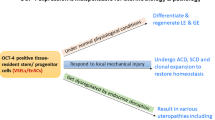
Abstract
We have earlier reported pluripotent, very small embryonic-like stem cells (VSELs) and slightly bigger endometrial stem cells (EnSCs) in adult mouse uterus and their regulation by gonadotropin and steroid hormones. VSELs can differentiate into cells of all three lineages in vitro; however, they neither expand readily in vitro nor compliment a developing embryo. In the present study, a robust protocol is described to enrich uterine stem/progenitor cells along with their characterization and variation across estrus cycle. After enzymatic digestion of adult mouse uterus, single-cell suspension obtained was spun at 1000 rpm (250 g) to pellet majority of cells. Stem cells remain buoyant at this speed and were pelleted by spinning supernatant at 3000 rpm (1000 g). Spherical, darkly stained VSELs (2–6 μm) with high nucleo-cytoplasmic ratio and EnSCs (> 6 μm) expressed OCT-4, NANOG, SSEA-1, SCA-1, and c-KIT. OCT-4-positive cells co-expressed SSEA-1, ERα, ERβ, PR, and FSHR. Transcripts specific for pluripotent state (Oct-4, Oct-4a, Sox-2, Nanog), primordial germ cells (Stella, Fragilis), and receptors for pituitary and steroid hormones (ERα, ERβ, PR, FSHR 1 and 3) were studied by RT-PCR in 3000 rpm pellet. Cell pellet collected at 3000 rpm showed 10-fold enrichment of VSELs (2–6 μm, viable cells with surface phenotype of LIN-CD45-SCA-1+) by flow cytometry and upregulation of pluripotent transcripts by qRT-PCR compared with 1000 rpm pellet. VSELs were maximal during estrus and metestrus phases of estrus cycle. To conclude, VSELs/EnSCs can be enriched from adult uterus using the strategy described here, vary in numbers across estrus cycle, and are vulnerable to endocrine disruption as they express steroid receptors.







Similar content being viewed by others
References
Gargett CE, Schwab KE, Deane JA. Endometrial stem/progenitor cells: the first 10 years. Hum Reprod Update. 2016;22:137–63.
Cousins FL, O DF, Gargett CE. Endometrial stem/progenitor cells and their role in the pathogenesis of endometriosis. Best Pract Res Clin Obstet Gynaecol. 2018;50:27–38.
Gargett CE, Gurung S, Darzi S, Werkmeister JA, Mukherjee S. Tissue engineering approaches for treating pelvic organ prolapse using a novel source of stem/stromal cells and new materials. Curr Opin Urol. 2019;29:450–7.
Darzi S, Werkmeister JA, Deane JA, Gargett CE. Identification and characterization of human endometrial mesenchymal stem/stromal cells and their potential for cellular therapy. Stem Cells Transl Med. 2016;5:1127–32.
Bhartiya D. An update on endometrial stem cells and progenitors. Hum Reprod Update. 2016;22(4):529–30.
Caplan AI. Mesenchymal stem cells: time to change the name! Stem Cells Transl Med. 2017;6:1445–51.
Sipp D, Robey PG, Turner L. Clear up this stem-cell mess. Nature. 2018;561:455–7.
Caplan AI. There is no “Stem Cell Mess”. Tissue Eng Part B Rev. 2019;25:291–3.
De Souza LE, Malta TM, Kashima Haddad S, Covas DT. Mesenchymal stem cells and pericytes: to what extent are they related? Stem Cells Dev. 2016;25:1843–52.
Taylor HS. Endometrial cells derived from donor stem cells in bone marrow transplant recipients. JAMA. 2004;292:81–5.
Ong YR, Cousins FL, Yang X, Mushafi AAAA, Breault DT, Gargett CE, et al. Bone marrow stem cells do not contribute to endometrial cell lineages in chimeric mouse models. Stem Cells. 2018;36:91–102.
Santamaria X, Mas A, Cervelló I, Taylor H, Simon C. Uterine stem cells: from basic research to advanced cell therapies. Hum Reprod Update. 2018;24:673–93.
Gunjal P, Bhartiya D, Metkari S, Manjramkar D, Patel H. Very small embryonic-like stem cells are the elusive mouse endometrial stem cells--a pilot study. J Ovarian Res. 2015;8:9.
James K, Bhartiya D, Ganguly R, Kaushik A, Gala K, Singh P, et al. Gonadotropin and steroid hormones regulate pluripotent very small embryonic-like stem cells in adult mouse uterine endometrium. J Ovarian Res. 2018;11:83.
Bhartiya D, James K. Very small embryonic-like stem cells (VSELs) in adult mouse uterine perimetrium and myometrium. J Ovarian Res. 2017;10:29.
Taichman RS, Wang Z, Shiozawa Y, Jung Y, Song J, Balduino A, et al. Prospective identification and skeletal localization of cells capable of multilineage differentiation in vivo. Stem Cells Dev. 2010;19:1557–70.
