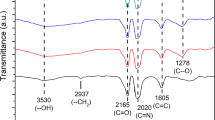
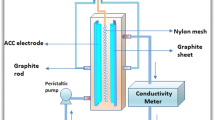
Abstract
Enhancing the capacitive deionization performance requires the inner structure expansion of porous activated carbon to facilitate the charge storage and electrolyte penetration. This work aimed to modify the porosity of coconut-shell activated carbon (AC) through CO2 activation at high temperature. The electrochemical performance of CO2-activated AC electrodes was evaluated by cyclic voltammetry, charge/discharge test and electrochemical impedance spectroscopy, which exhibited that AC-800 had the superior performance with the highest capacitance of 112 F/g at the rate of 0.1 A/g and could operate for up to 4000 cycles. Furthermore, in the capacitive deionization, AC-800 showed salt removal of 9.15 mg/g with a high absorption rate of 2.8 mg/g min and Ni(II) removal of 5.32 mg/g with a rate close to 1 mg/g.min. The results promote the potential application of CO2-activated AC for desalination as well as Ni-removal through capacitance deionization (CDI) technology.







Similar content being viewed by others
References
Zaid K, Suriani AB (2017) A review on electrode materials used in capacitive deionization processes for water treatment applications. Sci Int 29:285–289
Hemmatifar A, Palko JW, Stadermann M, Santiago JG (2016) Energy breakdown in capacitive deionization. Water Res 104:303–311. https://doi.org/10.1016/j.watres.2016.08.020
Qasim M, Badrelzaman M, Darwish NN et al (2019) Reverse osmosis desalination: A state-of-the-art review. Desalination 459:59–104. https://doi.org/10.1016/j.desal.2019.02.008
Sufiani O, Elisadiki J, Machunda RL, Jande YAC (2019) Modification strategies to enhance electrosorption performance of activated carbon electrodes for capacitive deionization applications. J Electroanal Chem 848:113328. https://doi.org/10.1016/j.jelechem.2019.113328
Qin M, Deshmukh A, Epsztein R et al (2019) Comparison of energy consumption in desalination by capacitive deionization and reverse osmosis. Desalination 455:100–114. https://doi.org/10.1016/j.desal.2019.01.003
Foo KY, Hameed BH (2009) A short review of activated carbon assisted electrosorption process: an overview, current stage and future prospects. J Hazard Mater 170:552–559. https://doi.org/10.1016/j.jhazmat.2009.05.057
Daer S, Kharraz J, Giwa A, Hasan SW (2015) Recent applications of nanomaterials in water desalination: a critical review and future opportunities. Desalination 367:37–48. https://doi.org/10.1016/j.desal.2015.03.030
Thamilselvan A, Nesaraj AS, Noel M (2016) Review on carbon-based electrode materials for application in capacitive deionization process. Int J Environ Sci Technol 13:2961–2976. https://doi.org/10.1007/s13762-016-1061-9
Lado JJ, Zornitta RL, Calvi FA et al (2017) Enhanced capacitive deionization desalination provided by chemical activation of sugar cane bagasse fly ash electrodes. J Anal Appl Pyrol 126:143–153. https://doi.org/10.1016/j.jaap.2017.06.014
Huynh LTN, Pham TN, Nguyen TH et al (2020) Coconut shell-derived activated carbon and carbon nanotubes composite: a promising candidate for capacitive deionization electrode. Synth Met 265:116415. https://doi.org/10.1016/j.synthmet.2020.116415
Kim M, Lim H, Xu X et al (2021) Sorghum biomass-derived porous carbon electrodes for capacitive deionization and energy storage. Microporous Mesoporous Mater 312:110757. https://doi.org/10.1016/j.micromeso.2020.110757
Itoi H, Hasegawa H, Iwata H, Ohzawa Y (2018) Non-polymeric hybridization of a TEMPO derivative with activated carbon for high-energy-density aqueous electrochemical capacitor electrodes. Sustain Energy Fuels 2:558–565. https://doi.org/10.1039/C7SE00541E
Itoi H, Suzuki R, Miyaji M et al (2021) Hybridization of a polymer inside the pores of activated carbon and pore structural characterization. ACS Appl Polym Mater 3:3603–3611. https://doi.org/10.1021/acsapm.1c00498
Kang C-S, Ko Y-I, Fujisawa K et al (2020) Hybridized double-walled carbon nanotubes and activated carbon as free-standing electrode for flexible supercapacitor applications. Carbon Lett 30:527–534. https://doi.org/10.1007/s42823-020-00122-4
