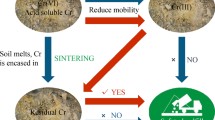
Abstract
Waste brownfield-site soils contaminated with heavy metals such as Zn and Cr are of critical environmental concern because of the rapid urbanization and industrialization that is occurring in China. Thermal treatment can fix heavy metals in specific mineral structures, which might be a promising technology for remediation and reutilization of the metal-contaminated soils. The objective of the present study was to elucidate the stabilization mechanisms of Zn and Cr through thermal treatment of mixtures of ZnO + Cr2O3 to form ZnCr2O4 and to confirm that Zn and Cr were incorporated simultaneously into the spinel structure. The incorporation efficiency for Zn was quantified, with the value ranging from 70.6 to 100% over the temperature range 700–1300°C. Leaching results further confirmed that ZnCr2O4 spinel was a superior product for Zn and Cr immobilization. Then, by artificially sintering Zn- and Cr-enriched soils, both Zn and Cr were immobilized effectively (with three orders of magnitude reduction in Zn leachability) in the ZnCr2O4 spinel as the predominant product phase. In addition, multiple heavy metals such as Zn, Cu, and Cr in the actual brownfield-site soils were well immobilized after sintering, which confirmed the potential for practical application of the thermal treatment technology utilized in this study.








Similar content being viewed by others
References
Basta, N. T., & McGowen, S. L. (2004). Evaluation of chemical immobilization treatments for reducing heavy metal transport in a smelter-contaminated soil. Environmental Pollution, 127, 73–82.
Bardos, R. P., Jones, S., Stephenson, I., Menger, P., Beumer, V., Neonato, F., Maring, L., Ferber, U., Track, T., & Wendler, K. (2016). Optimising value from the soft re-use of brownfield sites. Science of The Total Environment, 563–564, 769–782.
Bolan, N., Kunhikrishnan, A., Thangarajan, R., Kumpiene, J., Park, J., Makino, T., Kirkham, M. B., & Scheckel, K. (2014). Remediation of heavy metal(loid)s contaminated soils – to mobilize or to immobilize? Journal of Hazardous Materials, 266, 141–166.
Chen, S., Wu, Y., Cui, P., Chu, W., Chen, X., & Wu, Z. (2013). Cation distribution in ZnCr2O4 nanocrystals investigated by X-ray absorption fine structure spectroscopy. Journal of Physical Chemistry C, 117, 25019–25025.
Chen, Y., Hipel, K. W., Kilgour, D. M., & Zhu, Y. (2009). A strategic classification support system for brownfield redevelopment. Environmental Modelling & Software, 24, 647–654.
Capobianco, O., Costa, G., & Baciocchi, R. (2017). Assessment of the environmental sustainability of a treatment aimed at soil reuse in a brownfield regeneration context. Journal of Industrial Ecology, 22, 1027–1038.
Cameselle, C., & Gouveia, S. (2019). Phytoremediation of mixed contaminated soil enhanced with electric current. Journal of Hazardous Materials, 361, 95–102.
Coulon, F., Jones, K., Li, H., Hu, Q., Gao, J., Li, F., & Canning, K. (2016). China's soil and groundwater management challenges: lessons from the UK's experience and opportunities for China. Environment International, 91, 196–200.
De Kimpe, C. R., & Morel, J. (2000). Urban soil management: a growing concern. Soil Science, 165, 31–40.
Dermont, G., Bergeron, M., Mercier, G., & Richer-Laflèche, M. (2008). Soil washing for metal removal: A review of physical/chemical technologies and field applications. Journal of Hazardous Materials, 152, 1–31.
Dixit, T., Palani, I. A., & Singh, V. (2015). Investigation on the influence of dichromate ion on the ZnO nano-dumbbells and ZnCr2O4 nano-walls. Journal of Materials Science: Materials in Electronics, 26, 821–829.
