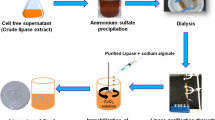
Abstract
Urease has a broad range of applications, however, the current studies on urease mainly focus on terrestrial plants or microbes. Thus, it is quite necessary to determine if marine-derived ureases have different characteristics from terrestrial origins since the finding of ureases with superior performance is of industrial interest. In this study, the marine urease produced by Penicillium steckii S4-4 derived from marine sponge Siphonochalina sp. was investigated. This marine urease exhibited a maximum specific activity of 1542.2 U mg protein−1. The molecular weight of the enzyme was 183 kDa and a single subunit of 47 kDa was detected, indicating that it was a tetramer. The N-terminal amino acid sequence of the urease was arranged as GPVLKKTKAAAV with greatest similarity to that from marine algae Ectocarpus siliculosus. This urease exhibited a Km of 7.3 mmol L−1 and a Vmax of 1.8 mmol urea min−1 mg protein−1. The optimum temperature, pH and salinity are 55 ℃, 8.5 and 10%, respectively. This urease was stable and more than 80% of its maximum specific activity was detected after incubating at 25–60 ℃ for 30 min, pH 5.5–10.0 or 0–25% salinity for 6 h. Compared with the terrestrial urease from Jack bean, this marine urease shows higher thermostability, alkaline preference and salinity tolerance, which extends the potential application fields of urease to a great extent.







Similar content being viewed by others
References
Baird ML, Garber ED (1981) The genetics and biochemistry of urease in Ustilago violacea. Biochem Gen 19:1101–1114
Balasubramanian A, Ponnuraj K (2010) Crystal structure of the first plant urease from jack bean: 83 years of journey from its first crystal to molecular structure. J Mol Biol 400:274–283
Benoit SL, Maier RJ (2011) Mua (HP0868) is a nickel-binding protein that modulates urease activity in Helicobacter pylori. mBio 2:e00039–e111
Bhatnagar L, Jain MK, Aubert JP, Zeikus JG (1984) Comparison of assimilatory organic nitrogen, sulfur, and carbon sources for growth of methanobacterium species. Appl Environ Microbiol 48:785–790
Blakesley RW, Boezi JA (1977) A new staining technique for proteins in polyacrylamide gels using coomassie brilliant blue G250. Anal Biochem 82:580–582
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Cai Y, Ni Y (1996) Purification, characterization, and pathogenicity of urease produced by Vibrio parahaemolyticus. J Clin Lab Anal 10:70–73
Carvajal N, Fernández M, Rodríguez JP, Donoso M (1982) Urease of Spirulina maxima. Phytochem 21:2821–2823
Collier JL, Brahamsha B, Palenik B (1999) The marine cyanobacterium Synechococcus sp. WH7805 requires urease (urea amidohydrolase, EC 3.5.1.5) to utilize urea as a nitrogen source: molecular-genetic and biochemical analysis of the enzyme. Microbiology 145:447–459
Creaser EH, Porter RL (1985) The purification of urease from Aspergillus nidulans. Int J Biochem 17:1339–1341
Das N, Kayastha AM, Srivastava PK (2002) Purification and characterization of urease from dehusked pigeonpea (Cajanus cajan L.) seeds. Phytochemistry 61:513–521
Dixon NE, Gazzola TC, Blakeley RL, Zermer B (1975) Letter: Jack bean urease (EC 3.5.1.5). A metalloenzyme. A simple biological role for nickel? J Am Chem Soc 97:4131–4133
El-Hefnawy ME, Sakran M, Ismail AI, Aboelfetoh EF (2014) Extraction, purification, kinetic and thermodynamic properties of urease from germinating Pisum sativum L. seeds. BMC Biochem 15:15
Feng G, Wei S, Zhang F, Karthik L, Li Z (2016) Inhabitancy of active Nitrosopumilus-like ammonia-oxidizing archaea and Nitrospira nitrite-oxidizing bacteria in the sponge Theonella swinhoei. Sci Rep 6:24966
