Abstract
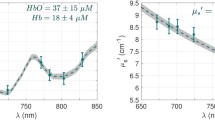
Obstetric management to prevent hypoxic ischemic encephalopathy (HIE) during labor is important to reduce the cerebral palsy incidence in neonates. A novel approach to monitor or predict fetal brain damage during labor is required. Diffuse reflectance spectroscopy is a noninvasive method routinely used to assess the intrinsic characteristics of tissues. This study investigated the time course of diffuse reflectance signals during an early stage of cerebral cortical damage in a neonatal rat HIE model (Vannucci’s model). In the model, an HIE lesion was induced by hypoxic exposure following ligation of the left common carotid artery. Using this model, we established an experimental system to detect diffuse light reflectance signals at time points of interest. Quantitative monitoring of total hemoglobin, oxygen saturation, and scattering amplitude was conducted to examine the basis of the diffused reflectance signals. During hypoxic exposure, which induced HIE damage in the left hemisphere after ligation, the oxygen saturation level decreased, but the difference between the two hemispheres was relatively small. During this period, total hemoglobin was increased in both hemispheres, but the change in the left hemisphere was significantly greater than that in the right, which is attributable to a vigorous compensation response. During hypoxia, scattering amplitude, which reflects cellular/subcellular morphology, revealed a remarkable difference between the two hemispheres. We confirmed that scattering amplitude levels negatively correlated with the extent of edema. These findings suggest that simultaneous monitoring of the scattering amplitude, in addition to hemodynamic parameters, is useful for detecting brain tissue alterations leading to HIE.





Similar content being viewed by others
References
Toyokawa S, Maeda E, Kobayashi Y. Estimation of the number of children with cerebral palsy using nationwide health insurance claims data in Japan. Dev Med Child Neurol. 2017;59:317–21.
Graham EM, Ruis KA, Hartman AL, Northington FJ, Fox HE. A systematic review of the role of intrapartum hypoxia-ischemia in the causation of neonatal encephalopathy. Am J Obstet Gynecol. 2008;199:587–95.
Himmelmann K, Hagberg G, Uvebrant P. The changing panorama of cerebral palsy in Sweden. X. Prevalence and origin in the birth-year period 1999-2002. Acta Paediatr. 2010;99:1337–43.
Clark SL, Hamilton EF, Garite TJ, Timmins A, Warrick PA, Smith S. The limits of electronic fetal heart rate monitoring in the prevention of neonatal metabolic acidemia. Am J Obstet Gynecol. 2017;216:163.e161–6.
Blix E. The admission CTG: is there any evidence for still using the test? Acta Obstet Gynecol Scand. 2013;92:613–9.
Nishidate I, Ishizuka T, Mustari A, et al. Evaluation of cerebral hemodynamics and tissue morphology of in vivo rat brain using spectral diffuse reflectance imaging. Appl Spectrosc. 2017;71:866–78.
Durduran T, Zhou C, Buckley EM, et al. Optical measurement of cerebral hemodynamics and oxygen metabolism in neonates with congenital heart defects. J Biomed Opt. 2010;15:037004.
Bale G, Elwell CE, Tachtsidis I. From Jobsis to the present day: a review of clinical near-infrared spectroscopy measurements of cerebral cytochrome-c-oxidase. J Biomed Opt. 2016;21:091307.
Farzam P, Buckley EM, Lin PY, et al. Shedding light on the neonatal brain: probing cerebral hemodynamics by diffuse optical spectroscopic methods. Sci Rep. 2017;7:15786.
Kawauchi S, Sato S, Uozumi Y, Nawashiro H, Ishihara M, Kikuchi M. Light-scattering signal may indicate critical time zone to rescue brain tissue after hypoxia. J Biomed Opt. 2011;16:027002.
Sato S, Kawauchi S, Okuda W, Nishidate I, Nawashiro H, Tsumatori G. Real-time optical diagnosis of the rat brain exposed to a laser-induced shock wave: observation of spreading depolarization, vasoconstriction and hypoxemia-oligemia. PLoS One. 2014;9:e82891.
