Abstract
Site fidelity is commonly observed in pinnipeds and has direct consequences for individual space use and population dynamics. Here, we used photo-identification recapture data to quantify site fidelity of the endangered Saimaa ringed seal (Pusa hispida saimensis) over four successive moulting seasons. We identified 337 seals based on their permanent fur patterns, and 192 of them were observed during at least 2 years. Over the study period, the median number of terrestrial haul-out sites used by an individual seal was four, and nearly 50% of the seals reused them over the years. Although eight seals performed movements (up to 48 km) between the different water basins of Lake Saimaa, most of the studied seals remained in the same water basin over the years. The median distance between successive moulting sites used by an individual seal was 643 m. While these distances were similar within years in both sexes, the distances between years were longer in females, suggesting post-nursing related behaviour. The extreme site fidelity of the Saimaa ringed seal has important implications for its conservation, especially in the land use management of the Lake Saimaa shoreline.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Site fidelity is defined as the tendency of animals to return to previously occupied locations in one or more stages of their life history (Switzer 1993). It has been documented for many migratory and non-migratory species (Greenwood 1980). Site fidelity is particularly common in terrestrial habitats, but it also occurs among many aquatic species, such as highly mobile pinnipeds. It may vary across multiple spatial and temporal scales, including very fine-scaled fidelity to breeding and moulting sites, daily to seasonal foraging areas or relatively coarse-scale annual and seasonal migration. By returning to familiar locations, individuals may benefit from more efficient exploitation of resources and improved reproductive success or survival (e.g., Campbell et al. 2008; Kelly et al. 2010; Oksanen et al. 2014; Cordes and Thompson 2015; Koivuniemi et al. 2016). Site fidelity can also have population-level effects, for example, on metapopulation dynamics (Matthiopoulos et al. 2005), social structure (Wolf and Trillmich 2007) and gene flow (Valtonen et al. 2012, 2014). While phocids are primarily aquatic, critical parts of their biological cycle—breeding, nursing and moulting—are connected to ice or terrestrial substrates. Therefore, understanding phocid site fidelity patterns in the use of these habitats, which are often threatened by anthropogenic activities, is important for their conservation.
Traditionally, various aspects of pinniped spatial ecology have been studied with telemetry (e.g., McConnell et al. 1992; Bjorge et al. 2002; Cunningham et al. 2009; Oksanen et al. 2014) or methods based on individual identification, such as flipper tagging (Pomeroy et al. 2000), painting (Griben et al. 1984), branding (Walker et al. 2010), genetics (Valtonen et al. 2014) and, more recently, photo-identification (Photo-ID) (Karlsson et al. 2005; Graham et al. 2011; Hastings et al. 2012; Koivuniemi et al. 2016; Sayer et al. 2019). Photo-ID is a non-invasive method, which relies on individual recognition via permanent natural markings, such as scars or pelage patterns. This approach can be applied to a wide range of studies, for instance, estimation of population size (Hiby et al. 2007; Koivuniemi et al. 2019) and reproductive success (Thompson and Wheeler 2008). It is also a powerful tool in the assessment of the degree of site fidelity (Cordes and Thompson 2015; Koivuniemi et al. 2016).
The Saimaa ringed seal (Pusa hispida saimensis, Appendix Fig. A1) is an endemic subspecies of ringed seal that is landlocked in Lake Saimaa, a freshwater lake in Eastern Finland (Fig. 1). Its current estimated population size is ~ 400 individuals (Metsähallitus 2020). Primary threats to the Saimaa ringed seal include incidental by-catch mortality, habitat loss and climate change (Sipilä 2003; Auttila 2015; Jounela et al. 2019; Kunnasranta et al. 2021). Compared to its marine relatives (Härkönen et al. 2008; Kelly et al. 2010; Oksanen et al. 2015), Saimaa ringed seal’s relatively small home range and its high site fidelity (e.g., Koskela et al. 2002; Koivuniemi et al. 2016; Niemi et al. 2012, 2019) are unparalleled in any other pinniped species and reflect the unique life-history of this endangered seal. During the last 9,500 years of being landlocked (Nyman et al. 2014), Saimaa ringed seals have adopted various strategies to cope with their lacustrine habitat.
Density of the Saimaa ringed seal moulting sites. The numbers on the map correspond to the different water basins composing Lake Saimaa. 1: Pohjois–Saimaa (PS), 2: Haukivesi (HV), 3: Joutenvesi (JV), 4: Pyyvesi–Enonvesi (PEV), 5: Kolovesi (KV), 6: Pihlajavesi (PV), 7: Puruvesi (PUV), 8: Katosselkä–Lepistönselkä–Haapaselkä (KS), 9: Lietvesi–Luonteri (LL), 10: Etelä–Saimaa (ES). The shaded areas are part of the European Union network of nature protected areas, Natura 2000
While arctic ringed seals are ice obligates (Kelly 2001) and depend on sea ice for breeding, resting and moulting, in Lake Saimaa, they give birth in snow lairs located along shorelines (Sipilä 1990) instead of marine ice pressure ridges (Smith and Stirling 1975). Moreover, the ice cover melts in early spring. Consequently, Saimaa ringed seals moult on terrestrial platforms, such as flat rocks on the shores of islands. They also use these for resting at night after moulting (Kunnasranta et al. 2002; Niemi et al. 2013a). Terrestrial habitat is, therefore, crucial for the Saimaa ringed seal. The subspecies is strictly protected through Annex II of the European Union Habitats Directive, in which article 12 especially frames the protection of its breeding and resting sites (Council directive 1992). Yet, anthropogenic land use is increasingly degrading and fragmenting ringed seal habitat in Lake Saimaa (Liukkonen et al. 2017). Therefore, understanding Saimaa ringed seal terrestrial habitat use, including site fidelity patterns, is essential for the design of conservation measures to ensure adequate protection of terrestrial moulting and resting habitat.
