Abstract
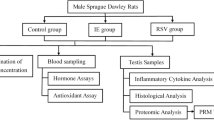
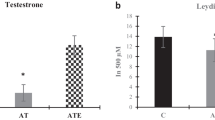
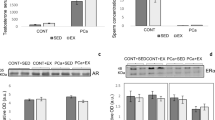
The hyperhomocysteinemia (HHcy) is toxic to the cells and associated with several diseases. Clinical studies have shown changes in plasma concentrations of Hcy after physical exercise. This study aimed to assess the effect of HHcy on testis, epididymis and sperm quality and to investigate whether voluntary exercise training protects this system against damage caused by HHcy in Swiss mice. In this study, 48 mice were randomly distributed in the control, HHcy, physical exercise, and HHcy combined with physical exercise groups. HHcy was induced by daily administration of dl-homocysteine thiolactone via gavage throughout the experimental period. Physical exercise was performed through voluntary running on the exercise wheels. The plasma concentrations of homocysteine (Hcy) and testosterone were determined. The testes and epididymis were used to assess the sperm count, histopathology, lipoperoxidation, cytokine levels, testicular cholesterol, myeloperoxidase, and catalase activity. Spermatozoa were analyzed for morphology, acrosome integrity, mitochondrial activity, and motility. In the testes, HHcy increased the number of abnormal seminiferous tubules, reduced the tubular diameter and the height of the germinal epithelium. In the epididymis, there was tissue remodeling in the head region. Ultimately, voluntary physical exercise training reduced plasma Hcy concentration but did not attenuate HHcy-induced testicular and epididymal disturbances.





Similar content being viewed by others
Data and materials availability
Not applicable.
Code Availability
Not applicable.
References
Kim J, Kim H, Roh H, Kwon Y. Causes of hyperhomocysteinemia and its pathological significance. Arch Pharm Res. 2018;41:372–83. https://doi.org/10.1007/s12272-018-1016-4.
Tinelli C, Di Pino A, Ficulle E, et al. Hyperhomocysteinemia as a risk factor and potential nutraceutical target for certain pathologies. Front Nutr. 2019;6:1–13. https://doi.org/10.3389/fnut.2019.00049.
Smith AD, Refsum H. Homocysteine, B Vitamins, and cognitive impairment. Annu Rev Nutr. 2016;36:211–39. https://doi.org/10.1146/annurev-nutr-071715-050947.
Moretti R, Caruso P. The controversial role of homocysteine in neurology: from labs to clinical practice. Int J Mol Sci 2019;20(1):231. https://doi.org/10.3390/ijms20010231
Rizzo A, Sciorsci RL. Role of homocysteine metabolism in animal reproduction: a review. Res Vet Sci. 2019;122:29–35. https://doi.org/10.1016/j.rvsc.2018.11.011.
World Health Organization. Prevention of cardiovascular disease: guidelines for assessment and management of total cardiovascular risk. World Health Organization. 2007. https://apps.who.int/iris/handle/10665/43685.
Peng H, Man C, Xu J, Fan Y. Elevated homocysteine levels and risk of cardiovascular and all-cause mortality: a meta-analysis of prospective studies. J Zhejiang Univ Sci B. 2015;16:78–86. https://doi.org/10.1631/jzus.B1400183.
Ji C, Kaplowitz N. Hyperhomocysteinemia, endoplasmic reticulum stress, and alcoholic liver injury. World J Gastroenterol. 2004;10:1699–708. https://doi.org/10.3748/wjg.v10.i12.1699.
Ge YF, Wang CH, Ouyang LX, et al. Determination of plasma homocysteine in oligospermia and/or asthenospermia patients. Zhonghua Nan Ke Xue. 2008;14:1112–4.
Ebisch IMW, Peters WHM, Thomas CMG, et al. Homocysteine, glutathione and related thiols affect fertility parameters in the (sub)fertile couple. Hum Reprod. 2006;21:1725–33. https://doi.org/10.1093/humrep/del081.
Aitken RJ, Flanagan HM, Connaughton H, et al. Involvement of homocysteine, homocysteine thiolactone, and paraoxonase type 1 (PON-1) in the etiology of defective human sperm function. Andrology. 2016;4:345–60. https://doi.org/10.1111/andr.12157.
Chen Z, Karaplis AC, Ackerman SL, et al. Mice deficient in methylenetetrahydrofolate reductase exhibit hyperhomocysteinemia and decreased methylation capacity, with neuropathology and aortic lipid deposition. Hum Mol Genet. 2001;10:433–43. https://doi.org/10.1093/hmg/10.5.433.
