Abstract
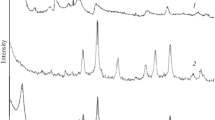
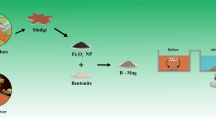
The presence of pharmaceutical pollutants in the environment is one of the most pressing environmental problems. Adsorption from solution is an effective way to remove pharmaceuticals from liquid media, but the problem then is to separate the adsorbent from the liquids. The objective of the present study was to remove nitrofurazone from aqueous solutions using a bentonite/magnetite composite, prepared by co-precipitation of magnetite with bentonite, which could then be collected by magnetic separation. The bentonite/magnetite composite was characterized using diverse techniques, such as X-ray diffraction, scanning electron microscopy, low-temperature N2 adsorption/desorption, laser diffraction, and magnetization measurements. The particle size of the composite material did not exceed 50 μm and the particle size distribution was mono-modal with a maximum at 3.2 μm. The strong hysteresis in the magnetization curve revealed that the bentonite/magnetite particles were ferromagnetic. Adsorption of nitrofurazone by the bentonite/magnetite composite from aqueous solutions was measured and the amount of nitrofurazone adsorbed was 3.2×10–2 mmol/g. The adsorption kinetics of nitrofurazone to the bentonite/magnetite composite followed a pseudo-second-order kinetics equation. Upon adsorption, hydrogen bonds were formed between the amide groups of nitrofurazone and oxygen groups in bentonite.












Similar content being viewed by others
References
Abidi, N., Duplay, J., Jada, A., Errais, E., Ghazi, M., Semhi, K., & Trabelsi-Ayadi, M. (2019). Removal of anionic dye from textile industries' effluents by using Tunisian clays as adsorbents. Ζeta potential and streaming-induced potential measurements. Comptes Rendus Chimie, 22, 113–125.
Alekseeva, O. V., Rodionova, A. N., Bagrovskaya, N. A., Agafonov, A. V., & Noskov, A. V. (2017). Effect of the organobentonite filler on structure and properties of composites based on hydroxyethyl cellulose. Journal of Chemistry, 2017, 1603937.
Alekseeva, O. V., Rodionova, A. N., Bagrovskaya, N. A., Agafonov, A. V., & Noskov, A. V. (2019). Effect of the bentonite filler on structure and properties of composites based on hydroxyethyl cellulose. Arabian Journal of Chemistry, 12, 398–404.
ALOthman, Z. A. (2012). A review: Fundamental aspects of silicate mesoporous materials. Materials, 5, 2874–2902.
Barraqué, F., Montes, M. L., Fernández, M. A., Mercader, R. C., Candal, R. J., & Sánchez, R. M. T. (2018). Synthesis and characterization of magnetic-montmorillonite and magneticorgano-montmorillonite: Surface sites involved on cobalt sorption. Journal of Magnetism and Magnetic Materials, 466, 376–384.
Bee, S.-L., Abdullah, M. A. A., Bee, S.-T., Sin, L. T., & Rahmat, A. R. (2018). Polymer nanocomposites based on silylated-montmorillonite: A review. Progress in Polymer Science, 85, 57–82.
Biosic, M., Skoric, I., Beganovic, J., & Babic, S. (2017). Nitrofurantoin hydrolytic degradation in the environment. Chemosphere, 186, 660–668.
Cazetta, A. L., Vargas, A. M. M., Nogami, E. M., Kunita, M. H., Guilherme, M. R., Martins, A. C., Silva, T. L., Moraes, J. C. G., & Almeida, V. C. (2011). NaOH-activated carbon of high surface area produced from coconut shell: Kinetics and equilibrium studies from the methylene blue adsorption. Chemical Engineering Journal, 174, 117–125.
Datta, S. M. (2013). Clay–polymer nanocomposites as a novel drug carrier: Synthesis, characterization and controlled release study of Propranolol Hydrochloride. Applied Clay Science, 80–81, 85–92.
Deng, L., Yuan, P., Liu, D., Annabi-Bergaya, F., Zhou, J., Chen, F., & Liu, Z. (2017). Effects of microstructure of clay minerals, montmorillonite, kaolinite and halloysite, on their benzene adsorption behaviors. Applied Clay Science, 143, 184–191.
Dordio, A. V., Miranda, S., Ramalho, J. P. P., & Carvalho, A. J. P. (2017). Mechanisms of removal of three widespread pharmaceuticals by two clay materials. Journal of Hazardous Materials, 323(Part A), 575–583.
Gamba, M., Kovář, P., Pospíšil, M., & Sánchez, R. M. T. (2017). Insight into thiabendazole interaction with montmorillonite and organically modified montmorillonites. Applied Clay Science, 137, 59–68.
Gil, A., Korili, S. A., & Vicente, M. A. (2008). Recent advances in the control and characterization of the porous structure of pillared clay catalysts. Catalysis Reviews, 50, 153–221.
Golubeva, O. Y. (2016). Effect of synthesis conditions on hydrothermal crystallization, textural characteristics and morphology of aluminum-magnesium montmorillonite. Microporous and Mesoporous Materials, 224, 271–276.