Epling, W. S., Hoflund, G. B., Hart, W. M., & Minahan, D. M. (1997). Reaction and surface characterization study of higher alcoholsynthesis catalysts. Journal of Catalysis, 169, 438–446.
Fonseca, B., Figueiredo, H., Rodrigues, J., Queiroz, A., & Tavares, T. (2011). Mobility of Cr, Pb, Cd, Cu and Zn in a loamy sand soil: A comparative study. Geoderma, 164, 232–237.
Fu, F., Xie, L., Tang, B., Wang, Q., & Jiang, S. (2012). Application of a novel strategy–Advanced Fenton-chemical precipitation to the treatment of strong stability chelated heavy metal containing wastewater. Chemical Engineering Journal, 189–190, 283–287.
Gallagher, F. J., Pechmann, I., Bogden, J. D., Grabosky, J., & Weis, P. (2008). Soil metal concentrations and vegetative assemblage structure in an urban brownfield. Environmental Pollution, 153, 351–361.
Gene, S. A., Saion, E. B., Shaari, A. H., Kamarudeen, M. A., & Al-Hada, N. M. (2015). Fabrication and characterization of nano spinel ZnCr2O4 using thermal treatment method. Advanced Materials Research, 1107, 301–307.
Gingasu, D., Mindru, I., Culita, D. C., Patron, L., Calderon-Moreno, J. M., Preda, S., Oprea, O., Osiceanu, P., & Morena Pineda, E. (2014). Investigation of nanocrystalline zinc chromite obtained by two soft chemical routes. Materials Research Bulletin, 49, 151–159.
Hsu, N., Wang, S., Lin, Y., Sheng, G. D., & Lee, J. (2009). Reduction of Cr(VI) by crop-residue-derived black carbon. Environmental Science & Technology, 43, 8801–8806.
Huynh, T., Tong, A. R., Singh, B., & Kennedy, B. J. (2003). Cd-substituted goethites – a structural investigation by synchrotron X-ray diffraction. Clays and Clay Minerals, 51, 397–402.
Kadir, A. A., Hassan, M. I. H., Salim, N. S. A., Sarani, N. A., Ahmad, S., & Rahmat, N. A. I. (2018). Stabilization of heavy metals in fired clay brick incorporated with wastewater treatment plant sludge: leaching analysis. Journal of Physics: Conference Series, 995, 1–10.
Kumpiene, J., Lagerkvist, A., & Maurice, C. (2008). Stabilization of As, Cr, Cu, Pb and Zn in soil using amendments–A review. Waste Management, 28, 215–225.
Lee, S., Lee, J., Jeong Choi, Y., & Kim, J. (2009). In situ stabilization of cadmium-, lead-, and zinc-contaminated soil using various amendments. Chemosphere, 77, 1069–1075.
Li, D., Li, R., Zhu, Z., Wu, X., Liu, F., Zhao, B., Cheng, J., & Wang, B. (2018). Elemental characteristics and paleoenvironment reconstruction: a case study of the Triassic lacustrine Zhangjiatan oil shale, southern Ordos Basin, China. Acta Geochimica, 37, 134–150.
Li, X., He, C., Bai, Y., Ma, B., Wang, G., & Tan, H. (2014). Stabilization/solidification on chromium (III). wastes by C3A and C3A hydrated matrix. Journal of Hazardous Materials, 268, 61–67.
Li, X., Liu, L., Wang, Y., Luo, G., Chen, X., Yang, X., Hall, M. H. P., Guo, R., Wang, H., Cui, J., & He, X. (2013). Heavy metal contamination of urban soil in an old industrial city (Shenyang) in Northeast China. Geoderma, 192, 50–58.
Lin, Y., & Wang, S. (2012). Chromium(VI) reactions of polysaccharide biopolymers. Chemical Engineering Journal, 181–182, 479–485.
Lombi, E., Zhao, F. J., Dunham, S. J., & McGrath, S. P. (2001). Phytoremediation of heavy metal–contaminated soils. Journal of Environmental Quality, 30, 1919–1926.
