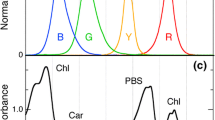
Abstract
Fluorescence recovery after photobleaching (FRAP) has been used to study the dynamics of the cyanobacterial photosynthesis apparatus since 1997. Fluorescence recovery of cyanobacteria during FRAP was conventionally interpreted as a result of phycobilisome (PBS) diffusion on the surface of the thylakoid membrane. The mechanism of state transition in cyanobacteria has been widely attributed to PBS diffusion. However, in red algae, another PBS-containing group, the intrinsic photoprocess was found to contribute greatly to the fluorescence recovery of PBS, which raises questions concerning the role of FRAP in red algal PBS. Therefore, it is important to re-evaluate the nature of PBS fluorescence recovery in cyanobacteria. In the present study, four cyanobacterial strains with different phenotypes and PBS compositions were used to investigate their FRAP characteristics. Fluorescence recovery of PBS was observed in wholly photobleached cells in all four cyanobacterial strains, in which the contribution of PBS diffusion to the fluorescence recovery was not possible. Moreover, the fluorescence recovered in isolated PBSs and PBS-thylakoid membranes after photobleaching further demonstrated the intrinsic photoprocess nature of fluorescence recovery. These findings suggest that the intrinsic photoprocess contributed to the fluorescence recovery following photobleaching when measured by the FRAP method.






Similar content being viewed by others
Change history
25 June 2021
A Correction to this paper has been published: https://doi.org/10.1007/s42995-021-00111-0
References
Adir N, Bar-Zvi S, Harris D (2020) The amazing phycobilisome. Biochim Biophys Acta 1861:148047
Allen MM (1968) Simple conditions for growth of unicellular blue-green algae on plates. J Phycol 4:1–4
Arteni AA, Liu LN, Aartsma TJ, Zhang YZ, Zhou BC, Boekema EJ (2008) Structure and organization of phycobilisomes on membranes of the red alga Porphyridium cruentum. Photosynth Res 95:169–174
Aspinwall CL, Sarcina M, Mullineaux CW (2004) Phycobilisome mobility in the cyanobacterium Synechococcus sp. PCC7942 is influenced by the trimerisation of photosystem I. Photosynth Res 79:179
Glazer AN (1988) Phycobilisomes. In: Packer L, Glazer AN (eds) Methods in enzymology. Academic Press, San Diego, pp 304–312
Goedheer JC (1960) Spectral and redox properties of bacteriochlorophyll in its natural state. Biochim Biophys Acta 38:389–399
Grossman AR, Schaefer MR, Chiang GG, Collier JL (1993) The phycobilisome, a light-harvesting complex responsive to environmental conditions. Microbiol Rev 57:725–749
Henderson JN, Ai H-W, Campbell RE, Remington SJ (2007) Structural basis for reversible photobleaching of a green fluorescent protein homologue. Proc Natl Acad Sci USA 104:6672–6677
Joshua S, Mullineaux CW (2004) Phycobilisome diffusion is required for light-state transitions in cyanobacteria. Plant Physiol 135:2112–2119
Kaňa R, Kotabová E, Lukeš M, Papáček Š, Matonoha C, Liu L-N, Prášil O, Mullineaux CW (2014) Phycobilisome mobility and its role in the regulation of light harvesting in red algae. Plant Physiol 165:1618–1631
Li W, Su H-N, Pu Y, Chen J, Liu L-N, Liu Q, Qin S (2019) Phycobiliproteins: molecular structure, production, applications, and prospects. Biotechnol Adv 37:340–353
Lippincott-Schwartz J, Snapp EL, Phair RD (2018) The development and enhancement of FRAP as a key tool for investigating protein dynamics. Biophys J 115:1146–1155
Liu LN, Chen XL, Zhang YZ, Zhou BC (2005) Characterization, structure and function of linker polypeptides in phycobilisomes of cyanobacteria and red algae: an overview. Biochim Biophys Acta 1708:133–142
Liu L-N, Aartsma TJ, Thomas J-C, Lamers GEM, Zhou B-C, Zhang Y-Z (2008) Watching the native supramolecular architecture of photosynthetic membrane in red algae: topography of phycobilisomes and their crowding, diverse distribution patterns. J Biol Chem 283:34946–34953
Liu LN, Aartsma TJ, Thomas JC, Zhou BC, Zhang YZ (2009) FRAP analysis on red alga reveals the fluorescence recovery is ascribed to intrinsic photoprocesses of phycobilisomes than large-scale diffusion. PLoS ONE 4:e5295
Livingston R, Stockman D (1962) A further study of the phototropy of chlorophyll in solution. J Phys Chem 66:2533–2537
MacColl R (1998) Cyanobacterial phycobilisomes. J Struct Biol 124:311–334