Ringed seals have distinctive and permanent fur patterns, which enables identification of individuals through their lifetime (Koivuniemi et al. 2016). In this study, our primary objectives were to investigate the moulting behaviour of the Saimaa ringed seal using the Photo-ID method, with the focus on estimating the degree of moulting site fidelity. For the first time, we include the entire distribution range of the Saimaa ringed seal to characterize their site fidelity at the population scale. We aim to quantify the movement patterns during the moulting seasons and the spatial and temporal factors affecting them. Finally, we discuss how these findings should be considered in the conservation of the Saimaa ringed seal.
Methods
Lake Saimaa (61° 05´ to 62° 36´ N, 27° 15´ to 30° 00´ E) is the largest lake of Finland extending over approximately 4,400 km2. The labyrinthine lake has a mean depth of 12 m and comprises 13,700 islands (Kuusisto 1999) and ten different water basins (Fig. 1). We collected Photo-ID data from 1 April through 30 June, spanning the seals’ annual moulting season, between 2016 and 2019. Our Photo-ID team (average of 17 photographers per year) covered the entire distribution range of the Saimaa ringed seal, which includes some 70% of the lake (Niemi et al. 2012) (see Supplementary Table S1 for details). We conducted surveys on outboard motor boats (< 6 m) by photographing the seals using digital single-lens reflex cameras with telephoto lenses (up to 300 mm). GPS coordinates of each seal location were recorded for every sighting. In addition, we set up game camera traps (average of 53 locations per year; Scout Guard 550VB and 560 K-8, UoVision UV785 superb full HD 12 MP) at some moulting sites mainly in Pihlajavesi (PV) water basin (Fig. 1 area 6). Game cameras were set to motion trigger activation and to take two pictures over a 0.5–2 min time span (see details in Koivuniemi et al. 2016), with the exception of 2019 when they were set to time lapse, two pictures every 10 min.
We stored the image data into a Wildbook (https://www.wildbook.org/doku.php) based catalogue (https://norppagalleria.wwf.fi/) managed since 2010. We identified individual seals by visually matching their unique ring-shaped fur patterns to images in the catalogue. We also determined the sex from ventral photographs when available. We identified nursing females by the presence of a pup in photographs taken immediately prior to and during the Photo-ID data collection period previously described. In addition, the data set was supplemented by images, only if associated with precise GPS coordinates, collected by the members of the public, who directly submitted them to the Norppagalleria platform.
For the site fidelity analyses, we only included individuals that had been photographed during at least two of the 4 years. For each individual seal, we merged moulting site locations that were within < 50 m of each other to account for a potential error of the GPS device and observer assessment of seal location. However, if the locations were situated clearly on different islands, different shorelines or bays of islands within this 50 m range, they were treated as distinct sites. For each seal, we calculated the number of years that it was observed, the number of overall sightings and the number of sightings per year. Furthermore, we calculated the number of different moulting sites used per seal as well as the number of sites used within each year. In addition, we determined the total number of seals observed per year, the portion of individuals reusing the same moulting sites over the years and the number of seals sharing moulting sites.
As common measures of site fidelity are difficult to apply in a restricted lake system, we assessed, in this study, site fidelity by calculating the distance between each successive moulting site (within and between years) for each individual seal, using the R package geosphere (Hijmans 2019). We tested the effect of sex (Sex), water basin (Water_Basin), year (Year, between or within) and nursing (Pup, absence or presence) on the distance between successive moulting sites (logarithm of distance, log_Distance) using the R package glmmTMB (Brooks et al. 2017). We accounted for individual variation (and pseudoreplication) in distances by adding a random intercept of seal ID (1|Seal_ID) in the models. We used Akaike’s Information Criterion (AIC) values to select the variables that best explained the variation in the distances, based on maximum likelihood estimation (REML = FALSE). Individuals of unknown sex were excluded from the models as including them would not have been biologically meaningful. We ran models with Gaussian, Gamma and Tweedie distributed errors on untransformed data to find the best fitting distribution. In addition, we ran a Gaussian error model on log-transformed distances after converting zero distances (46 cases, where the seal used the same site in successive years, resulting in a distance between successive moulting sites of 0 m) to 10 m to normalize the error residuals. We chose the model with the best fitting error distribution by inspecting the error residual plots drawn with the function ‘check_model’ from the R package performance (Lüdecke et al. 2020).
Because of the low number of seals observed in other water basins, only the data from the main distribution areas—Haukivesi (Fig. 1 area 2) and Pihlajavesi (Fig. 1 area 6)—were included to further investigate the combined influence of sex and water basin (as an interaction of the two) on the distance between moulting sites. We ran a Gaussian error model on the previously described log-transformed distances and verified the fit by inspecting the error residual plots. Finally, the effect of nursing was examined using only females from all water basins.