Lu, X., & Shih, K. (2012). Metal stabilization mechanism of incorporating lead-bearing sludge in kaolinite-based ceramics. Chemosphere, 86, 817–821.
Luo, X., Yu, S., Zhu, Y., & Li, X. (2012). Trace metal contamination in urban soils of China. Science of the Total Environment, 421–422, 17–30.
Mao, L., Cui, H., An, H., Wang, B., Zhai, J., Zhao, Y., & Li, Q. (2014). Stabilization of simulated lead sludge with iron sludge via formation of PbFe12O19 by thermal treatment. Chemosphere, 117, 745–752.
Marinković, Z., Mančić, L., Vulić, P., & Milošević, O. (2004). The influence of mechanical activation on the stoichiometry and defect structure of a sintered ZnO-Cr2O3 system. Materials Science Forum, 453–454, 423–428.
Mulligan, C. N., Yong, R. N., & Gibbs, B. F. (2001). Remediation technologies for metal-contaminated soils and groundwater: an evaluation. Engineering Geology, 60, 193–207.
Naumkin, A.V., Kraut-Vass, A., & Powell, C.J. (2008). NIST X-ray photoelectron spectroscopy database. Measurement Services Division of the National Institute of Standards and Technology (NIST). Technology Services.
Prabhukumar, G., Matsumoto, M., Mulchandani, A., & Chen, W. (2004). Cadmium removal from contaminated soil by tunable biopolymers. Environmental Science & Technology, 38, 3148–3152.
Sarıkaya, Y., Ada, K., & Önal, M. (2008). A model for initial-stage sintering thermodynamics of an alumina powder. Powder Technology, 188, 9–12.
Shih, K., White, T., & Leckie, J. O. (2006). Nickel stabilization efficiency of aluminate and ferrite spinels and their leaching behavior. Environmental Science & Technology, 40, 5520–5526.
Smol, M., Kulczycka, J., Henclik, A., Gorazda, K., & Wzorek, Z. (2015). The possible use of sewage sludge ash (SSA) in the construction industry as a way towards a circular economy. Journal of Cleaner Production, 95, 45–54.
Snellings, R. (2015). Surface chemistry of calcium aluminosilicate glasses. Journal of the American Ceramic Society, 98, 303–314.
Song, H., Laudenschleger, D., Carey, J. J., Ruland, H., & Nolan, M. (2017). Spinel-structured ZnCr2O4 with excess Zn is the active ZnO/Cr2O3 catalyst for high-temperature methanol synthesis. ACS Catalysis, 7, 7610–7622.
Stephen, S. E., Lawrence, L. M., Roy, R. A., & Wallace, W. D. (2007). Thermodynamics of Cr2O3, FeCr2O4, ZnCr2O4, and CoCr2O4. The Journal of Chemical Thermodynamics, 39, 1474–1492.
Su, M., Liao, C., Chan, T., Shih, K., Xiao, T., Chen, D., Kong, L., & Song, G. (2017). Incorporation of cadmium and nickel into ferrite spinel solid solution: XRD and EXAFS study. Environmental Science & Technology, 52, 775–782.
Su, M., Tang, J., Liao, C., Kong, L., Xiao, T., Shih, K., Song, G., Chen, D., & Zhang, H. (2018). Cadmium stabilization via silicates formation: Efficiency, reaction routes and leaching behavior of products. Environmental Pollution, 239, 571–578.
Sun, Y., Li, Y., Xu, Y., Liang, X., & Wang, L. (2015). In situ stabilization remediation of cadmium (Cd) and lead (Pb) co-contaminated paddy soil using bentonite. Applied Clay Science, 105–106, 200–206.
Tang, Y., Chan, S., & Shih, K. (2014). Copper stabilization in beneficial use of waterworks sludge and copper-laden electroplating sludge for ceramic materials. Waste Management, 34, 1085–1091.
