Abstract
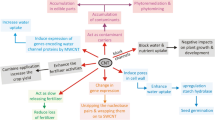
Recent years have witnessed remarkable development in the field of nanotechnology and it has been affirmed that carbon-based nanomaterials have wide applications in agriculture, industrial, biomedical and environmental sectors. Due to distinctive physicochemical properties of the carbon nanotubes (CNTs), they have been extensively utilized in plant science as a growth promoter, and thus, could be a boon for biomass production of agricultural products. Studies suggest that CNTs help increase the plant’s ability to absorb water and essential nutrients, thereby increasing growth. Apart from this, CNTs have been scrutinized for their utilization in genetic engineering for the delivery of genes, proteins or drugs. However, the literature discloses mixed effects of CNTs exposure on plants like in inducing oxidative stress by generating reactive oxygen species (ROS). Moreover, studies concerning CNTs interaction with plant system is at a nascent stage and needs further investigations to explore the mechanisms influencing the growth and toxicity in plants. Therefore, this review attempts to highlight the current literature on CNTs (including both single walled and multi walled) exposure on plants. It also explores unresolved challenges, as well as recommendations to ensure sustainable development of CNTs while minimizing any possible adverse health impacts.



Similar content being viewed by others
References
Endo M, Strano MS, Ajayan PM (2007) Potential applications of carbon nanotubes. carbon nanotubes. Springer, Berlin, Heidelberg, pp 13–62
Herrero-Latorre C, Álvarez-Méndez J, Barciela-García J, García-Martín S, Peña-Crecente R (2015) Characterization of carbon nanotubes and analytical methods for their determination in environmental and biological samples: A review. Anal Chim Acta 853:77–94
Gandhi K, Lari S, Tripathi D, Kanade G (2016) Advanced oxidation processes for the treatment of chlorpyrifos, dimethoate and phorate in aqueous solution. J Water Reuse Desalin 6(1):195–203
Ren X, Chen C, Nagatsu M, Wang X (2011) Carbon nanotubes as adsorbents in environmental pollution management: a review. Chem Eng J 170(2–3):395–410
Ambrosi A, Pumera M (2010) Regulatory peptides are susceptible to oxidation by metallic impurities within carbon nanotubes. Chem European J 16(6):1786–1792
Tiwari D, Dasgupta-Schubert N, Cendejas LV, Villegas J, Montoya LC, García SB (2014) Interfacing carbon nanotubes (CNT) with plants: enhancement of growth, water and ionic nutrient uptake in maize (Zea mays) and implications for nanoagriculture. Appl Nanosci 4(5):577–591
Khodakovskaya MV, De Silva K, Biris AS, Dervishi E, Villagarcia H (2012) Carbon nanotubes induce growth enhancement of tobacco cells. ACS nano 6(3):2128–2135
Verma SK, Das AK, Gantait S, Kumar V, Gurel E (2019) Applications of carbon nanomaterials in the plant system: a perspective view on the pros and cons. Sci Total Environ 667:485–499
Tripathi D, Singh K, Mansoori MA (2019) Soil Pollution: Health Implications and Management. In: Kumar M, Tiwari RR (eds) Recent Trends and Advances in Environmental Health, vol 1. Nova Science Publishers. New York, USA, pp 41–64
Khot LR, Sankaran S, Maja JM, Ehsani R, Schuster EW (2012) Applications of nanomaterials in agricultural production and crop protection: a review. Crop Protec 35:64–70
Khodakovskaya M, Dervishi E, Mahmood M, Xu Y, Li Z, Watanabe F, Biris AS (2009) Carbon nanotubes are able to penetrate plant seed coat and dramatically affect seed germination and plant growth. ACS Nano 3(10):3221–3227
Khodakovskaya MV, Kim BS, Kim JN, Alimohammadi M, Dervishi E, Mustafa T, Cernigla CE (2013) Carbon nanotubes as plant growth regulators: effects on tomato growth, reproductive system, and soil microbial community. Small 9(1):115–123
Kwak S-Y, Lew TTS, Sweeney CJ, Koman VB, Wong MH, Bohmert-Tatarev K, Snell KD, Seo JS, Chua N-H, Strano MS (2019) Chloroplast-selective gene delivery and expression in planta using chitosan-complexed single-walled carbon nanotube carriers. Nat Nanotechnol 14(5):447–455
