.1016/j.molcel.2014.08.019.
Zhang XO, Dong R, Zhang Y, Zhang JL, Luo Z, Zhang J, et al. Diverse alternative back-splicing and alternative splicing landscape of circular RNAs. Genome Res. 2016;26(9):1277–87. https://doi.org/10.1101/gr.202895.115.
Starke S, Jost I, Rossbach O, Schneider T, Schreiner S, Hung LH, et al. Exon Circularization Requires Canonical Splice Signals. Cell Rep. 2015;10(1):103–11. https://doi.org/10.1016/j.celrep.2014.12.002.
Zhang Y, Xue W, Li X, Zhang J, Chen SY, Zhang JL, et al. The Biogenesis of Nascent Circular RNAs. Cell Rep. 2016;15(3):611–24. https://doi.org/10.1016/j.celrep.2016.03.058.
Zhang XO, Wang HB, Zhang Y, Lu XH, Chen LL, Yang L. Complementary Sequence-mediated exon circularization. Cell. 2014;159(1):134–47. https://doi.org/10.1016/j.cell.2014.09.001.
Daniel C, Behm M, Ohman M. The role of Alu elements in the cis-regulation of RNA processing. Cell Mol Life Sci. 2015;72(21):4063–76. https://doi.org/10.1007/s00018-015-1990-3.
Kristensen LS, Andersen MS, Stagsted LVW, Ebbesen KK, Hansen TB, Kjems J. The biogenesis, biology and characterization of circular RNAs. Nat Rev Genet. 2019;20(11):675–91. https://doi.org/10.1038/s41576-019-0158-7.
Wang Y, Wang ZF. Efficient backsplicing produces translatable circular mRNAs. RNA. 2015;21(2):172–9. https://doi.org/10.1261/rna.048272.114.
Kelly S, Greenman C, Cook PR, Papantonis A. Exon skipping is correlated with exon circularization. J Mol Biol. 2015;427(15):2414–7. https://doi.org/10.1016/j.jmb.2015.02.018.
Koh W, Gonzalez V, Natarajan S, Carter R, Brown PO, Gawad C. Dynamic ASXL1 Exon skipping and alternative circular splicing in single human cells. PloS ONE. 2016;11(10), ARTN e0164085. https://doi.org/10.1371/journal.pone.0164085.
Suzuki H, Aoki Y, Kameyama T, Saito T, Masuda S, Tanihata J, et al. Endogenous multiple exon skipping and back-splicing at the DMD Mutation Hotspot. Int J Mol Sci. 2016;17(10),ARTN 1722. https://doi.org/10.3390/ijms17101722.
Conn SJ, Pillman KA, Toubia J, Conn VM, Salmanidis M, Phillips CA, et al. The RNA Binding Protein Quaking Regulates Formation of circRNAs. Cell. 2015;160(6):1125–34. https://doi.org/10.1016/j.cell.2015.02.014.
Aktas T, Ilik IA, Maticzka D, Bhardwaj V, Rodrigues CP, Mittler G, et al. DHX9 suppresses RNA processing defects originating from the Alu invasion of the human genome. Nature. 2017;544(7648):115-+. https://doi.org/10.1038/nature21715.
Li X, Liu CX, Xue W, Zhang Y, Jiang S, Yin QF, et al. Coordinated circRNA Biogenesis and Function with NF90/NF110 in Viral Infection. Mol Cell. 2017;67(2):214-27 e7. https://doi.org/10.1016/j.molcel.2017.05.023.
Hong XH, Liu N, Liang YL, He QM, Yang XJ, Lei Y, et al. Circular RNA CRIM1 functions as a ceRNA to promote nasopharyngeal carcinoma metastasis and docetaxel chemoresistance through upregulating FOXQ1. Mol Cancer. 2020;19(1), ARTN 33. https://doi.org/10.1186/s12943-020-01149-x.
Ma NN, Pan J, Ye XY, Yu B, Zhang W, Wan J. Whole-transcriptome analysis of APP/PS1 Mouse Brain and Identification of circRNA-miRNA-mRNA Networks to Investigate AD Pathogenesis. Mol Ther-Nucl Acids. 2019;18:1049–62. https://doi.org/10.1016/j.omtn.2019.10.030.
Zhang F, Zhang RY, Zhang XY, Wu YN, Li XY, Zhang S, et al. Comprehensive analysis of circRNA expression pattern and circRNA-miRNA-mRNA network in the pathogenesis of atnerosclerosis in rabbits. Aging-Us. 2018;10(9):2266–83. https://doi.org/10.18632/aging.101541.