Li L, Clevers H. Coexistence of quiescent and active adult stem cells in mammals. Science. 2010;327:542–5.
De Rosa L, De Luca M. Cell biology: dormant and restless skin stem cells. Nature. 2012;489:215–7.
Clevers H, Watt FM. Defining adult stem cells by function, not by phenotype. Annu Rev Biochem. 2018;87:1015–27.
Post Y, Clevers H. Defining adult stem cell function at its simplest: the ability to replace lost cells through mitosis. Cell Stem Cell. 2019;25(2):174–83.
Bhartiya D, Shaikh A, Anand S, Patel H, Kapoor S, Sriraman K, et al. Endogenous, very small embryonic-like stem cells: critical review, therapeutic potential and a look ahead. Hum Reprod Update. 2016;23:41–76.
Lahlil R, Scrofani M, Barbet R, Tancredi C, Aries A, Hénon P. VSELs maintain their pluripotency and competence to differentiate after enhanced ex vivo expansion. Stem Cell Rev Rep. 2018;14(4):510–24.
Monti M, Imberti B, Bianchi N, Pezzotta A, Morigi M, Del Fante C, et al. A novel method for isolation of pluripotent stem cells from human umbilical cord blood. Stem Cells Dev. 2017;26(17):1258–69.
Shaikh A, Anand S, Kapoor S, Ganguly R, Bhartiya D. Mouse bone marrow VSELs exhibit differentiation into three embryonic germ lineages and germ & hematopoietic cells in culture. Stem Cell Rev Rep. 2017;13:202–16.
Kucia M, Reca R, Campbell FR, Zuba-Surma E, Majka M, Ratajczak J, et al. A population of very small embryonic-like (VSEL) CXCR4(+)SSEA-1(+)Oct-4+ stem cells identified in adult bone marrow. Leukemia. 2006;20(5):857–69.
Shin DM, Liu R, Wu W, Waigel SJ, Zacharias W, Ratajczak MZ, et al. Global gene expression analysis of very small embryonic-like stem cells reveals that the Ezh2-dependent bivalent domain mechanism contributes to their pluripotent state. Stem Cells Dev. 2012;21(10):1639–52.
Surani MA, Durcova-Hills G, Hajkova P, Hayashi K, Tee WW. Germ line, stem cells, and epigenetic reprogramming. Cold Spring Harb Symp Quant Biol. 2008;73:9–15.
Shin DM, Liu R, Klich I, Ratajczak J, Kucia M, Ratajczak MZ. Molecular characterization of isolated from murine adult tissues very small embryonic/epiblast like stem cells (VSELs). Mol Cells. 2010;29(6):533–8.
Ratajczak MZ, Ratajczak J, Kucia M. Very small embryonic-like stem cells (VSELs). Circ Res. 2019;124:208–10.
Abbott A. Doubt cast over tiny stem cells. Nature. 2013;499:390.
Hapangama DK, Drury J, Da Silva L, Al-Lamee H, Earp A, Valentijn AJ, et al. Abnormally located SSEA1+/SOX9+ endometrial epithelial cells with a basalis-like phenotype in the eutopic functionalis layer may play a role in the pathogenesis of endometriosis. Hum Reprod. 2019;34:56–68.
Zuba-Surma EK, Kucia M, Wu W, Klich I, Lillard JW Jr, Ratajczak J, et al. Very small embryonic-like stem cells are present in adult murine organs: Image Stream-based morphological analysis and distribution studies. Cytometry A. 2008;73A:1116–27.
Caligioni C. Assessing reproductive status/stages in mice. Curr Protoc Neurosci. 2009;APPENDIX:Appendix–4I.
Sairam MR, Babu PS. The tale of follitropin receptor diversity: a recipe for fine tuning gonadal responses? Mol Cell Endocrinol. 2007;260–262:163–71.
Patel H, Bhartiya D, Parte S, Gunjal P, Yedurkar S, Bhatt M. Follicle stimulating hormone modulates ovarian stem cells through alternately spliced receptor variant FSH-R3. J Ovarian Res. 2013;20(6):52.
Patel H, Bhartiya D. Testicular stem cells express follicle-stimulating hormone receptors and are directly modulated by FSH. Reprod Sci. 2016;23(11):1493–508.
Ratajczak MZ, Zuba-Surma EK, Shin DM, Ratajczak J, Kucia M. Very small embryonic-like (VSEL) stem cells in adult organs and their potential role in rejuvenation of tissues and longevity. Exp Gerontol. 2008;43:1009–17.
Bhartiya D. Pluripotent stem cells in adult tissues: struggling to be acknowledged over two decades. Stem Cell Rev Rep. 2017;13(6):713–24.
Wagner M, Yoshihara M, Douagi I, Damdimopoulos A, Panula S, Petropoulos S, et al. Single-cell analysis of human ovarian cortex identifies distinct cell populations but no oogonial stem cells. Nat Commun. 2020;11(1):1147.