Begum P, Fugetsu B (2012) Phytotoxicity of multi-walled carbon nanotubes on red spinach (Amaranthus tricolor L) and the role of ascorbic acid as an antioxidant. J Hazard Mater 243:212–222
Lin C, Fugetsu B, Su Y, Watari F (2009) Studies on toxicity of multi-walled carbon nanotubes on Arabidopsis T87 suspension cells. J Hazard Mater 170(2–3):578–583. https://doi.org/10.1016/j.jhazmat.2009.05.025
Chen M, Zhou S, Zhu Y, Sun Y, Zeng G, Yang C, Xu P, Yan M, Liu Z, Zhang W (2018) Toxicity of carbon nanomaterials to plants, animals and microbes: Recent progress from 2015-present. Chemosphere 206:255–264
Hao Y, Ma C, Zhang Z, Song Y, Cao W, Guo J, Zhou G, Rui Y, Liu L, Xing B (2018) Carbon nanomaterials alter plant physiology and soil bacterial community composition in a rice-soil-bacterial ecosystem. Environ Pollut 232:123–136
Simon-Deckers A, Loo S, Mayne-L'hermite M, Herlin-Boime N, Menguy N, Reynaud C, Gouget B, Carriere M (2009) Size-, composition- and shape-dependent toxicological impact of metal oxide nanoparticles and carbon nanotubes toward bacteria. Environ Sci Technol 43(21):8423–8429. https://doi.org/10.1021/es9016975
Su W-C, Cheng YS (2014) Carbon nanotubes size classification, characterization and nasal airway deposition. Inhalation Toxicol 26(14):843–852
Jackson P, Jacobsen NR, Baun A, Birkedal R, Kühnel D, Jensen KA, Vogel U, Wallin H (2013) Bioaccumulation and ecotoxicity of carbon nanotubes. Chem Cent J 7(1):154
Ganesh E (2013) Single walled and multi walled carbon nanotube structure, synthesis and applications. Int J Innov Technol Explor Eng 2(4):311–320
Tománek D, Jorio A, Dresselhaus MS, Dresselhaus G (2007) Introduction to the important and exciting aspects of carbon-nanotube science and technology. Carbon Nanotubes. Springer, Berlin, Heidelberg, pp 1–12
Eatemadi A, Daraee H, Karimkhanloo H, Kouhi M, Zarghami N, Akbarzadeh A, Abasi M, Hanifehpour Y, Joo SW (2014) Carbon nanotubes: properties, synthesis, purification, and medical applications. Nanoscale Res Lett 9(1):393
Chico L, Crespi VH, Benedict LX, Louie SG, Cohen ML (1996) Pure carbon nanoscale devices: nanotube heterojunctions. Phys Rev Lett 76(6):971
Vander Wal RL, Berger G, Ticich T (2003) Carbon nanotube synthesis in a flame using laser ablation for in situ catalyst generation. Appl Phys A 77(7):885–889
Ajayan P, Ebbesen T (1997) Nanometre-size tubes of carbon. Rep Prog Phys 60(10):1025
Huczko A (2002) Synthesis of aligned carbon nanotubes. Appl Phys A 74(5):617–638
Iijima S (1991) Helical microtubules of graphitic carbon. Nature 354(6348):56–58
Thess A, Lee R, Nikolaev P, Dai H, Petit P, Robert J, Xu C, Lee YH, Kim SG, Rinzler AG (1996) Crystalline ropes of metallic carbon nanotubes. Science 273(5274):483–487
Cheng H, Li F, Su G, Pan H, He L, Sun X, Dresselhaus M (1998) Large-scale and low-cost synthesis of single-walled carbon nanotubes by the catalytic pyrolysis of hydrocarbons. Appl Phys Lett 72(25):3282–3284
Szabó A, Perri C, Csató A, Giordano G, Vuono D, Nagy JB (2010) Synthesis methods of carbon nanotubes and related materials. Materials 3(5):3092–3140
Prasek J, Drbohlavova J, Chomoucka J, Hubalek J, Jasek O, Adam V, Kizek R (2011) Methods for carbon nanotubes synthesis. J Mater Chem 21(40):15872–15884
Tiwari DK, Valenzuela J, Tripathi D, Mathew S (2020) Fabrication of carbon based nanostructured materials on Si/SiO2substrate and their growth mechanism over different catalysts. Nanomed Nanotechnol Open Access https://doi.org/10.23880/nnoa-16000175
Tendeloo G, Nagy J (1998) Purification of catalytically produced multi-wall nanotubes. J Chem Soc Faraday Trans 94(24):3753–3758
Djordjević V, Djustebek J, Cvetićanin J, Velićknović S, Veljković M, Bokorov M, Stojić BB, Nešković O (2006) Methods of purification and characterization of carbon nanotubes. J Optoelectron Adv Mater 8(4):1631–1634