Results
A total of 337 individual seals were identified from 2164 sightings (36% from game cameras) containing sufficient data for identification. We were able to determine the sex of 102 females and 69 males, but the sex of 166 seals remained undetermined. In total, 192 seals (76 females, 55 males and 61 unknown) were sighted during at least two of the 4 years. As expected, the median number of seals observed per year was the highest in Pihlajavesi (61) and Haukivesi (34) water basins (Table S2), corresponding to the current abundance estimates (Metsähallitus 2020). We identified 788 different moulting sites, the majority of which were located in the Pihlajavesi (44%) and Haukivesi (29%) water basins (Table S3). The total number of sites observed per year was relatively consistent over the study period (2016 = 233; 2017 = 193; 2018 = 181; 2019 = 181). Overall, 53 moulting sites were used by multiple seals, mainly within the same year (87% of the cases). This involves a total of 73 seals (32 females, 24 males and 17 unknown), but does not systematically imply that more than one seal was observed simultaneously at a given site. The median number of moulting sites used by an individual was four (range 1–13), while the median number per year was one (range 1–7). Almost half of the seals (49.5%) used at least one (range 1–3) specific site during more than 1 year.
The median distance between the successive moulting sites used by individual seals (N = 192) was 643 m (range 0–47,550 m) (Table S4, Fig. A2). The median distance within years was shorter (494 m) than the median distance between years (982 m). In the mixed models that accounted for the random effect of the individual seal, the Gaussian distribution (and log-transformed response) had the best fit to the data out of all the candidate models with no observed significant lack of fit in the error residuals. The best GLMM fitted to the data set of successive distances from males and females from all water basins included the factors Water_Basin, Sex and Year as well as the Sex*Year interaction. Of the fixed effects, Water_Basin had the greatest influence on the successive distance, with medians ranging from 109 m in Kolovesi (Fig. 1 area 5) to 23,103 m in Haukivesi/Pihlajavesi (Fig. 1 movements between area 2 and 6) (Table A1, Fig. 2A). Eight individuals (five females and three males) moved between water basins, resulting in the longest median distances. When omitting these individuals, the maximum median drops to 2,058 m in Joutenvesi (Fig. 1 area 3). The Sex*Year interaction was also significant; while the distances within years were relatively similar in both sexes (females median: 445 m; males median: 594 m), the distances between years were longer in females than in males, with median distances of 126 m and 842 m, respectively (Fig. 2B).
Distance between successive moulting sites used by known sex Saimaa ringed seals between 2016 and 2019. A Comparison between the different water basins composing Lake Saimaa. Abbreviation of the water basins: PS = Pohjois–Saimaa, KV = Kolovesi, JV = Joutenvesi, HV = Haukivesi, PV = Pihlajavesi, KS = Katosselkä–Lepistönselkä–Haapaselkä, LL = Lietvesi–Luonteri, PUV = Puruvesi, ES = Etelä–Saimaa. Abbreviations separated by a slash correspond to movements between the two water basins. The numbers above each water basin correspond to the number of individuals within each water basin. B Comparison between male and female for the whole Lake Saimaa. The “Between” series represents the distances between successive moulting sites used in different years. The “Within” series represent the distances between successive moulting sites used within the same year. The numbers above each sex categories correspond to the number of individuals within each sex group. C Comparison between male and female for the main distribution area of the Saimaa ringed seal. Abbreviation of the water basins: HV = Haukivesi, PV = Pihlajavesi. The numbers above each sex categories correspond to the number of individuals within each sex group for each water basin. The figure was drawn using the R package ggplot2 (Wickham 2016)
The best fitting model including only Haukivesi and Pihlajavesi data comprised the factors Water_Basin and Year and their interactions with Sex and accounted for the random effect of the individual seal. Distances in Haukivesi were longer than in Pihlajavesi, with a median of 880 m compared to a median of 473 m (Table A2). Females moved shorter distances between their moulting sites within years compared to between years (median of 416 m and 1,350 m, respectively), while male distances were, in general, more constant (median of 585 m and 626 m, respectively) (Fig. 2C). Both males and females moved longer distances between their moulting sites in Haukivesi (median of 917 m and 829 m, respectively) compared to Pihlajavesi (median of 486 m and 450 m, respectively). However, the Sex*Water_Basin interaction was not statistically significant.
The best fitting model including only females from all the water basins comprised the factors Water_Basin, Year and Pup. Distances were shorter within years (445 m) compared to between years (1,126 m) (Table A3). Water basins had an effect on the distances between moulting sites, with medians ranging from 104 m in Kolovesi (Fig. 1 area 5) to 14,676 m in Pihlajavesi/Puruvesi (Fig. 1 movements between area 6 and 7). However, nursing did not have an effect on the distances between moulting sites.
Discussion
Over the study period of 4 years, individual seals used a median of four moulting sites, and nearly half of the individuals reused at least one site. Some seals even used the exact same rocks during several successive years. In addition, the median distance between successive moulting sites was just over half a kilometre. Our study, which incorporates data from the whole distribution area, not only confirms earlier findings on the site fidelity of the Saimaa ringed seal (Koskela et al. 2002; Valtonen et al. 2012; Koivuniemi et al. 2016) but further defines the extreme extent of such behaviour. Moreover, our results bring additional evidence to the site fidelity behaviour of the ringed seal in general (McLaren 1958; Smith and Hammill 1981; Härkönen et al. 2008). Kelly et al. (2010) reported an analogous degree of high site fidelity in Arctic ringed seals, where several individuals returned to the vicinity of their breeding sites (3–54 km) in the following year after moving up to 1,800 km. However, in the case of Saimaa ringed seals, the specificity of their habitat and the scale in which such behaviour is observed suggest extreme fidelity to moulting sites.