George S, Chellapandian M, Sivasankar B, Jayaraman K (1997) A new process for the treatment of fertilizer effluent using immobilized urease. Bioprocess Eng 16:83–85
Geweely NSI (2006) Purification and characterization of intracellular urease enzyme isolated from Rhizopus oryzae. Biotechnology 5:358–364
Hirayama C, Sugimura M, Saito H, Nakamura M (2000) Purification and properties of urease from the leaf of mulberry, Morus alba. Phytochemistry 53:325–330
Jahns T (1995) Purification and properties of urease from Sporobolomyces roseus. Anton van Leeuw 68:209–214
Jones BD, Mobley HL (1988) Proteus mirabilis urease: genetic organization, regulation, and expression of structural genes. J Bacteriol 170:3342–3349
Kakimoto S, Sumino Y, Kawahara K, Yamazaki E, Nakatsui I (1990) Purification and characterization of acid urease from Lactobacillus fermentum. Appl Microbiol Biotechnol 32:538–543
Kester DR, Duedall IW, Connors DN, Pytkowicz RM (1967) Preparation of artificial seawater. Limnol Oceanogr 12:176–179
Kostecka-Madalska O, Noculak A (1973) Urease activity in seeds of Cucurbitaceae and Papilionaceae plants. Acta Pol Pharm 30:533–537
Krajewska B (2009) Ureases I. Functional, catalytic and kinetic properties: a review. J Mol Catal B Enzym 59:9–21
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Liu Q, Chen Y, Yuan M, Du G, Chen J, Kang Z (2017a) A Bacillus paralicheniformis iron-containing urease reduces urea concentrations in rice wine. Appl Environ Microbiol 83:e01258–e1317
Liu X, Zhang Q, Zhou N, Tian Y (2017b) Expression of an acid urease with urethanase activity in E. coli and analysis of urease gene. Mol Biotechnol 59:84–97
Lubbers MW, Rodriguez SB, Honey NK, Thornton RJ (1996) Purification and characterization of urease from Schizosaccharomyces pombe. Can J Microbiol 42:132–140
Mcdonald JA, Vorhaben JE, Campbell JW (1980) Invertebrate urease: purification and properties of the enzyme from a land snail, Otala lactea. Comp Biochem Physiol Part B Comp Biochem 66:223–231
Menegassi A, Wassermann GE, Olivera-Severo D, Becker-Ritt AB, Martinelli AH, Feder V, Carlini CR (2008) Urease from cotton (Gossypium hirsutum) seeds: isolation, physicochemical characterization, and antifungal properties of the protein. J Agric Food Chem 56:4399–4405
Mirbod F, Schaller RA, Cole GT (2002) Purification and characterization of urease isolated from the pathogenic fungus Coccidioides immitis. Med Mycol 40:35–44
Mobley HL, Hausinger RP (1989) Microbial ureases: significance, regulation, and molecular characterization. Microbiol Rev 53:85–108
Mobley HL, Jones BD, Jerse AE (1986) Cloning of urease gene sequences from Providencia stuartii. Infect Immun 54:161–169
Mobley HL, Island MD, Hausinger RP (1995) Molecular biology of microbial ureases. Microbiol Rev 59:451–480
Palinska KA, Jahns T, Rippka R, Tandeau De Marsac N (2000) Prochlorococcus marinus strain PCC 9511, a picoplanktonic cyanobacterium, synthesizes the smallest urease. Microbiol 146:3099–3107
Phillips A, Pretorius GH, Du Toit PJ (1991) A survey of yeast ureases and characterisation of partially purified Rhodosporidium paludigenum urease. FEMS Microbiol Lett 63:21–25
Prakash O, Bhushan G (1997) Isolation, purification and partial characterisation of urease from seeds of water melon (Citrullus vulgaris). J Plant Biochem Biotechnol 6:45–47
Preininger C (1999) The enzymatic determination of mercury and copper using acid urease. The effects of buffers. Microchim Acta 130:209–214
Qin Y, Cabral JMS (2002) Properties and applications of urease. Biocatal Biotransform 20:1–14