Chen XY, Mao R, Su WM, Yang X, Geng QQ, Guo CF, et al. Circular RNA circHIPK3 modulates autophagy via MIR124-3p-STAT3-PRKAA/AMPK alpha signaling in STK11 mutant lung cancer. Autophagy. 2020;16(4):659–71. https://doi.org/10.1080/15548627.2019.1634945.
Lu Q, Liu TY, Feng HJ, Yang R, Zhao XZ, Chen W, et al. Circular RNA circSLC8A1 acts as a sponge of miR-130b/miR-494 in suppressing bladder cancer progression via regulating PTEN. Mol Cancer. 2019;18, ARTN 111. https://doi.org/10.1186/s12943-019-1040-0.
Huang XX, Li Z, Zhang Q, Wang WZ, Li BW, Wang L, et al. Circular RNA AKT3 upregulates PIK3R1 to enhance cisplatin resistance in gastric cancer via miR-198 suppression. Mol Cancer. 2019;18, ARTN 71. https://doi.org/10.1186/s12943-019-0969-3.
Cheng ZA, Yu CT, Cui SH, Wang H, Jin HJ, Wang C, et al. circTP63 functions as a ceRNA to promote lung squamous cell carcinoma progression by upregulating FOXM1. Nat Commun. 2019;10, ARTN 3200. https://doi.org/10.1038/s41467-019-11162-4.
Tang HL, Huang XJ, Wang J, Yang L, Kong YA, Gao GF, et al. circKIF4A acts as a prognostic factor and mediator to regulate the progression of triple-negative breast cancer. Mol Cancer. 2019;18, ARTN 23. https://doi.org/10.1186/s12943-019-0946-x.
Zhu ZL, Rong ZY, Luo Z, Yu ZL, Zhang J, Qiu ZJ, et al. Circular RNA circNHSL1 promotes gastric cancer progression through the miR-1306–3p/SIX1/vimentin axis. Mol Cancer. 2019;18(1), ARTN 126. https://doi.org/10.1186/s12943-019-1054-7.
Zhang PF, Wei CY, Huang XY, Peng R, Yang X, Lu JC, et al. Circular RNA circTRIM33–12 acts as the sponge of MicroRNA-191 to suppress hepatocellular carcinoma progression. Mol Cancer. 2019;18, ARTN 105. https://doi.org/10.1186/s12943-019-1031-1.
Li ZY, Huang C, Bao C, Chen L, Lin M, Wang XL, et al. Exon-intron circular RNAs regulate transcription in the nucleus. Nat Struct Mol Biol. 2015;22(3):256–64. https://doi.org/10.1038/nsmb.2959.
Conn VM, Hugouvieux V, Nayak A, Conos SA, Capovilla G, Cildir G, et al. A circRNA from SEPALLATA3 regulates splicing of its cognate mRNA through R-loop formation. Nat Plants. 2017;3(5), ARTN 17053. https://doi.org/10.1038/nplants.2017.53.
Yang Q, Du WW, Wu N, Yang W, Awan FM, Fang L, et al. A circular RNA promotes tumorigenesis by inducing c-myc nuclear translocation. Cell Death Differ. 2017;24(9):1609–20. https://doi.org/10.1038/cdd.2017.86.
Yang ZG, Awan FM, Du WW, Zeng Y, Lyu JJ, Wu D, et al. The Circular RNA Interacts with STAT3, Increasing Its nuclear translocation and wound repair by modulating Dnmt3a and miR-17 function. Mol Ther. 2017;25(9):2062–74. https://doi.org/10.1016/j.ymthe.2017.05.022.
Song C, Zhang Y, Huang W, Shi J, Huang Q, Jiang M, et al. Circular RNA Cwc27 contributes to Alzheimer’s disease pathogenesis by repressing Pur-alpha activity. Cell Death Differ. 2022;29(2):393–406. https://doi.org/10.1038/s41418-021-00865-1.
Wang LY, Long HY, Zheng QH, Bo XT, Xiao XH, Li B. Circular RNA circRHOT1 promotes hepatocellular carcinoma progression by initiation of NR2F6 expression. Mol Cancer. 2019;18, ARTN 119. https://doi.org/10.1186/s12943-019-1046-7.
Zhu YJ, Zheng B, Luo GJ, Ma XK, Lu XY, Lin XM, et al. Circular RNAs negatively regulate cancer stem cells by physically binding FMRP against CCAR1 complex in hepatocellular carcinoma. Theranostics. 2019;9(12):3526–40. https://doi.org/10.7150/thno.32796.