It is important to consider that the definition of moulting site fidelity and the scale of home range applied to marine populations (Kelly et al. 2010) may not be applicable in the case of the Saimaa ringed seal due to the restricted yet highly complex lake habitat. In fact, a similar spatial range, which in a more open marine environment would typically represent only one site, may cover five or more different moulting sites at different islands in the case of the Saimaa ringed seal (Fig. 3). Therefore, in this study, we estimate site fidelity by quantifying the distance between moulting sites, which provides a fine-scale measure of fidelity, more suitable for the lake system. We found that the distances between moulting sites were 400 m less in Pihlajavesi than in Haukivesi. This may be attributable to the distinctive landscapes of these two basins, with the labyrinthine archipelago of Pihlajavesi offering a higher density of potential haulout locations, thus underlining the effect of a restricted but complex habitat on reducing the moulting range (i.e., higher site fidelity). Because of the restricted lake system, sense of scale is primordial when studying the behaviour of the Saimaa ringed seal. For instance, the average post-moult home range of Baltic ringed seals (P. h. botnica) is almost twice as big as the surface area of Lake Saimaa (Oksanen et al. 2015), while it is only 90 km2 for Saimaa ringed seals (Niemi et al. 2012). Our results further emphasize that Saimaa ringed seal behaviour plays out at a fine scale, especially when considering their moulting range. This is accentuated by the fact that nearly half of the seals in this study were re-using the exact same locations throughout the years. We suggest that this extremely fine-scale site fidelity could be the adaptational result from the highly labyrinthine and limited lake habitat.
Example of four Saimaa ringed seals using multiple moulting sites located at different islands within the restricted yet highly complex lake habitat. Individuals Phs125 and Phs149 are males; individuals Phs164 and Phs174 are females. White areas are land. This map represents a small area of Pihlajavesi (PV) water basin
Our study revealed that the females’ distances between successive moulting sites were larger between years (1,126 m) compared to within years (445 m), while no similar effect was observed for males. This could be explained by the reproductive status of females. Females’ breeding and moulting areas are not necessarily the same, and females may leave their weaned pups and breeding sites to moult in different locations. Saimaa ringed seal pups tend to stay in their nursing area shortly after weaning (Niemi et al. 2013b), which may induce females to leave the site. Moreover, Reder et al. (2003) reported female dispersion and behavioural changes following lactation in high Arctic harbour seals (Phoca vitulina vitulina). Therefore, we could have observed nursing females in their breeding sites before they moved to their moulting locations, leading to larger distances between years. However, the low number of confirmed reproductive status of females in our study may hinder our ability to make definitive conclusions. Female ringed seals nurse their offspring in snow lairs (Sipilä 1990) that are difficult to monitor before the moulting season. Thus, a substantial number of them might have been misidentified as non-nursing in our study. Besides, females may not give birth every year (Sipilä 2003), which makes the reproductive status designation even more challenging. Finally, we may lack post-weaning observations of identified nursing females and thus underestimate the movements within years that would be expected. Further research combining Photo-ID and genetics, based on placentas for instance, could help to better understand the effect of nursing on female moulting site fidelity.
Our study shows that Saimaa ringed seals exhibit restricted moulting range, as the majority of the seals had their interannual moulting sites situated within a few kilometres. We observed only a few movements across water basins, with only eight individuals undertaking trips of up to 50 km. Such sporadic trans-basin movements of adults (see also Niemi et al. 2012) are an interesting phenomenon, because they are more typical of juveniles (Niemi et al. 2013b). Most of these movements took place over two moulting seasons, which may reflect a recolonisation of previously occupied habitats. One example is a female that travelled approximately 30 km from Pihlajavesi to Puruvesi and subsequently gave birth there in 2018, which was the first pup for that region for several decades (Metsähallitus 2020).
Unlike many other pinnipeds that gather in colonies for breeding or moulting, ringed seals are generally considered solitary (McLaren 1958). Exceptions to this include some low latitude ringed seals from Lake Ladoga (P. h. ladogensis) in Russia (Sipilä et al. 1996; Kunnasranta et al. 1996, 2001), and to some extent from the Baltic Sea (Härkönen et al. 1998), that are occasionally found hauling out in gregarious groups. Although our study reveals that Saimaa ringed seals are usually solitary, almost 40% of the seals observed for at least 2 years were using the same moulting sites with one or two of their conspecifics, suggesting that some associative behaviour might also exist within Lake Saimaa.
The Photo-ID method, which was used to collect data in this study, was originally implemented for population abundance estimation (Koivuniemi et al. 2019), as the traditional lair census method (see Sipilä 2003) is hampered by mild winters. However, our study has shown that it has further applications in the research on other aspects of seal ecology and is a well-suited method for a complex and unique environment, such as Lake Saimaa. Telemetry is a powerful tool for the monitoring of individuals at a very fine spatio-temporal scale and has revealed information on the home range and habitat use of Saimaa ringed seals (Hyvärinen et al. 1995; Koskela et al. 2002; Kunnasranta et al. 2002; Niemi et al. 2012, 2013a, 2019). However, the applicability of such a method during the moulting season and on a larger scale is financially, technically and logistically challenging. For instance, the tag will detach as the seal renews its fur annually. Thus, the Photo-ID method is the most appropriate tool, up to date, to study seals during the moulting season in their whole distribution area. Seal sightings are also reported year-round by the public through a citizen science programme, opening perspectives to use this technique in a larger time frame. Nonetheless, manual matching of thousands of images is time and labour consuming, and an automatic identification tool that takes variations in seal posture into consideration is urgently needed. Some preliminary developments with automatic matching approaches have already been carried out (Zhelezniakov et al. 2015; Chehrsimin et al. 2017; Nepovinnykh et al. 2020) but are still in progress.