Turmuzi M, Daud WRW, Tasirin SM et al (2004) Production of activated carbon from candlenut shell by CO2 activation. Carbon 42:453–455. https://doi.org/10.1016/j.carbon.2003.11.015
Guo S, Peng J, Li W et al (2009) Effects of CO2 activation on porous structures of coconut shell-based activated carbons. Appl Surf Sci 255:8443–8449. https://doi.org/10.1016/j.apsusc.2009.05.150
Şahin Ö, Yardim Y, Baytar O, Saka C (2020) Enhanced electrochemical double-layer capacitive performance with CO2 plasma treatment on activated carbon prepared from pyrolysis of pistachio shells. Int J Hydrog Energy 45:8843–8852. https://doi.org/10.1016/j.ijhydene.2020.01.128
Zhang Y, Chen L, Mao S et al (2019) Fabrication of porous graphene electrodes via CO2 activation for the enhancement of capacitive deionization. J Colloid Interface Sci 536:252–260. https://doi.org/10.1016/j.jcis.2018.10.063
Rashidi NA, Yusup S (2017) A review on recent technological advancement in the activated carbon production from oil palm wastes. Chem Eng J 314:277–290. https://doi.org/10.1016/j.cej.2016.11.059
Ogungbenro AE, Quang DV, Al-Ali K, Abu-Zahra MRM (2017) Activated carbon from date seeds for CO2 capture applications. Energy Procedia 114:2313–2321. https://doi.org/10.1016/j.egypro.2017.03.1370
Nowicki P, Pietrzak R, Wachowska H (2010) Sorption properties of active carbons obtained from walnut shells by chemical and physical activation. Catal Today 150:107–114. https://doi.org/10.1016/j.cattod.2009.11.009
Pietrzak R (2010) Sawdust pellets from coniferous species as adsorbents for NO2 removal. Biores Technol 101:907–913. https://doi.org/10.1016/j.biortech.2009.09.017
Ryu SK, Jin H, Gondy D et al (1993) Activation of carbon fibres by steam and carbon dioxide. Carbon 31:841–842. https://doi.org/10.1016/0008-6223(93)90025-6
Tchounwou PB, Yedjou CG, Patlolla AK, Sutton DJ (2012) Heavy metal toxicity and the environment. Exp Suppl 101:133–164. https://doi.org/10.1007/978-3-7643-8340-4_6
Chen R, Sheehan T, Ng JL et al (2020) Capacitive deionization and electrosorption for heavy metal removal. Environ Sci Water Res Technol 6:258–282. https://doi.org/10.1039/C9EW00945K
Nguyen TT, Huynh LTN, Pham TN et al (2021) Enhanced capacitive deionization performance of activated carbon derived from coconut shell electrodes with low content carbon nanotubes–graphene synergistic hybrid additive. Mater Lett 292:129652. https://doi.org/10.1016/j.matlet.2021.129652
Le VH, Huynh LTN, Tran TN et al (2021) Comparative desalination performance of activated carbon from coconut shell waste/carbon nanotubes composite in batch mode and single-pass mode. J Appl Electrochem 51:1313–1322. https://doi.org/10.1007/s10800-021-01575-9
Jiang C, Yakaboylu GA, Yumak T et al (2020) Activated carbons prepared by indirect and direct CO2 activation of lignocellulosic biomass for supercapacitor electrodes. Renew Energy 155:38–52. https://doi.org/10.1016/j.renene.2020.03.111
Yang K, Peng J, Xia H et al (2010) Textural characteristics of activated carbon by single step CO2 activation from coconut shells. J Taiwan Inst Chem Eng 41:367–372. https://doi.org/10.1016/j.jtice.2009.09.004
Dantas TLP, Rodrigues AE, Moreira RFPM (2012) Separation of carbon dioxide from flue gas using adsorption on porous solids. In: Separation of carbon dioxide from flue gas using adsorption on porous solids. IntechOpen
Reddy KSK, Al Shoaibi A, Srinivasakannan C (2012) A comparison of microstructure and adsorption characteristics of activated carbons by CO2 and H3PO4 activation from date palm pits. New Carbon Mater 27:344–351. https://doi.org/10.1016/S1872-5805(12)60020-1
Ghouma I, Jeguirim M, Sager U et al (2017) The potential of activated carbon made of agro-industrial residues in NOx immissions abatement. Energies 10:1508. https://doi.org/10.3390/en10101508
Wenzhong S, Zhijie L, Yihong L (2007) Surface chemical functional groups modification of porous carbon. Recent Pat Chem Eng 1:27–40
Allwar A, Noor AM, Nawi MAM (2011) Preparation and characterization of microporous activated carbon from oil palm shell by physical activation using purified nitrogen. EKSAKTA: J Sci Data Anal 12(2):1–3