Grenni, P., Ancona, V., & Caracciolo, A. B. (2018). Ecological effects of antibiotics on natural ecosystems: A review. Microchemical Journal, 136, 25–39.
Ho, Y.-S. (2004). Citation review of Lagergren kinetic rate equation on adsorption reactions. Scientometrics, 59, 171–177.
Jaynes, W. F., & Boyd, S. A. (1991). Clay mineral type and organic compound sorption by hexadecyltrimethlyammonium-exchanged clays. Soil Science Society of America Journal, 55, 43–48.
Khashirova, S. Y., Musaev, Y., Mikitaev, A. K., Malkanduev, Y. A., & Ligidov, M. K. (2009). Hybrid nanocomposites based on guanidine methacrylate monomer and polymer and layered aluminosilicates: Synthesis, structure and properties. Polymer Science. Series B, 51, 377–382.
Lee, C. H., Kato, M., & Usuki, A. (2011). Preparation and properties of bio-based polycarbonate/clay nanocomposites. Journal of Materials Chemistry, 21, 6844–6847.
Liao, M. H., & Chen, D. H. (2002). Preparation and characterization of a novel magnetic nano-adsorbent. Journal of Materials Chemistry, 12, 3654–3659.
Lin, S., Zhou, T., & Yin, S. (2017). Properties of thermally treated granular montmorillonite-palygorskite adsorbent (GMPA) and use to remove Pb2+ and Cu2+ from aqueous solutions. Clays and Clay Minerals, 65, 184–192.
Lou, Z., Zhou, Z., Zhang, W., Zhang, X., Hu, X., Liu, P., & Zhang, H. (2015). Magnetized bentonite by Fe3O4 nanoparticles treated as adsorbent for methylene blue removal from aqueous solution: Synthesis, characterization, mechanism, kinetics and regeneration. Journal of the Taiwan Institute of Chemical Engineers, 49, 199–205.
Ma, M., Zhang, Y., Yu, W., Shen, H., Zhang, H., & Gu, N. (2003). Preparation and characterization of magnetite nanoparticles coated by amino silane. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 212, 219–226.
Martinez-Costa, J. I., Leyva-Ramos, R., & Padilla-Ortega, E. (2018). Sorption of diclofenac from aqueous solution on an organobentonite and adsorption of cadmium on organobentonite saturated with diclofenac. Clays and Clay Minerals, 66, 515–528.
Park, K. W., Jung, J. H., Kim, J. D., Kim, S. K., & Kwon, O. Y. (2009). Preparation of mesoporous silica-pillared H+-titanosilicates. Microporous and Mesoporous Materials, 118, 100–105.
Pastukhov, A. V., Davankov, V. A., Lubentsova, K. I., Kosandrovich, E. G., & Soldatov, V. S. (2013). Structure and properties of magnetic composite sorbents based on hypercrosslinked polystyrenes. Russian Journal of Physical Chemistry A, 87, 1702–1708.
Ruiz-Hitzky, E., Aranda, P., Dardera, M., & Rytwo, G. (2010). Hybrid materials based on clays for environmental and biomedical applications. Journal of Materials Chemistry, 20, 9306–9321.
Sahnoun, S., Boutahala, M., Tiar, C., & Kahoul, A. (2018). Adsorption of tartrazine from an aqueous solution by octadecyltrimethylammonium bromide-modified bentonite: Kinetics and isotherm modeling. Comptes Rendus Chimie, 21, 391–398.
Sebayang, P., Kurniawan, C., Aryanto, D., Setiadi, E. A., Tamba, K., Djuhana, & Sudiro, T. (2018). Preparation of Fe3O4/bentonite nanocomposite from natural iron sand by co-precipitation method for adsorbents materials. IOP Conference Series: Materials Science and Engineering, 316, 012053.
Yu, W. H., Li, N., Tong, D. S., Zhou, C. H., Lin, C. X., & Xu, C. Y. (2013). Adsorption of proteins and nucleic acids on clay minerals and their interactions: A review. Applied Clay Science, 80–81, 443–452.
Zhang, W. H., Ding, Y. J., Boyd, S. A., Teppen, B. J., & Li, H. (2010). Sorption and desorption of carbamazepine from water by smectite clays. Chemosphere, 81, 954–960.
Zhou, C. H., Shen, Z. F., Liu, L. H., & Liu, S. M. (2011). Preparation and functionality of clay-containing films. Journal of Materials Chemistry, 21, 15132–15153.
Acknowledgments
The work was supported by the Russia Foundation for Basic Research (Grant N 18-43-370015-a). Measurements were performed in the centre for joint use of scientific equipment “The Upper Volga Region Centre of Physicochemical Research.”
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of Interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
(Received 14 May 2019; revised 4 October 2019; AE: William F. Jaynes)
Rights and permissions
About this article
Cite this article
Alekseeva, O.V., Rodionova, A.N., Noskov, A.V. et al. Bentonite/Magnetite Composite for Removal of Nitrofurazone. Clays Clay Miner. 67, 471–480 (2019). https://doi.org/10.1007/s42860-019-00037-w
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42860-019-00037-w