Understanding site fidelity patterns of the Saimaa ringed seal, occurring in both breeding (Valtonen et al. 2012; Niemi et al. 2019) and moulting seasons (Koskela et al. 2002; Koivuniemi et al. 2016; this study), is of paramount importance for the effective implementation of conservation actions. High site fidelity and restricted ranging patterns makes marine mammals more prone to population declines due to local anthropogenic threats, such as habitat degradation and human-caused mortalities (e.g., Rojas-Bracho et al. 2006; Campbell et al. 2008; Atkins et al. 2016). These risks are even more acute for the Saimaa ringed seal because of its restricted lake habitat and high proximity to humans. While it is commonly accepted that ringed seals are most vulnerable to disturbance during the breeding season (Sipilä 2003), we would also like to highlight their sensitivity during the moulting season, which is considered as an energetically demanding period (Paterson et al. 2012). Niemi et al. (2013a) also reported that Saimaa ringed seals rest in the same terrestrial platforms outside of the moulting season, which further highlights their importance as a key habitat. Our study not only emphasizes the high conservation value of the current haul-out sites in the core distribution area (see Fig. 1) but further underlines the need to extend the protection to all suitable habitats and safeguard future recolonisation of previously used areas. Indeed, the effective conservation measures implemented for decades have resulted in the Saimaa ringed seal population growth, which will logically expand its range from the current core areas to more peripheral areas of the lake. However, in the context of climate change, together with low genetic diversity (Valtonen et al. 2014) and limited suitable lake habitat (Liukkonen et al. 2017), the high site fidelity makes this subspecies especially vulnerable to changes in its environment.
This study, together with earlier findings, shows that Saimaa ringed seals exhibit strong site-fidelity and relatively limited mobility across water basins. Such affiliation to their breeding and moulting areas is already affected by habitat degradation and disturbance resulting from growing human activities. High site fidelity of this subspecies is a major biological factor that should be considered in the conservation strategies that integrate anthropogenic activities, particularly in land use planning, for safeguarding key habitats of the Saimaa ringed seal.
Availability of data and materials
The data sets generated and analysed during the current study are not publicly available due to the protection by law of the location of the haulout sites of the Saimaa ringed seal but are available from corresponding authors on reasonable request.
Change history
22 December 2022
Supplementary Information was updated.
References
Atkins S, Cantor M, Pillay N, Cliff G, Keith M, Parra GJ (2016) Net loss of endangered humpback dolphins: Integrating residency, site fidelity, and bycatch in shark nets. Mar Ecol Prog Ser 555:249–260. https://doi.org/10.3354/meps11835
Auttila M (2015) The endangered Saimaa ringed seal in a changing climate—challenges for conservation and monitoring. PhD thesis, University of Eastern Finland, Finland.
Bjorge A, Bekkby T, Bryant EB (2002) Summer home range and habitat selection of harbor seal (Phoca vitulina) pups. Mar Mamm Sci 18:438–454. https://doi.org/10.1111/j.1748-7692.2002.tb01047.x
Brooks ME, Kristensen K, van Benthem KJ, Magnusson A, Berg CW, Nielsen A, Skaug HJ, Maechler M, Bolker BM (2017) glmmTMB Balances Speed and Flexibility Among Packages for Zero-inflated Generalized Linear Mixed Modeling. R J 9:378–400. https://doi.org/10.3929/ethz-b-000240890
Campbell RA, Gales NJ, Lento GM, Baker CS (2008) Islands in the sea: extreme female natal site fidelity in the Australian sea lion, Neophoca cinerea. Biol Lett 4:139–142. https://doi.org/10.1098/rsbl.2007.0487
Chehrsimin T, Eerola T, Zhelezniakov A, Koivuniemi M, Auttila M, Levänen R, Niemi M, Kunnasranta M, Kälviäinen H (2017) Automatic individual identification of Saimaa ringed seals. IET Comput Vision. https://doi.org/10.1049/iet-cvi.2017.0082
Cordes LS, Thompson PM (2015) Mark-resight estimates of seasonal variation in harbor seal abundance and site fidelity. Popul Ecol 57:467–472. https://doi.org/10.1007/s10144-015-0496-z
Council Directive 92/43/EEC of 21 May 1992 on the conservation of natural habitats and of wild fauna and flora OJ L 206, 22.7.1992. http://data.europa.eu/eli/dir/1992/43/oj. Accessed 15 Oct 2020