Aitken RJ. Reactive oxygen species as mediators of sperm capacitation and pathological damage. Mol Reprod Dev. 2017;84:1039–52. https://doi.org/10.1002/mrd.22871.
Brown BM, Peiffer J, Rainey-Smith SR. Exploring the relationship between physical activity, beta-amyloid and tau: a narrative review. Ageing Res Rev. 2019;50:9–18. https://doi.org/10.1016/j.arr.2019.01.003.
Carvalho T, Nóbrega A, Lazzoli JK, et al. Posição oficial da Sociedade Brasileira de Medicina do Esporte: atividade física e saúde. Bras Med Esporte. 1996;2:79–81.
Deminice R, Rosa FT, Franco GS, et al. Effects of creatine supplementation on oxidative stress and inflammatory markers after repeated-sprint exercise in humans. Nutrition. 2013;29:1127–32. https://doi.org/10.1016/j.nut.2013.03.003.
Vincent HK, Bourguignon C, Vincent KR. Resistance training lowers exercise-induced oxidative stress and homocysteine levels in overweight and obese older adults. Obesity. 2006;14:1921–30. https://doi.org/10.1038/oby.2006.224.
Neuman JC, Albright KA, Schalinske KL. Exercise prevents hyperhomocysteinemia in a dietary folate-restricted mouse model. Nutr Res. 2013;33:487–93. https://doi.org/10.1016/j.nutres.2013.04.008.
Deminice R, Vannucchi H, Simões-Ambrosio LM, Jordao AA. Creatine supplementation reduces increased homocysteine concentration induced by acute exercise in rats. Eur J Appl Physiol. 2011;111:2663–70. https://doi.org/10.1007/s00421-011-1891-6.
Deminice R, Ribeiro DF, Frajacomo FTT. The effects of acute exercise and exercise training on plasma homocysteine: a meta-analysis. PLoS ONE. 2016;11:1–17. https://doi.org/10.1371/journal.pone.0151653.
Silva ADSE, Da Mota MPG. Effects of physical activity and training programs on plasma homocysteine levels: a systematic review. Amino Acids. 2014;46:1795–804. https://doi.org/10.1007/s00726-014-1741-z.
Punhagui APF, Teixeira GR, de Freitas MC, et al. Intermittent resistance exercise and obesity, considered separately or combined, impair spermatic parameters in adult male Wistar rats. Int J Exp Pathol. 2018;99:95–102. https://doi.org/10.1111/iep.12270.
Dutra Gonçalves G, Antunes Vieira N, Rodrigues Vieira H, et al. Role of resistance physical exercise in preventing testicular damage caused by chronic ethanol consumption in UChB rats. Microsc Res Tech. 2017;80:378–86. https://doi.org/10.1002/jemt.22806.
Ibáñez CA, Erthal RP, Ogo FM, et al. A high fat diet during adolescence in male rats negatively programs reproductive and metabolic function which is partially ameliorated by exercise. Front Physiol. 2017;8:1–12. https://doi.org/10.3389/fphys.2017.00807.
Gomes M, Freitas MJ, Fardilha M. Physical activity, exercise, and mammalian testis function: emerging preclinical protein biomarker and integrative biology insights. Omi A J Integr Biol. 2015;19:499–511. https://doi.org/10.1089/omi.2015.0065.
Celotto AC, Fukada SY, Laurindo FRM, et al. Chronic hyperhomocysteinemia impairs vascular function in ovariectomized rat carotid arteries. Amino Acids. 2010;38:1515–22. https://doi.org/10.1007/s00726-009-0368-y.
Jean-Faucher C, El WN, Berger M, et al. Ontogeny of the secretory pattern of LH and FSH in male mice during sexual maturation. Int J Androl. 1983;6:575–84. https://doi.org/10.1111/j.1365-2605.1983.tb00348.x.
Goh J, Ladiges W. Voluntary wheel running in mice. Curr Protoc Mouse Biol. 2015;5:283–90. https://doi.org/10.1002/9780470942390.mo140295.
Fernandes GSA, Fernandez CDB, Campos KE, et al. Vitamin C partially attenuates male reproductive deficits in hyperglycemic rats. Reprod Biol Endocrinol. 2011;9:1–9. https://doi.org/10.1186/1477-7827-9-100.
Akkaya H, Eyuboglu S, Erkanlı Senturk G, Yilmaz B. Investigation of the effects of kisspeptin-10 in methionine-induced lipid peroxidation in testicle tissue of young rats. J Biochem Mol Toxicol. 2017;31:1–7. https://doi.org/10.1002/jbt.21881.
Wang H, Yang LL, Ji YL, et al. Different fixative methods influence histological morphology and TUNEL staining in mouse testes. Reprod Toxicol. 2016;60:53–61. https://doi.org/10.1016/j.reprotox.2016.01.006.