Bhartiya D, Sharma D. Ovary does harbor stem cells—size of the cells matter! J Ovarian Res. 2020;13(1):39.
Bhartiya D, Ali Mohammad S, Guha A, Singh P, Sharma D, Kaushik A. Evolving definition of adult stem/progenitor cells. Stem Cell Rev Rep. 2019;15(3):456–8.
Syed SM, Kumar M, Ghosh A, Tomasetig F, Ali A, et al. Endometrial Axin2+ cells drive epithelial homeostasis, regeneration, and cancer following oncogenic transformation. Cell Stem Cell. 2020;26(1):64–80.e13.
Jin S. Bipotent stem cells support the cyclical regeneration of endometrial epithelium of the murine uterus. Proc Natl Acad Sci U S A. 2019;116(14):6848–57.
Johnson J, Bagley J, Skaznik-Wikiel M, Lee HJ, Adams GB, Niikura Y, et al. Oocyte generation in adult mammalian ovaries by putative germ cells in bone marrow and peripheral blood. Cell. 2005;122:303–15.
Nayernia K, Lee JH, Drusenheimer N, Nolte J, Wulf G, Dressel R, et al. Derivation of male germ cells from bone marrow stem cells. Lab Investig. 2006;86:654–63.
Bhartiya D. Being pluripotent, bone marrow very small embryonic-like stem cells rather than hematopoietic stem cells have the potential to regenerate other adult organs. Stem Cells. 2018;36:807–8.
Deane JA, Ong YR, Cousins FL, Gargett CE. In reply to letter to the editor from Bhartiya: transplantation of whole bone marrow indicates that bone marrow very small embryonic-like cells do not contribute to endometrial lineages. Stem Cells. 2018;36:809.
Bhartiya D. Clinical translation of stem cells for regenerative medicine. Circ Res. 2019;124(6):840–2.
Bhartiya D, Shaikh A, Nagvenkar P, Kasiviswanathan S, Pethe P, Pawani H, et al. Very small embryonic-like stem cells with maximum regenerative potential get discarded during cord blood banking and bone marrow processing for autologous stem cell therapy. Stem Cells Dev. 2012;21(1):1–6.
Winuthayanon W, Hewitt SC, Orvis GD, Behringer RR, Korach KS. Uterine epithelial estrogen receptor alpha is dispensable for proliferation but essential for complete biological and biochemical responses. Proc Natl Acad Sci U S A. 2010;107:19272–7.
Cooke PS, Buchanan DL, Lubahn DB, Cunha GR. Mechanism of estrogen action: lessons from the estrogen receptor-alpha knockout mouse. Biol Reprod. 1998;59:470–5.
Cooke PS, Buchanan DL, Young P, Setiawan T, Brody J, Korach KS, et al. Stromal estrogen receptors mediate mitogenic effects of estradiol on uterine epithelium. Proc Natl Acad Sci U S A. 1997;94:6535–40.
Chung D, Gao F, Jegga AG, Das SK. Estrogen mediated epithelial proliferation in the uterus is directed by stromal Fgf10 and Bmp8a. Mol Cell Endocrinol. 2015;400:48–60.
Zhu L, Pollard JW. Estradiol-17beta regulates mouse uterine epithelial cell proliferation through insulin-like growth factor 1 signaling. Proc Natl Acad Sci U S A. 2007;104:15847–51.
Murphy LJ, Ghahary A. Uterine insulin-like growth factor-1: regulation of expression and its role in estrogen-induced uterine proliferation. Endocr Rev. 1990;11:443–53.
Nelson KG, Takahashi T, Lee DC, Luetteke NC, Bossert NL, Ross K, et al. Transforming growth factor-alpha is a potential mediator of estrogen action in the mouse uterus. Endocrinology. 1992;131:1657–64.
Winuthayanon W, Lierz SL, Delarosa KC, Sampels SR, Donoghue LJ, Hewitt SC, et al. Juxtacrine activity of estrogen receptor α in uterine stromal cells is necessary for estrogen-induced epithelial cell proliferation. Sci Rep. 2017;7:8377.
Acknowledgments
The help from Ms Gayatri and Ms Sushma for flow cytometry studies and Ms Reshma and Ms Shobha for confocal studies is acknowledged. (NIRRH/MS/RA/849/12-2019)
Funding
The study was supported by core funds provided by the Indian Council of Medical Research, Government of India, New Delhi. PS acknowledges the DST-INSPIRE fellowship (IF170144).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The study was approved by the Institute Animal Ethics Committee.
Conflict of Interest
The authors declare that they have no conflicts of interest
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
ESM 1
(PDF 2014 kb).
Rights and permissions
About this article
Cite this article
Singh, P., Bhartiya, D. Pluripotent Stem (VSELs) and Progenitor (EnSCs) Cells Exist in Adult Mouse Uterus and Show Cyclic Changes Across Estrus Cycle. Reprod. Sci. 28, 278–290 (2021). https://doi.org/10.1007/s43032-020-00250-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43032-020-00250-2