Fang L, Du WW, Awan FM, Dong J, Yang BB. The circular RNA circ-Ccnb1 dissociates Ccnb1/Cdk1 complex suppressing cell invasion and tumorigenesis. Cancer Lett. 2019;459:216–26. https://doi.org/10.1016/j.canlet.2019.05.036.
Legnini I, Di Timoteo G, Rossi F, Morlando M, Briganti F, Sthandier O, et al. Circ-ZNF609 is a circular RNA that can be translated and functions in myogenesis. Mol Cell. 2017;66(1):22–37, https://doi.org/10.1016/j.molcel.2017.02.017.
Matsumoto A, Nakayama KI. Hidden peptides encoded by putative noncoding RNAs. Cell Struct Funct. 2018;43(1):75–83. https://doi.org/10.1247/csf.18005.
Lu Y, Li Z, Lin C, Zhang J, Shen Z. Translation role of circRNAs in cancers. J Clin Lab Anal. 2021;35(7):e23866. https://doi.org/10.1002/jcla.23866.
Liang WC, Wong CW, Liang PP, Shi M, Cao Y, Rao ST, et al. Translation of the circular RNA circbeta-catenin promotes liver cancer cell growth through activation of the Wnt pathway. Genome Biol. 2019;20(1):84. https://doi.org/10.1186/s13059-019-1685-4.
Zhang ML, Zhao K, Xu XP, Yang YB, Yan S, Wei P, et al. A peptide encoded by circular form of LINC-PINT suppresses oncogenic transcriptional elongation in glioblastoma. Nat Commun. 2018:9, ARTN 4475. https://doi.org/10.1038/s41467-018-06862-2.
Zhao J, Li Y, Wang C, Zhang HT, Zhang H, Jiang B, et al. IRESbase: A Comprehensive database of experimentally validated internal ribosome entry sites. Genom Proteom Bioinf. 2020;18(2):129–39. https://doi.org/10.1016/j.gpb.2020.03.001.
Yang Y, Wang Z. IRES-mediated cap-independent translation, a path leading to hidden proteome. J Mol Cell Biol. 2019;11(10):911–9. https://doi.org/10.1093/jmcb/mjz091.
Yang Y, Fan X, Mao M, Song X, Wu P, Zhang Y, et al. Extensive translation of circular RNAs driven by N(6)-methyladenosine. Cell Res. 2017;27(5):626–41. https://doi.org/10.1038/cr.2017.31.
Zhao J, Lee EE, Kim J, Yang R, Chamseddin B, Ni C, et al. Transforming activity of an oncoprotein-encoding circular RNA from human papillomavirus. Nat Commun. 2019;10(1):2300. https://doi.org/10.1038/s41467-019-10246-5.
Ali MM, Li F, Zhang ZQ, Zhang KX, Kang DK, Ankrum JA, et al. Rolling circle amplification: a versatile tool for chemical biology, materials science and medicine. Chem Soc Rev. 2014;43(10):3324–41. https://doi.org/10.1039/c3cs60439j.
Abe N, Matsumoto K, Nishihara M, Nakano Y, Shibata A, Maruyama H, et al. Rolling circle translation of circular RNA in living human cells. Sci Rep-Uk. 2015;5, ARTN 16435. https://doi.org/10.1038/srep16435.
Li J, Zhang G, Liu CG, Xiang X, Le MTN, Sethi G, et al. The potential role of exosomal circRNAs in the tumor microenvironment: insights into cancer diagnosis and therapy. Theranostics. 2022;12(1):87–104. https://doi.org/10.7150/thno.64096.
Practice Committee of the American Society for Reproductive Medicine. Definitions of infertility and recurrent pregnancy loss: a committee opinion. Fertil Steril. 2020;113(3):533–5. https://doi.org/10.1016/j.fertnstert.2019.11.025.
Rai R, Regan L. Recurrent miscarriage. Lancet. 2006;368(9535):601–11. https://doi.org/10.1016/S0140-6736(06)69204-0.
Sugiura-Ogasawara M, Ozaki Y, Suzumori N. Management of recurrent miscarriage. J Obstet Gynaecol Res. 2014;40(5):1174–9. https://doi.org/10.1111/jog.12388.