Cunningham L, Baxter JM, Boyd IL, Duck CD, Lonergan M, Moss SE, McConnell B (2009) Harbour seal movements and haul-out patterns: implications for monitoring and management. Aquat Conserv 19:398–407. https://doi.org/10.1002/aqc.983
Graham IM, Harris RN, Matejusova I, Middlemas SJ (2011) Do ‘rogue’ seals exist? Implications for seal conservation in the UK. Anim Conserv 14:587–598. https://doi.org/10.1111/j.1469-1795.2011.00469.x
Greenwood PJ (1980) Mating systems, philopatry and dispersal in birds and mammals. Anim Behav 28:1140–1162. https://doi.org/10.1016/S0003-3472(80)80103-5
Griben MR, Johnson HR, Gallucci BB, Gallucci VF (1984) A New Method to Mark Pinnipeds as Applied to the Northern Fur Seal. J Wildl Manag 48:945–949. https://doi.org/10.2307/3801444
Härkönen T, Stenman O, Jussi M, Jussi I, Sagitov R, Verevkin M (1998) Population size and distribution of the Baltic ringed seal (Phoca hispida botnica). In: Heide-Jørgensen M-P, Lydersen C (eds) Ringed seals of the North Atlantic. NAMMCO Sci Publ, Tromsø, pp 167–180. https://doi.org/10.7557/3.2986
Härkönen T, Jüssi M, Jüssi I, Verevkin M, Dmitrieva L, Helle E et al (2008) seasonal activity budget of adult baltic ringed seals. PLoS ONE 3:2006. https://doi.org/10.1371/journal.pone.0002006 (pmid:18414676)
Hastings KK, Small RJ, Pendelton GW (2012) Sex- and age-specific survival of harbor seals (Phoca vitulina) from Tugidak Island, Alaska. J Mamm 93:1368–1379. https://doi.org/10.1644/11-MAMM-A-291.1
Hiby L, Lundberg T, Karlsson O, Watkins J, Jüssi M, Jüssi I, Helander B (2007) Estimates of the size of the Baltic grey seal population based on photo-identification data. NAMMCO Sci Publ 6:163–175. https://doi.org/10.7557/3.2731
Hijmans RJ (2019) geosphere: spherical trigonometry. R package version 1.5–10. https://CRAN.R-project.org/package=geosphere. Accessed 15 Oct 2020
Hyvärinen H, Hämäläinen E, Kunnasranta M (1995) Diving behavior of the Saimaa ringed seal (Phoca hispida saimensis Nordq.). Mar Mamm Sci 11:324–334. https://doi.org/10.1111/j.1748-7692.1995.tb00288.x
Jounela P, Sipilä T, Koskela J, Tiilikainen R, Auttila M, Niemi M, Kunnasranta M (2019) Incidental bycatch mortality and fishing restrictions: impacts on juvenile survival in the Endangered Saimaa ringed seal Pusa hispida saimensis. Endanger Species Res 38:91–99. https://doi.org/10.3354/esr00939
Karlsson O, Hiby L, Lundberg T, Jüssi M, Jüssi I, Helander B (2005) Photo-identification, site fidelity, and movement of female grey seals (Halichoerus grypus) between haul-outs in the Baltic Sea. Ambio 34:628–634. https://doi.org/10.1579/0044-7447-34.8.628
Kelly BP (2001) Climate change and ice breeding pinnipeds. In: Walther G-R, Burga CA, Edwards PJ (eds) “Fingerprints” of climate change. Kluwer Academic/ Plenum Publishers, New York, pp 43–55
Kelly BP, Badajos OH, Kunnasranta M, Moran JR, Martinez-Bakker M, Wartzok D, Boveng P (2010) Seasonal home ranges and fidelity to breeding sites among ringed seals. Polar Biol 33:1095–1109. https://doi.org/10.1007/s00300-010-0796-x
Koivuniemi M, Auttila M, Niemi M, Levänen R, Kunnasranta M (2016) Photo-ID as a tool for studying and monitoring the endangered Saimaa ringed seal. Endanger Species Res 30:29–36. https://doi.org/10.3354/esr00723
Koivuniemi M, Kurkilahti M, Niemi M, Auttila M, Kunnasranta M (2019) A mark–recapture approach for estimating population size of the endangered ringed seal (Phoca hispida saimensis). PLoS ONE 14:e0214269. https://doi.org/10.1371/journal.pone.0214269
Koskela JT, Kunnasranta M, Hämäläinen E, Hyvärinen H (2002) Movements and use of haul-out sites of radio-tagged Saimaa ringed seal (Phoca hispida saimensis Nordq.) during the open-water season. Ann Zool Fenn 39:59–67
Kunnasranta M, Hyvärinen H, Sorjonen J (1996) Underwater vocalizations of Ladoga ringed seals (Phoca hispida ladogensis Nordq.) in summertime. Mar Mamm Sci 12:611–618. https://doi.org/10.1111/j.1748-7692.1996.tb00076.x
Kunnasranta M, Hyvärinen H, Sipilä T, Medvedev N (2001) Breeding habitat and lair structure of the ringed seal (Phoca hispida ladogensis) in northern Lake Ladoga in Russia. Polar Biol 24:171–174. https://doi.org/10.1007/s003000000192
Kunnasranta M, Hyvärinen H, Häkkinen J, Koskela JT (2002) Dive types and circadian behaviour patterns of Saimaa ringed seals Phoca hispida saimensis during the open-water season. Acta Theriol 47:63–72. https://doi.org/10.1007/BF03193567
Kunnasranta M, Niemi M, Auttila M, Valtonen M, Kammonen J, Nyman T (2021) Sealed in a lake - biology and conservation of the endangered Saimaa ringed seal: a review. Biol Conserv 253:108908. https://doi.org/10.1016/j.biocon.2020.108908
Kuusisto E (ed) (1999) Saimaa, a living lake. Tammi, Helsinki, Finland.