Tang, Y., Chui, S. S., Shih, K., & Zhang, L. (2011a). Copper stabilization via spinel formation during the sintering of simulated copper-laden sludge with aluminum-rich ceramic precursors. Environmental Science & Technology, 45, 7609–7610.
Tang, Y., & Shih, K. (2014). Mechanisms of zinc incorporation in aluminosilicate crystalline structures and the leaching behavior of product phases. Environmental Technology, 36, 2977–2986.
Tang, Y., Shih, K., Li, M., & Wu, P. (2016). Zinc immobilization in simulated aluminum-rich waterworks sludge systems. Procedia Environmental Sciences, 31, 691–697.
Tang, Y., Shih, K., Wang, Y., & Chong, T. (2011b). Zinc stabilization efficiency of aluminate spinel structure and its leaching behavior. Environmental Science & Technology, 45, 10544–10550.
Tang, Y., Xin, X., Shih, K., & Wang, Z. (2018). Effect of crystal size on zinc stabilization in aluminum-rich ceramic matrix. Journal of Material Cycles and Waste Management, 20, 2110–2116.
Velasco, P. M., Ortíz, M. M., Giró, M. M., & Velasco, L. M. (2014). Fired clay bricks manufactured by adding wastes as sustainable construction material–A review. Construction and Building Materials, 63, 97–107.
Woetzel, J., Mendonca, L., Devan, J., Negri, S., Hu, Y., Jordan, L., Li, X., Maasry, A., Tsen, G., & Yu, F. (2009). Preparing for China's urban billion. McKinsey Global Institute.
Wuana, R. A., & Okieimen, F. E. (2011). Heavy metals in contaminated soils: A review of sources, chemistry, risks and best available strategies for remediation. ISRN Ecology, 2011, 1–20.
Yao, Z., Li, J., Xie, H., & Yu, C. (2012). Review on remediation technologies of soil contaminated by heavy metals. Procedia Environmental Sciences, 16, 722–729.
Zhou, C., & Keeling, J. (2013). Fundamental and applied research on clay minerals: From climate and environment to nanotechnology. Applied Clay Science, 74, 3–9.
Zhou, C., Zhao, L., Chen, T., & He, H. (2016). Current fundamental and applied research into clay minerals in China. Applied Clay Science, 119, 3–7.
Zhou, G., Yin, X., Zhou, J., & Cheng, W. (2017). Speciation and spatial distribution of Cr in chromite ore processing residue site, Yunnan, China. Acta Geochimica, 36, 1–7.
Acknowledgments
This research was supported by the National Key Research and Development Program of China (2018YFC1801402), the Science and Technology Foundation of Guangdong, China (2016B020242006 and 2016TX03Z086), the Science and Technology Foundation of Guangzhou, China (201704020200 and 201804010197), the National Natural Science Foundations of China (U1701241, U1612442, and 21707063), and the Frontier Science Research Programme of CAS (QYZDB-SSW-DQC046).
Author information
Authors and Affiliations
Corresponding author
Additional information
This paper was originally presented during the World Forum on Industrial Minerals, held in Qing Yang, China, October 2018
Electronic supplementary material
ESM 1
(DOCX 18 kb)
ESM 2
Figure S1. TEM-EDX pattern of ZnCr2O4 for a sample calcined at 1350°C. (PNG 80 kb)
ESM 3
Figure S2. pH values of leachates after leaching by an extraction liquid with initial pH value of 2.9and a leaching period of 0.75 to 21 d. Samples for leaching experiments included (a) ZnO, ZnCr2O4, and (b) the mixtures after sintering at 700~1300°C. (PNG 313 kb)
Rights and permissions
About this article
Cite this article
Wu, F., Tang, Y., Lu, X. et al. Simultaneous Immobilization of Zn(II) and Cr(III) in Spinel Crystals from Beneficial Utilization of Waste Brownfield-Site Soils. Clays Clay Miner. 67, 315–324 (2019). https://doi.org/10.1007/s42860-019-00053-w
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42860-019-00053-w