Rice JE 3rd, Vannucci RC, Brierley JB. The influence of immaturity on hypoxic-ischemic brain damage in the rat. Ann Neurol. 1981;9:131–41.
Yager JY, Ashwal S. Animal models of perinatal hypoxic-ischemic brain damage. Pediatr Neurol. 2009;40:156–67.
Bloom SL, Spong CY, Thom E, et al. Fetal pulse oximetry and cesarean delivery. N Engl J Med. 2006;355:2195–202.
East CE, Begg L, Colditz PB, Lau R. Fetal pulse oximetry for fetal assessment in labour. Cochrane Database Syst Rev. 2014:Cd004075.
Kawauchi S, Nishidate I, Uozumi Y, Nawashiro H, Ashida H, Sato S. Diffuse light reflectance signals as potential indicators of loss of viability in brain tissue due to hypoxia: charge-coupled-device-based imaging and fiber-based measurement. J Biomed Opt. 2013;18:15003.
Beard P. Biomedical photoacoustic imaging. Interface Focus. 2011;1:602–31.
Tuchin V. Tissue optics: light scattering methods and instruments for medical diagnosis. 2nd ed. Bellingham: SPIE Press; 2007.
Wang X, Zhang J, Yang Y, et al. Progesterone attenuates cerebral edema in neonatal rats with hypoxic-ischemic brain damage by inhibiting the expression of matrix metalloproteinase-9 and aquaporin-4. Exp Ther Med. 2013;6:263–7.
Yu L, Fan SJ, Liu L, et al. Effect of ischemic postconditioning on cerebral edema and the AQP4 expression following hypoxic-ischemic brain damage in neonatal rats. World J Pediatr. 2015;11:165–70.
Buckley EM, Patel SD, Miller BF, Franceschini MA, Vannucci SJ. In vivo monitoring of cerebral hemodynamics in the immature rat: effects of hypoxia-ischemia and hypothermia. Dev Neurosci. 2015;37:407–16.
Wood T, Smit E, Maes E, et al. Monitoring of cerebral blood flow during hypoxia-ischemia and resuscitation in the neonatal rat using laser speckle imaging. Phys Rep. 2016;4:e12749.
James IM, Millar RA, Pruves MJ. Observations on the extrinsic neural control of cerebral blood flow in the Baboon. Circ Res. 1969;25:77–93.
McDowall DG. Interrelationships between blood oxygen tensions and cerebral blood flow. Int Anesthesiol Clin. 1966;4:205–19.
Ainslie PN, Ogoh S. Regulation of cerebral blood flow in mammals during chronic hypoxia: a matter of balance. Exp Physiol. 2010;95:251–62.
Willie CK, Macleod DB, Shaw AD, et al. Regional brain blood flow in man during acute changes in arterial blood gases. J Physiol. 2012;590:3261–75.
Dubowitz DJ, Dyer EAW, Theilmann RJ, Buxton RB, Hopkins SR. Early brain swelling in acute hypoxia. J Appl Physiol. 2009;107:244–52.
Davis C, Hackett P. Advances in the prevention and treatment of high altitude illness. Emerg Med Clin North Am. 2017;35:241–60.
Ueda Y, Sato S, Ashida H, et al. Transcranial measurement of diffuse light reflectance from cold-injured brains in rats. J Biomed Opt. 2005;10:064010.
Acknowledgments
We thank Mai Miyaki and Keiichiro Yoshida for technical assistance and optical data analysis.
Funding
This work was supported by JSPS KAKENHI (Grant Number 15K15595).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All procedures were approved by the ethics committee of the National Defense Medical College of Japan (No. 17022).
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kinoshita, S., Kawauchi, S., Nagamatsu, T. et al. Real-time Monitoring of Hypoxic-Ischemic Brain Damage in Neonatal Rats Using Diffuse Light Reflectance Spectroscopy. Reprod. Sci. 27, 172–181 (2020). https://doi.org/10.1007/s43032-019-00020-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43032-019-00020-9