Liukkonen L, Rautio A, Sipilä T, Niemi M, Auttila M, Koskela J, Kunnasranta M (2017) Long-term effects of land use on perinatal mortality in the Endangered Saimaa ringed seal population. Endanger Species Res 34:283–291. https://doi.org/10.3354/esr00856
Lüdecke D, Makowski P, Waggoner, Patil I, Ben-Shachar MS (2020). Assessment of regression models performance. CRAN. https://easystats.github.io/performance/. Accessed 15 Oct 2020
Matthiopoulos J, Harwood J, Thomas LEN (2005) Metapopulation consequences of site fidelity for colonially breeding mammals and birds. J Anim Ecol 74:716–727. https://doi.org/10.1111/j.1365-2656.2005.00970.x
McConnell BJ, Chambers C, Nicholas KS, Fedak MA (1992) Satellite Tracking of Grey Seals (Halichoerus grypus). J Zool 226:271–282. https://doi.org/10.1111/j.1469-7998.1992.tb03839.x
McLaren IA (1958) The biology of the ringed seal (Phoca hispida Schreber) in the eastem Canadian arctic. Fish Res Board Can Bull No. 118
Metsähallitus (2020) Norppakannan tila 2020 https://www.metsa.fi/luonto-ja-kulttuuriperinto/lajien-ja-luontotyyppien-suojelu/lajien-suojelu/saimaannorppa/ In Finnish. Accessed 1 Dec 2020
Nepovinnykh E, Eerola T, Kalviainen H (2020) Siamese network based pelage pattern matching for ringed seal re-identification. In: The IEEE Winter Conference on Applications of Computer Vision (WACV) Workshops, 2020, pp 25–34. https://doi.org/10.1109/WACVW50321.2020.9096935
Niemi M, Auttila M, Viljanen M, Kunnasranta M (2012) Movement data and their application for assessing the current distribution and conservation needs of the endangered Saimaa ringed seal. Endanger Species Res 19:99–108. https://doi.org/10.3354/esr00468
Niemi M, Auttila M, Valtonen A, Viljanen M, Kunnasranta M (2013a) Haulout patterns of Saimaa ringed seals and their response to boat traffic during the moulting season. Endanger Species Res 22:115–124. https://doi.org/10.3354/esr00541
Niemi M, Auttila M, Viljanen M, Kunnasranta M (2013b) Home range, survival and dispersal of endangered Saimaa ringed seal pups: implications for conservation. Mar Mamm Sci 29:1–13. https://doi.org/10.1111/j.1748-7692.2011.00521.x
Niemi M, Liukkonen L, Koivuniemi M, Auttila M, Rautio A, Kunnasranta M (2019) Winter behavior of Saimaa ringed seals: Non-overlapping core areas as indicators of avoidance in breeding females. PLoS ONE 14:e0210266. https://doi.org/10.1371/journal.pone.0210266
Nyman T, Valtonen M, Aspi J, Ruokonen M, Kunnasranta M, Palo JU (2014) Demographic histories and genetic diversities of Fennoscandian marine and landlocked ringed seal subspecies. Ecol Evol 4:3420–3434. https://doi.org/10.1002/ece3.1193
Oksanen SM, Ahola MP, Lehtonen E, Kunnasranta M (2014) Using movement data of Baltic grey seals to examine foraging-site fidelity: Implications for seal-fishery conflict mitigation. Mar Ecol Prog Ser 507:297–308. https://doi.org/10.3354/meps10846
Oksanen SM, Niemi M, Ahola MP, Kunnasranta M (2015) Identifying foraging habitats of Baltic ringed seals using movement data. Mov Ecol 3:33. https://doi.org/10.1186/s40462-015-0058-1
Paterson W, Sparling CE, Thompson D, Pomeroy PP, Currie JI, McCafferty DJ (2012) Seals like it hot: Changes in surface temperature of harbour seals (Phoca vitulina) from late pregnancy to moult. J Therm Biol 37:454–461. https://doi.org/10.1016/j.jtherbio.2012.03.004
Pomeroy PP, Twiss SD, Duck CD (2000) Expansion of a grey seal (Halichoerus grypus) breeding colony: Changes in pupping site use at the Isle of May, Scotland. J Zool 250:1–12. https://doi.org/10.1111/j.1469-7998.2000.tb00573.x
Reder S, Lydersen C, Arnold W, Kovacs KM (2003) Haulout behaviour of High Arctic harbour seals (Phoca vitulina vitulina) in Svalbard, Norway. Polar Biol 27:6–16. https://doi.org/10.1007/s00300-003-0557-1
Rojas-Bracho L, Reeves RR, Jaramillo-Legorreta A (2006) Conservation of the vaquita Phocoena sinus. Mammal Rev 36:179–216. https://doi.org/10.1111/j.1365-2907.2006.00088.x
Sayer S, Allen R, Hawkes LA, Hockley K, Jarvis D, Witt MJ (2019) Pinnipeds, people and photo identification: the implications of grey seal movements for effective management of the species. J Mar Biol Assoc UK 99:1221–1230. https://doi.org/10.1017/S0025315418001170
Sipilä T (1990) Lair structure and breeding habitat of the Saimaa ringed seal (Phoca hispida saimensis Nordq.) in Finland. Finn Game Res 47:11–20
Sipilä T (2003) Conservation biology of Saimaa ringed seal (Phoca hispida saimensis) with reference to other European seal populations. PhD thesis, University of Helsinki, Finland.