Leblond CP, Clermont Y. Spermiogenesis of rat, mouse, hamster and guinea pig as revealed by the “periodic acid-fuchsin sulfurous acid” technique. Am J Anat. 1952;90:167–215. https://doi.org/10.1002/aja.1000900202.
Favareto APA, de Toledo FC, Kempinas WDG. Paternal treatment with cisplatin impairs reproduction of adult male offspring in rats. Reprod Toxicol. 2011;32:425–33. https://doi.org/10.1016/j.reprotox.2011.10.003.
Miller RJ, Killian GJ. Morphometric analyses of the epididymis from normal and vasectomized rats. J Androl. 1987;8:279–91. https://doi.org/10.1002/j.1939-4640.1987.tb00962.x.
WEIBEL ER, . Principles and methods for the morphometric study of the lung and other organs. Lab Invest. 1963;12:131–55.
Casagrande R, Georgetti SR, Verri WA, et al. Protective effect of topical formulations containing quercetin against UVB-induced oxidative stress in hairless mice. J Photochem Photobiol B Biol. 2006;84:21–7. https://doi.org/10.1016/j.jphotobiol.2006.01.006.
Slater TF, Sawyer BC. The stimulatory effects of carbon tetrachloride and other halogenoalkanes on peroxidative reactions in rat liver fractions in vitro. General features of the systems used. Biochem J. 1971;123:805–14. https://doi.org/10.1042/bj1230805.
Beers RF, Sizer IW. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J Biol Chem. 1952;195:133–40. https://doi.org/10.1093/jxb/48.2.181.
Fernandes GSA, Arena AC, Fernandez CDB, et al. Reproductive effects in male rats exposed to diuron. Reprod Toxicol. 2007;23:106–12. https://doi.org/10.1016/j.reprotox.2006.09.002.
Cheng FP, Fazeli A, Voorhout WF, et al. Use of peanut agglutinin to assess the acrosomal status and the zona pellucida-induced acrosome reaction in stallion spermatozoa. J Androl. 1996;17:674–82.
Siervo GEML, Vieira HR, Ogo FM, et al. Spermatic and testicular damages in rats exposed to ethanol: influence of lipid peroxidation but not testosterone. Toxicology. 2015;330:1–8. https://doi.org/10.1016/j.tox.2015.01.016.
HRUDKA F, . Cytochemical and ultracytochemical demonstration of cytochrome c oxidase in spermatozoa and dynamics of its changes accompanying ageing or induced by stress. Int J Androl. 1987;10:809–28. https://doi.org/10.1111/j.1365-2605.1987.tb00385.x.
Rennie MJ, Tipton KD. Protein and amino acid metabolism during and after exercise and the effects of nutrition. Annu Rev Nutr. 2000;20:457–83.
Leeuwenburgh C, Hollander J, Leichtweis S, et al. Adaptations of glutathione antioxidant system to endurance training are tissue and muscle fiber specific. Am J Physiol - Regul Integr Comp Physiol. 1997;272:363–9. https://doi.org/10.1152/ajpregu.1997.272.1.r363.
Unt E, Zilmer K, Mägi A, et al. Homocysteine status in former top-level male athletes: possible effect of physical activity and physical fitness. Scand J Med Sci Sport. 2008;18:360–6. https://doi.org/10.1111/j.1600-0838.2007.00674.x.
Fon Tacer K, Pompon D, Rozman D. Adaptation of cholesterol synthesis to fasting and TNF-α: profiling cholesterol intermediates in the liver, brain, and testis. J Steroid Biochem Mol Biol. 2010;121:619–25. https://doi.org/10.1016/j.jsbmb.2010.02.026.
Akpovi CD, Murphy BD, Erickson RP, Pelletier RM. Dysregulation of testicular cholesterol metabolism following spontaneous mutation of the niemann-pick C1 gene in mice. Biol Reprod. 2014;91:1–8. https://doi.org/10.1095/biolreprod.114.119412.
Alves MG, Socorro S, Silva J, et al. In vitro cultured human Sertoli cells secrete high amounts of acetate that is stimulated by 17β-estradiol and suppressed by insulin deprivation. Biochim Biophys Acta - Mol Cell Res. 2012;1823:1389–94.
Hermo L, Pelletier RM, Cyr DG, Smith CE. Surfing the wave, cycle, life history, and genes/proteins expressed by testicular germ cells. part 5: intercellular junctions and contacts between germs cells and Sertoli cells and their regulatory interactions, testicular cholesterol, and genes/proteins. Microsc Res Tech. 2010;73:409–94. https://doi.org/10.1002/jemt.20786.