Fan X, Zhang X, Wu X, Guo H, Hu Y, Tang F, et al. Single-cell RNA-seq transcriptome analysis of linear and circular RNAs in mouse preimplantation embryos. Genome Biol. 2015;16:148. https://doi.org/10.1186/s13059-015-0706-1.
Li SQ, Li X, Xue W, Zhang L, Yang LZ, Cao SM, et al. Screening for functional circular RNAs using the CRISPR-Cas13 system. Nat Methods. 2021;18(1):51–59, https://doi.org/10.1038/s41592-020-01011-4.
Qian YT, Wang X, Ruan HJ, Rui C, Mao PY, Cheng Q, et al. Circular RNAs expressed in chorionic villi are probably involved in the occurrence of recurrent spontaneous abortion. Biomed Pharmacother. 2017;88:1154–62. https://doi.org/10.1016/j.biopha.2017.01.172.
Li C, Chen X, Liu X, Liu X, He J, Ding Y, et al. CircRNA expression profiles in decidual tissue of patients with early recurrent miscarriage. Genes Dis. 2020;7(3):414–23. https://doi.org/10.1016/j.gendis.2019.06.003.
Arck PC, Hecher K. Fetomaternal immune cross-talk and its consequences for maternal and offspring’s health. Nat Med. 2013;19(5):548–56. https://doi.org/10.1038/nm.3160.
Zhou W, Wang H, Yang J, Long W, Zhang B, Liu J, et al. Down-regulated circPAPPA suppresses the proliferation and invasion of trophoblast cells via the miR-384/STAT3 pathway. Biosci Rep. 2019;39(9). https://doi.org/10.1042/BSR20191965.
Li ZF, Zhou GJ, Tao FB, Cao YX, Han WH, Li Q. circ-ZUFSP regulates trophoblasts migration and invasion through sponging miR-203 to regulate STOX1 expression. Biochem Bioph Res Co. 2020;531(4):472–9. https://doi.org/10.1016/j.bbrc.2020.06.117.
Zhu LH, Shi LJ, Ye WF, Li SP, Liu XM, Zhu ZH. Circular RNA PUM1 (CircPUM1) attenuates trophoblast cell dysfunction and inflammation in recurrent spontaneous abortion via the MicroRNA-30a-5p (miR-30a-5p)/JUNB axis. Bioengineered. 2021;12(1):6878–90. https://doi.org/10.1080/21655979.2021.1973207.
Tang M, Bai L, Wan Z, Wan S, Xiang Y, Qian Y, et al. circRNA-DURSA regulates trophoblast apoptosis via miR-760-HIST1H2BE axis in unexplained recurrent spontaneous abortion. Mol Ther Nucleic Acids. 2021;26:1433–45. https://doi.org/10.1016/j.omtn.2021.06.012.
Berkane N, Liere P, Oudinet JP, Hertig A, Lefevre G, Pluchino N, et al. From Pregnancy to Preeclampsia: A Key Role for Estrogens. Endocr Rev. 2017;38(2):123–44. https://doi.org/10.1210/er.2016-1065.
Okoth K, Chandan JS, Marshall T, Thangaratinam S, Thomas GN, Nirantharakumar K, et al. Association between the reproductive health of young women and cardiovascular disease in later life: umbrella review. BMJ-Brit Med J. 2020;371, ARTN m3502. https://doi.org/10.1136/bmj.m3502.
Raymond D, Peterson E. A critical review of early-onset and late-onset preeclampsia. Obstet Gynecol Surv. 2011;66(8):497–506. https://doi.org/10.1097/OGX.0b013e3182331028.
Raguema N, Moustadraf S, Bertagnolli M. Immune and apoptosis mechanisms regulating placental development and vascularization in preeclampsia. Front Physiol. 2020;11:98. https://doi.org/10.3389/fphys.2020.00098.
Sun N, Qin S, Zhang L, Liu S. Roles of noncoding RNAs in preeclampsia. Reprod Biol Endocrinol. 2021;19(1):100. https://doi.org/10.1186/s12958-021-00783-4.
Gong S, Gaccioli F, Dopierala J, Sovio U, Cook E, Volders PJ, et al. The RNA landscape of the human placenta in health and disease. Nat Commun. 2021;12(1):2639. https://doi.org/10.1038/s41467-021-22695-y.
Chen D, He B, Zheng P, Wang S, Zhao X, Liu J, et al. Identification of mRNA-, circRNA- and lncRNA- Associated ceRNA Networks and Potential Biomarkers for Preeclampsia From Umbilical Vein Endothelial Cells. Front Mol Biosci. 2021;8:652250. https://doi.org/10.3389/fmolb.2021.652250.