Yeh C-L, Hsi H-C, Li K-C, Hou C-H (2015) Improved performance in capacitive deionization of activated carbon electrodes with a tunable mesopore and micropore ratio. Desalination 367:60–68. https://doi.org/10.1016/j.desal.2015.03.035
Aslan M, Zeiger M, Jäckel N et al (2016) Improved capacitive deionization performance of mixed hydrophobic/hydrophilic activated carbon electrodes. J Phys Condens Matter 28:114003. https://doi.org/10.1088/0953-8984/28/11/114003
Sheng K, Sun Y, Li C et al (2012) Ultrahigh-rate supercapacitors based on eletrochemically reduced graphene oxide for ac line-filtering. Sci Rep 2:247. https://doi.org/10.1038/srep00247
Taberna PL, Simon P, Fauvarque JF (2003) Electrochemical characteristics and impedance spectroscopy studies of carbon–carbon supercapacitors. J Electrochem Soc 150:A292. https://doi.org/10.1149/1.1543948
Mei B-A, Munteshari O, Lau J et al (2018) Physical interpretations of nyquist plots for EDLC electrodes and devices. J Phys Chem C 122:194–206. https://doi.org/10.1021/acs.jpcc.7b10582
Sidhu NK, Rastogi AC (2016) Bifacial carbon nanofoam-fibrous PEDOT composite supercapacitor in the 3-electrode configuration for electrical energy storage. Synth Met 219:1–10. https://doi.org/10.1016/j.synthmet.2016.04.012
Manoharan S, Sahoo S, Pazhamalai P, Kim SJ (2018) Supercapacitive properties of activated carbon electrode using ammonium based proton conducting electrolytes. Int J Hydrog Energy 43:1667–1674. https://doi.org/10.1016/j.ijhydene.2017.11.121
Gaikwad MS, Balomajumder C (2017) Tea waste biomass activated carbon electrode for simultaneous removal of Cr(VI) and fluoride by capacitive deionization. Chemosphere 184:1141–1149. https://doi.org/10.1016/j.chemosphere.2017.06.074
Hai A, Bharath G, Babu KR et al (2019) Date seeds biomass-derived activated carbon for efficient removal of NaCl from saline solution. Process Saf Environ Prot 129:103–111. https://doi.org/10.1016/j.psep.2019.06.024
Elisadiki J, Kibona TE, Machunda RL et al (2020) Biomass-based carbon electrode materials for capacitive deionization: a review. Biomass Convers Biorefin 10:1327–1356. https://doi.org/10.1007/s13399-019-00463-9
Zeng A, Shrestha M, Wang K et al (2017) Plasma treated active carbon for capacitive deionization of saline water. J Nanomater 2017:e1934724. https://doi.org/10.1155/2017/1934724
Chang L, Hang HuY (2019) 3D Channel-structured graphene as efficient electrodes for capacitive deionization. J Colloid Interface Sci 538:420–425. https://doi.org/10.1016/j.jcis.2018.11.105
Agartan L, Akuzum B, Mathis T et al (2018) Influence of thermal treatment conditions on capacitive deionization performance and charge efficiency of carbon electrodes. Sep Purif Technol 202:67–75. https://doi.org/10.1016/j.seppur.2018.02.039
Zornitta RL, Srimuk P, Lee J et al (2018) Charge and potential balancing for optimized capacitive deionization using lignin-derived, low-cost activated carbon electrodes. Chemsuschem 11:2101–2113. https://doi.org/10.1002/cssc.201800689
Liu L, Qiu G, Suib SL et al (2017) Enhancement of Zn2+ and Ni2+ removal performance using a deionization pseudocapacitor with nanostructured birnessite and its carbon nanotube composite electrodes. Chem Eng J 328:464–473. https://doi.org/10.1016/j.cej.2017.07.066
Iftekhar S, Farooq MU, Sillanpää M et al (2017) Removal of Ni(II) using multi-walled carbon nanotubes electrodes: relation between operating parameters and capacitive deionization performance. Arab J Sci Eng 42:235–240. https://doi.org/10.1007/s13369-016-2301-5
Li P, Gui Y, Blackwood DJ (2018) Development of a nanostructured α-MnO2/carbon paper composite for removal of Ni2+/Mn2+ ions by electrosorption. ACS Appl Mater Interfaces 10:19615–19625. https://doi.org/10.1021/acsami.8b02471
Acknowledgements
This research is funded by Vietnam National University, Ho Chi Minh City (VNU-HCM) under Grant number of 562-2020-18-06.
Author information
Authors and Affiliations
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Huynh, L.T.N., Tran, T.N., Ho, T.T.N. et al. Enhanced electrosorption of NaCl and nickel(II) in capacitive deionization by CO2 activation coconut-shell activated carbon. Carbon Lett. 32, 1531–1540 (2022). https://doi.org/10.1007/s42823-022-00387-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42823-022-00387-x