Keber R, Rozman D, Horvat S. Sterols in spermatogenesis and sperm maturation. J Lipid Res. 2013;54:20–33. https://doi.org/10.1194/jlr.R032326.
Akpovi CD, Suk RY, Vitale ML, Pelletier RM. The predominance of one of the SR-BI isoforms is associated with increased esterified cholesterol levels not apoptosis in mink testis. J Lipid Res. 2006;47:2233–47. https://doi.org/10.1194/jlr.M600162-JLR200.
Jakubowski H. The pathophysiological hypothesis of homocysteine thiolactone-mediated vascular disease. J Physiol Pharmacol. 2008;59:155–67.
Huleihel M, Lunenfeld E. Regulation of spermatogenesis by paracrine/autocrine testicular factors. Asian J Androl. 2004;6:259–68.
Xia W, Mruk DD, Lee WM, Cheng CY. Cytokines and junction restructuring during spermatogenesis — a lesson to learn from the testis. Cytokine Growth Factor Rev. 2005;16:469–93. https://doi.org/10.1016/j.cytogfr.2005.05.007.
Sarkar O, Bahrainwala J, Chandrasekaran S, et al. Impact of inflammation on male fertility. Front Biosci. 2011;E3:89–95.
Hong CY, Park JH, Ahn RS, et al. Molecular mechanism of suppression of testicular steroidogenesis by proinflammatory cytokine tumor necrosis factor alpha. Mol Cell Biol. 2004;24:2593–604. https://doi.org/10.1128/mcb.24.7.2593-2604.2004.
Fraczek M, Kurpisz M. Inflammatory mediators exert toxic effects of oxidative stress on human spermatozoa. J Androl. 2007;28:325–33. https://doi.org/10.2164/jandrol.106.001149.
Cornwall GA. New insights into epididymal biology and function. Hum Reprod Update. 2009;15:213–27. https://doi.org/10.1093/humupd/dmn055.
Cuasnicú PS, Cohen DJ, Ellerman DA, et al. Changes in specific sperm proteins during epididymal maturation. Epididymis From Mol to Clin Pract. 2002;389–403. https://doi.org/10.1007/978-1-4615-0679-9_22.
Da Ros VG, Munuce MJ, Cohen DJ, et al. Bicarbonate is required for migration of sperm epididymal protein DE (CRISP-1) to the equatorial segment and expression of rat sperm fusion ability. Biol Reprod. 2004;70:1325–32. https://doi.org/10.1095/biolreprod.103.022822.
Barrios B, Fernández-Juan M, Muiño-Blanco T, Cebrián-Pérez JA. Immunocytochemical localization and biochemical characterization of two seminal plasma proteins that protect ram spermatozoa against cold shock. J Androl. 2005;26:539–49. https://doi.org/10.2164/jandrol.04172.
Al Mutairi F. Hyperhomocysteinemia: clinical insights. J Cent Nerv Syst Dis. 2020;12:117957352096223. https://doi.org/10.1177/1179573520962230.
Crisóstomo L, Videira RA, Jarak I, et al. Diet during early life defines testicular lipid content and sperm quality in adulthood. Am J Physiol Metab. 2020;319:E1061–73. https://doi.org/10.1152/ajpendo.00235.2020.
Crisóstomo L, Rato L, Jarak I, et al. A switch from high-fat to normal diet does not restore sperm quality but prevents metabolic syndrome. Reproduction. 2019;158:377–87. https://doi.org/10.1530/REP-19-0259.
Acknowledgements
This study was partially funded by the Coordination for the Improvement of Postgraduate Studies in Higher Education (CAPES / PROEX—Financial Code 001) and for providing a Master’s scholarship to DP dos Santos. This paper represents part of the Master’s thesis by DP dos Santos (Experimental pathology—State University of Londrina—Brazil) under the supervision of GSA Fernandes.
Funding
This study is supported by the CAPES (Coordinating Body for the Improvement of Postgraduate Studies in Higher Education) and by State University of Londrina.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics Approval and Consent to Participate
The animal care and manipulation procedures followed the recommendations of the Brazilian Code for the Use of Laboratory Animals and the experiment was approved by the Animal Use Ethics Committee of the State University of Londrina (OF. CIRC. CEUA nº102/2017).
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare no competing interests.
Rights and permissions
About this article
Cite this article
dos Santos, D.P., Ribeiro, D.F., Frigoli, G.F. et al. Voluntary Exercise Attenuates Hyperhomocysteinemia, But Does not Protect Against Hyperhomocysteinemia-Induced Testicular and Epididymal Disturbances. Reprod. Sci. 29, 277–290 (2022). https://doi.org/10.1007/s43032-021-00704-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43032-021-00704-1