Cao M, Wen J, Bu C, Li C, Lin Y, Zhang H, et al. Differential circular RNA expression profiles in umbilical cord blood exosomes from preeclampsia patients. BMC Pregnancy Childb. 2021;21(1):303. https://doi.org/10.1186/s12884-021-03777-7.
Shen XY, Zheng LL, Huang J, Kong HF, Chang YJ, Wang F, et al. CircTRNC18 inhibits trophoblast cell migration and epithelial-mesenchymal transition by regulating miR-762/Grhl2 pathway in pre-eclampsia. RNA Biol. 2019;16(11):1565–73. https://doi.org/10.1080/15476286.2019.1644591.
Qi TT, Zhang D, Shi XT, Li MH, Xu HB. Decreased circUBAP2 Expression is associated with preeclampsia by limiting trophoblast cell proliferation and migration. Reprod Sci. 2021;28(8):2237–45. https://doi.org/10.1007/s43032-020-00450-w.
Liu SW, Xie X, Lei HJ, Zou BY, Xie L. Identification of key circRNAs/lncRNAs/miRNAs/mRNAs and pathways in preeclampsia using bioinformatics analysis. Med Sci Monitor. 2019;25:1679–93. https://doi.org/10.12659/Msm.912801.
Malik A, Pal R, Gupta SK. Interdependence of JAK-STAT and MAPK signaling pathways during EGF-mediated HTR-8/SVneo cell invasion. PLoS ONE. 2017;12(5), ARTN e0178269. https://doi.org/10.1371/journal.pone.0178269.
Yin NL, Zhang H, Luo X, Ding YB, Xiao XQ, Liu XR, et al. IL-27 Activates human trophoblasts to express IP-10 and IL-6: implications in the immunopathophysiology of preeclampsia. Mediat Inflamm. 2014;Artn 926875. https://doi.org/10.1155/2014/926875.
Zhang Y, Yang H, Long Y, Zhang Y, Chen R, Shi J, et al. circRNA N6-methyladenosine methylation in preeclampsia and the potential role of N6-methyladenosine-modified circPAPPA2 in trophoblast invasion. Sci Rep. 2021;11(1):24357. https://doi.org/10.1038/s41598-021-03662-5.
Zondervan KT, Becker CM, Missmer SA. Endometriosis. New Engl J Med. 2020;382(13):1244–56. https://doi.org/10.1056/NEJMra1810764.
Taylor HS, Kotlyar AM, Flores VA. Endometriosis is a chronic systemic disease: clinical challenge and novel innovations. Lancet. 2021;397(10276):839–52.
Wang WT, Sun YM, Huang W, He B, Zhao YN, Chen YQ. Genome-wide long non-coding RNA analysis identified circulating LncRNAs as novel non-invasive diagnostic biomarkers for gynecological disease. Sci Rep-Uk. 2016;6, ARTN 23343. https://doi.org/10.1038/srep23343.
Cui D, Ma JY, Liu Y, Lin KQ, Jiang XX, Qu Y, et al. Analysis of long non-coding RNA expression profiles using RNA sequencing in ovarian endometriosis. Gene. 2018;673:140–8. https://doi.org/10.1016/j.gene.2018.06.046.
Huang H, Zhu ZY, Song Y. Downregulation of lncrna uca1 as a diagnostic and prognostic biomarker for ovarian endometriosis. Rev Assoc Med Bras. 2019;65(3):336–41. https://doi.org/10.1590/1806-9282.65.3.336.
Xu XX, Jia SZ, Dai Y, Zhang JJ, Li XY, Shi JH, et al. Identification of circular RNAs as a novel biomarker for ovarian endometriosis. Chinese Med J-Peking. 2018;131(5):559–66. https://doi.org/10.4103/0366-6999.226070.
Xu XX, Jia SZ, Dai Y, Zhang JJ, Li XY, Shi JH, et al. The relationship of circular RNAs with ovarian endometriosis. Reprod Sci. 2018;25(8):1292–300. https://doi.org/10.1177/1933719118759439.
Zhang MM, Wang SX, Tang L, Wang X, Zhang TT, Xia XM, et al. Downregulated circular RNA hsa_circ_0067301 regulates epithelial-mesenchymal transition in endometriosis via the miR-141/Notch signaling pathway. Biochem Bioph Res Co. 2019;514(1):71–7. https://doi.org/10.1016/j.bbrc.2019.04.109.