Rai AK (1989) Purification and properties of urease from a cyanobacterium Anabaena doliolum. FEMS Microbiol Lett 61:319–322
Rando D, Steglitz U, Morsdorf G, Kaltwasser H (1990) Nickel availability and urease expression in Proteus mirabilis. Arch Microbiol 154:428–432
Rodriguez-Marconi S, De la Iglesia R, Diez B, Fonseca CA, Hajdu E, Trefault N (2015) Characterization of bacterial, archaeal and eukaryote symbionts from Antarctic sponges reveals a high diversity at a three-domain level and a particular signature for this ecosystem. PLoS ONE 10:e0138837
Sharma V, Chaudhary R, Khurana JM, Muralidhar K (2008) In-gel detection of urease activity by nitroprusside-thiol reaction. Phytochem Anal 19:99–103
Simmons TL, Coates RC, Clark BR, Engene N, Gonzalez D, Esquenazi E, Dorrestein PC, Gerwick WH (2008) Biosynthetic origin of natural products isolated from marine microorganism-invertebrate assemblages. P NATL ACAD SCI USA 105:4587–4594
Smith PT, King AD, Goodman N (1993) Isolation and characterization of urease from Aspergillus niger. J Gen Microbiol 139:957–962
Su J, Jin L, Jiang Q, Sun W, Zhang F, Li Z (2013) Phylogenetically diverse ureC genes and their expression suggest the urea utilization by bacterial symbionts in marine sponge Xestospongia testudinaria. PLoS ONE 8:e64848
Thompson JD, Higgins DG, Gibson TJ (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucl Acids Res 22:4673–4680
Todd MJ, Hausinger RP (1987) Purification and characterization of the nickel-containing multicomponent urease from Klebsiella aerogenes. J Biol Chem 262:5963–5967
Turbett GR, Høj PB, Horne R, Mee BJ (1992) Purification and characterization of the urease enzymes of Helicobacter species from humans and animals. Infect Immun 60:5259–5266
Weatherburn MW (1967) Phenol-hypochlorite reaction for determination of ammonia. Anal Chem 39:971–974
Wittekindt E, Werner M, Reinicke A, Herbert A, Hansen P (1996) A microtiter-plate urease inhibition assay-sensitive, rapid and cost-effective screening for heavy metals in water. Environ Technol Lett 17:597–603
Yang Y, Kang Z, Zhou J, Chen J, Du G (2015) High-level expression and characterization of recombinant acid urease for enzymatic degradation of urea in rice wine. Appl Microbiol Biotechnol 99:301–308
Yao LL, Ye BC (2016) Reciprocal regulation of GlnR and PhoP in response to nitrogen and phosphate limitations in Saccharopolyspora erythraea. Appl Environ Microbiol 82:409–420
Zhylyak GA, Dzyadevich SV, Korpan YI, Soldatkin AP, El'Skaya AV (1995) Application of urease conductometric biosensor for heavy-metal ion determination. Sens Actuators B Chem 24:145–148
Acknowledgements
This work was supported by the National Key Research and Development Program of China (2018YFC030980504). The authors would like to thank BiotechPack Co. Ltd for the determination of N-terminal amino acid sequence.
Author information
Authors and Affiliations
Contributions
CL finished the experiments and wrote the manuscript, YX analyzed the data, YX wrote the manuscript, ZL designed the experiments and revised the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Animal and human rights statement
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Edited by Chengchao Chen.
Rights and permissions
About this article
Cite this article
Liu, C., Xiao, Y., Xiao, Y. et al. Marine urease with higher thermostability, pH and salinity tolerance from marine sponge-derived Penicillium steckii S4-4. Mar Life Sci Technol 3, 77–84 (2021). https://doi.org/10.1007/s42995-020-00076-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42995-020-00076-6