McConnell MD, Koop R, Vasil’ev S, Bruce D (2002) Regulation of the distribution of chlorophyll and phycobilin-absorbed excitation energy in cyanobacteria: a structure-based model for the light state transition. Plant Physiol 130:1201–1212
Mullineaux CW, Emlyn-Jones D (2005) State transitions: an example of acclimation to low-light stress. J Exp Bot 56:389–393
Mullineaux CW, Tobin MJ, Jones GR (1997) Mobility of photosynthetic complexes in thylakoid membranes. Nature 390:421–424
Mustardy L, Cunningham FX Jr, Gantt E (1992) Photosynthetic membrane topography: quantitative in situ localization of photosystems I and II. Proc Natl Acad Sci USA 89:10021–10025
Ohki K, Fujita Y (1979) In vivo transformation of phycobiliproteins during photobleaching of Tolypothrix tenuis to forms active in photoreversible absorption changes. Plant Cell Physiol 20:1341–1347
Rayan G, Guet J-E, Taulier N, Pincet F, Urbach W (2010) Recent applications of fluorescence recovery after photobleaching (FRAP) to membrane bio-macromolecules. Sensors (Basel) 10:5927–5948
Samsonoff WA, MacColl R (2001) Biliproteins and phycobilisomes from cyanobacteria and red algae at the extremes of habitat. Arch Microbiol 176:400–405
Sarcina M, Mullineaux CW (2000) Effects of tubulin assembly inhibitors on cell division in prokaryotes in vivo. FEMS Microbiol Lett 191:25–29
Sarcina M, Tobin MJ, Mullineaux CW (2001) Diffusion of phycobilisomes on the thylakoid membranes of the cyanobacterium Synechococcus 7942: effects of phycobilisome size, temperature, and membrane lipid composition. J Biol Chem 276:46830–46834
Siebzehnrubl S, Fischer R, Kufer W, Scheer H (1989) Photochemistry of phycobiliproteins: reciprocity of reversible photochemistry and aggregation in phycoerythrocyanin from Mastigocladus laminosus. Photochem Photobiol 49:753–761
Verkman AS (2002) Solute and macromolecule diffusion in cellular aqueous compartments. Trends Biochem Sci 27:27–33
Watanabe M, Ikeuchi M (2013) Phycobilisome: architecture of a light-harvesting supercomplex. Photosynth Res 116:265–276
Yang S, Su Z, Li H, Feng J, Xie J, Xia A, Gong Y, Zhao J (2007) Demonstration of phycobilisome mobility by the time- and space-correlated fluorescence imaging of a cyanobacterial cell. Biochim Biophys Acta 1767:15–21
Zhao K-H, Scheer H (1995) Type I and type II reversible photochemistry of phycoerythrocyanin α-subunit from Mastigocladus laminosus both involve Z, E isomerization of phycoviolobilin chromophore and are controlled by sulfhydryls in apoprotein. Biochim Biophys Acta 1228:244–253
Zhao K-H, Haessner R, Cmiel E, Scheer H (1995) Type I reversible photochemistry of phycoerythrocyanin involves Z/E-isomerization of α-84 phycoviolobilin chromophore. Biochim Biophys Acta 1228:235–243
Zhao L-S, Su H-N, Li K, Xie B-B, Liu L-N, Zhang X-Y, Chen X-L, Huang F, Zhou B-C, Zhang Y-Z (2016) Supramolecular architecture of photosynthetic membrane in red algae in response to nitrogen starvation. Biochim Biophys Acta 1857:1751–1758
Acknowledgements
We thank Haiyan Yu and Xiaomin Zhao from Core Facilities for Life and Environmental Sciences of Shandong University for technical help. This work was supported by the National Natural Science Foundation of China (no. 31900023), National Key R&D Program of China (no. 2018YFC1406701), Program of Shandong Taishan Scholars (no. tspd20181203), Natural Science Foundation of Shandong (no. ZR2017LD013), AoShan Talents Cultivation Program (no. 2017ASTCP-OS14), State Key Laboratory of Microbial Technology Open Projects Fund (no. M2019-07), and Young Scholars Program of Shandong University (no. 2017WLJH22).
Author information
Authors and Affiliations
Contributions
YZZ and HNS conceived this work; NZ, HNS, and KL performed experiments; HNS, BBX, XLC, and BCZ analyzed data; NZ and HNS prepared the figures and wrote the manuscript. All authors approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Animal and human rights statement
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Edited by Jiamei Li.
The original online version of this article was revised: A funding number was incorrect in the Acknowledgements section.
Rights and permissions
About this article
Cite this article
Zhang, N., Li, K., Xie, BB. et al. Fluorescence recovery after photobleaching: analyses of cyanobacterial phycobilisomes reveal intrinsic fluorescence recovery. Mar Life Sci Technol 3, 427–433 (2021). https://doi.org/10.1007/s42995-021-00104-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42995-021-00104-z