Sipilä T, Medvedev NV, Hyvärinen H (1996) The Ladoga seal (Phoca hispida ladogensis Nordq.). Hydrobiologia 322:193–198. https://doi.org/10.1007/978-94-009-1655-5_30
Smith TG, Stirling I (1975) The breeding habitat of the ringed seal (Phoca hispida). The birth lair and associated structures. Can J Zool 53:1195–1378. https://doi.org/10.1139/z75-155
Smith TG, Hammill MO (1981) Ecology of the ringed seal, Phoca hispida, in its fast ice breeding habitat. Can J Zool 59:966–981. https://doi.org/10.1139/z81-135
Switzer PV (1993) Site fidelity in predictable and unpredictable habitats. Evol Ecol 7:533–555. https://doi.org/10.1007/BF01237820
Thompson PM, Wheeler H (2008) Photo-ID-based estimates of reproductive patterns in female harbor seals. Mar Mamm Sci 24:138–146. https://doi.org/10.1111/j.1748-7692.2007.00179.x
Valtonen M, Palo J, Ruokonen M, Kunnasranta M, Nyman T (2012) Spatial and temporal variation in genetic diversity of an endangered freshwater seal. Conserv Genet 13:1231–1245. https://doi.org/10.1007/s10592-012-0367-5
Valtonen M, Palo JU, Aspi J, Ruokonen M, Kunnasranta M, Nyman T (2014) Causes and consequences of fine-scale population structure in a critically endangered freshwater seal. BMC Ecol 14:22. https://doi.org/10.1186/1472-6785-14-22
Walker KA, Mellish J-AE, Weary DM (2010) Behavioural responses of juvenile Steller sea lions to hot-iron branding. Appl Anim Behav Sci 122:58–62. https://doi.org/10.1016/j.applanim.2009.11.013
Wickham H (2016) ggplot2: elegant graphics for data analysis. Springer-Verlag, New York
Wolf JBW, Trillmich F (2007) Beyond habitat requirements: individual fine-scale site fidelity in a colony of the Galápagos sea lion (Zalophus californianus wollebaeki) creates conditions for social structuring. Oecologia 152:553–567. https://doi.org/10.1007/s00442-007-0665-7
Zhelezniakov A, Eerola T, Koivuniemi M, Auttila M, Levänen R, Niemi M, Kunnasranta M, Kälviäinen H (2015) Segmentation of Saimaa ringed seals for identification purposes. In: Bebis G, Boyle R, Parvin B, Koracin D, Pavlidis I, Feris RS, McGraw T, Elendt M, Kopper R, Ragan E, Ye Z, Weber G (eds) Advances in visual computing, Lecture Notes in Computer Science, vol, 9475. Springer, Cham, pp 227–236, https://doi.org/10.1007/978-3-319-27863-6_21
Acknowledgements
The authors wish to thank all the photographers involved in the data collection and the persons involved in the demanding manual photo-identification process, especially seal identification specialists Meeri Koivuniemi and Piia Mutka. V.B. was funded by the Maj ja Tor Nessling Foundation. This study was supported by WWF Finland, the Raija and Ossi Tuuliainen Foundation and was part of the South–East Finland–Russia CBC 2014–2020 programme funded by the European Union, the Russian Federation and the Republic of Finland. Anonymous reviewers, Handling Editor Stephen C.Y. Chan and Subject Editor Leszek Karczmarski improved this article with valuable criticisms of an earlier draft.
Funding
Open access funding provided by University of Eastern Finland (UEF) including Kuopio University Hospital. Vincent Biard was funded by the Maj ja Tor Nessling Foundation. This study was supported by WWF Finland, the Raija and Ossi Tuuliainen Foundation and was part of the South–East Finland–Russia CBC 2014–2020 programme funded by the European Union, the Russian Federation and the Republic of Finland.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Data analysis was performed by Vincent Biard and Milaja Nykänen. The first draft of the manuscript was written by Vincent Biard and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflicts of interest
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Ethics approval
Data collection was done under permits by the Finnish environmental authorities ELY centre (ESAELY/1290/2015, POKELY/1232/2015, KASELY/2014/2015 and POSELY/313/07.01/2012) and Metsähallitus (MH 5813/2013 and MH 6377/2018/05.04.01).
Additional information
Handling editors: Stephen C.Y. Chan and Leszek Karczmarski.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is a contribution to the special issue on “Individual Identification and Photographic Techniques in Mammalian Ecological and Behavioural Research – Part 2: Field Studies and Applications” — Editors: Leszek Karczmarski, Stephen C.Y. Chan, Scott Y.S. Chui and Elissa Z. Cameron.
Supplementary Information was updated.
Supplementary Information
Below is the link to the electronic supplementary material.
Appendix
Appendix
See Figs. A1, A2 and Tables A1, A2 and A3.
Photograph of a female Saimaa ringed seal (Phs229) at its moulting site in Pihlajavesi water basin. The highly labyrinthine water basin of Pihlajavesi is home to nearly half of the Saimaa ringed seal population. From mid April to mid June, the seals are moulting on terrestrial platforms, the only period when they are easily observable. Despite the thousands of islands in Lake Saimaa, the seals present restricted moulting range and often reuse the exact same locations over years. Photographer: Vincent Biard
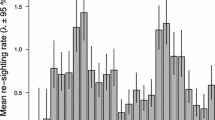
Distance between successive moulting sites used by both sexed and unsexed Saimaa ringed seals in different water basins of Lake Saimaa between 2016 and 2019. The “Between” series represents the distances between successive moulting sites used in different years. The “Within” series represent the distances between successive moulting sites used within the same year. Abbreviation of the water basins: PS = Pohjois–Saimaa, PEV = Pyyvesi–Enonvesi, KV = Kolovesi, JV = Joutenvesi, HV = Haukivesi, PV = Pihlajavesi, KS = Katosselkä–Lepistönselkä–Haapaselkä, LL = Lietvesi–Luonteri, PUV = Puruvesi, ES = Etelä–Saimaa. Abbreviations separated by a slash correspond to movements between the two water basins. Abbreviation of the sex categories: F = Females, M = Males, U = Unknown sex. The numbers above each sex categories correspond to the number of individuals within each sex group for each water basin. The figure was drawn using the R package ggplot2 (Wickham 2016)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Biard, V., Nykänen, M., Niemi, M. et al. Extreme moulting site fidelity of the Saimaa ringed seal. Mamm Biol 102, 1483–1495 (2022). https://doi.org/10.1007/s42991-021-00209-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42991-021-00209-z