Dong L, Zhang L, Liu H, Xie M, Gao J, Zhou X, et al. Circ_0007331 knock-down suppresses the progression of endometriosis via miR-200c-3p/HiF-1alpha axis. J Cell Mol Med. 2020;24(21):12656–66. https://doi.org/10.1111/jcmm.15833.
Sun L, Wei Y, Wang J. Circular RNA PIP5K1A (circPIP5K1A) accelerates endometriosis progression by regulating the miR-153-3p/Thymosin Beta-4 X-Linked (TMSB4X) pathway. Bioengineered. 2021;12(1):7104–18. https://doi.org/10.1080/21655979.2021.1978618.
Yang Y, Ban D, Zhang C, Shen L. Downregulation of circ_0000673 promotes Cell proliferation and migration in endometriosis via the Mir-616-3p/PTEN axis. Int J Med Sci. 2021;18(15):3506–15. https://doi.org/10.7150/ijms.63564.
Liu D, Liang Y, Chen M, Yang F, Yao S. Knockdown of circ_0075503 suppresses cell migration and invasion by regulating miR-15a-5p and KLF12 in endometriosis. Mol Cell Biochem. 2021;476(10):3845–56. https://doi.org/10.1007/s11010-021-04202-5.
Du Y, Zhang Z, Xiong W, Li N, Liu H, He H, et al. Estradiol promotes EMT in endometriosis via MALAT1/miR200s sponge function. Reproduction. 2019;157(2):179–88. https://doi.org/10.1530/REP-18-0424.
He X, Liu N, Mu T, Lu D, Jia C, Wang S, et al. Oestrogen induces epithelial-mesenchymal transition in endometriosis via circ_0004712/miR-148a-3p sponge function. J Cell Mol Med. 2020;24(17):9658–66. https://doi.org/10.1111/jcmm.15495.
Zhang MM, Ren CT, Xiao YN, Xia XM, Fang XL. Expression Profile analysis of circular RNAs in ovarian endometriosis by microarray and bioinformatics. Med Sci Monitor. 2018;24:9240–50. https://doi.org/10.12659/Msm.913885.
Wang D, Luo Y, Wang G, Yang Q. CircATRNL1 promotes epithelial-mesenchymal transition in endometriosis by upregulating Yes-associated protein 1 in vitro. Cell Death Dis. 2020;11(7):594. https://doi.org/10.1038/s41419-020-02784-4.
Jiang N, Pan WW, Li JH, Cao TF, Shen HM. Upregulated circular RNA hsa_circ_0008433 regulates pathogenesis in endometriosis via miRNA. Reprod Sci. 2020;27(11):2002–17. https://doi.org/10.1007/s43032-020-00219-1.
Tu J, Yang H, Chen Y, Chen Y, Chen H, Li Z, et al. Current and future roles of circular RNAs in normal and pathological endometrium. Front Endocrinol (Lausanne). 2021;12:668073. https://doi.org/10.3389/fendo.2021.668073.
Wu J, Fang X, Huang H, Huang W, Wang L, Xia X. Construction and topological analysis of an endometriosis-related exosomal circRNA-miRNA-mRNA regulatory network. Aging (Albany NY). 2021;13(9):12607–30. https://doi.org/10.18632/aging.202937.
Acknowledgements
We thank Professor Chen Lingling from the Institute of Biochemistry and Cell Biology, SIBS, CAS, for helpful comments and revising the manuscript.
Funding
This work was supported by grant from the Nature Science Foundation of China (NSFC) (81630036, 91542116 and 31570920 to MRD), the Strategic Collaborative Research Program of the Ferring Institute of Reproductive Medicine Supported by Ferring Pharmaceuticals and Chinese Academy of Sciences (FIRMX200504 to MRD), the Innovation oriented Science and Technology Grant from NPFPC Key Laboratory of Reproduction Regulation (CX2017-2 to MRD), the Nature Science Foundation of Shanghai (21ZR1410500 to SCW), and the Shanghai Sailing Program (19YF1404100 to LYC).
Author information
Authors and Affiliations
Contributions
MDL, SCW, and LYC wrote the manuscript and drafted the figures, which were corrected and modified by JPZ and MRD. All authors approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, M., Cui, L., Zhang, J. et al. The Critical Roles of Circular RNAs in Basic Research and Clinical Application of Female Reproductive–Related Diseases. Reprod. Sci. 30, 1421–1434 (2023). https://doi.org/10.1007/s43032-022-01070-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43032-022-01070-2