Shanov V, Yun Y-H, Schulz MJ (2006) Synthesis and characterization of carbon nanotube materials. J University Chem Technol Metall 41(4):377–390
Belin T, Epron F (2005) Characterization methods of carbon nanotubes: a review. Mater Sci Eng, B 119(2):105–118
Mahalingam P, Parasuram B, Maiyalagan T, Sundaram S (2012) Chemical Methods for purification of carbon nanotubes–a review. J Environ Nanotechnol 1(1):53–61
Hou P-X, Liu C, Cheng H-M (2008) Purification of carbon nanotubes. Carbon 46(15):2003–2025
Du J, Wang S, You H, Zhao X (2013) Understanding the toxicity of carbon nanotubes in the environment is crucial to the control of nanomaterials in producing and processing and the assessment of health risk for human: a review. Environ Toxicol Pharmacol 36(2):451–462
Sun Y-P, Fu K, Lin Y, Huang W (2002) Functionalized carbon nanotubes: properties and applications. Acc Chem Res 35(12):1096–1104
Wepasnick KA, Smith BA, Bitter JL, Fairbrother DH (2010) Chemical and structural characterization of carbon nanotube surfaces. Anal Bioanal Chem 396(3):1003–1014
Li J, He Y, Han Y, Liu K, Wang J, Li Q, Fan S, Jiang K (2012) Direct identification of metallic and semiconducting single-walled carbon nanotubes in scanning electron microscopy. Nano Lett 12(8):4095–4101
García-Gutiérrez MC, Nogales A, Hernández JJ, Rueda DR, Ezquerra TA (2007) X-ray scattering applied to the analysis of carbon nanotubes, polymers and nanocomposites. Opt Pura Apl 40(2):195–205
Lopez-Lorente A, Simonet B, Valcárcel M (2014) Raman spectroscopic characterization of single walled carbon nanotubes: influence of the sample aggregation state. Analyst 139(1):290–298
Zang Z, Hu Z, Li Z, He Q, Chang X (2009) Synthesis, characterization and application of ethylenediamine-modified multiwalled carbon nanotubes for selective solid-phase extraction and preconcentration of metal ions. J Hazard Mater 172(2–3):958–963
Attal S, Thiruvengadathan R, Regev O (2006) Determination of the concentration of single-walled carbon nanotubes in aqueous dispersions using UV− Visible absorption spectroscopy. Anal Chem 78(23):8098–8104
Wenseleers W, Vlasov II, Goovaerts E, Obraztsova ED, Lobach AS, Bouwen A (2004) Efficient isolation and solubilization of pristine single-walled nanotubes in bile salt micelles. Adv Func Mater 14(11):1105–1112
Haggenmueller R, Rahatekar SS, Fagan JA, Chun J, Becker ML, Naik RR, Krauss T, Carlson L, Kadla JF, Trulove PC (2008) Comparison of the quality of aqueous dispersions of single wall carbon nanotubes using surfactants and biomolecules. Langmuir 24(9):5070–5078
Grossiord N, Regev O, Loos J, Meuldijk J, Koning CE (2005) Time-dependent study of the exfoliation process of carbon nanotubes in aqueous dispersions by using UV− Visible spectroscopy. Anal Chem 77(16):5135–5139
Schierz A, Parks AN, Washburn KM, Chandler GT, Ferguson PL (2012) Characterization and quantitative analysis of single-walled carbon nanotubes in the aquatic environment using near-infrared fluorescence spectroscopy. Environ Sci Technol 46(22):12262–12271
Pang LS, Saxby JD, Chatfield SP (1993) Thermogravimetric analysis of carbon nanotubes and nanoparticles. J Phys Chem 97(27):6941–6942
Chen C-M, Chen M, Leu F-C, Hsu S-Y, Wang S-C, Shi S-C, Chen C-F (2004) Purification of multi-walled carbon nanotubes by microwave digestion method. Diam Relat Mater 13(4–8):1182–1186
Mansfield E, Kar A, Hooker SA (2010) Applications of TGA in quality control of SWCNTs. Anal Bioanal Chem 396(3):1071–1077
Duesberg G, Blau W, Byrne H, Muster J, Burghard M, Roth S (1999) Chromatography of carbon nanotubes. Synth Met 103(1–3):2484–2485
Flavel BS, Moore KE, Pfohl M, Kappes MM, Hennrich F (2014) Separation of single-walled carbon nanotubes with a gel permeation chromatography system. ACS Nano 8(2):1817–1826
Lopez-Pastor M, Domínguez-Vidal A, Ayora-Canada M, Simonet B, Lendl B, Valcarcel M (2008) Separation of single-walled carbon nanotubes by use of ionic liquid-aided capillary electrophoresis. Anal Chem 80(8):2672–2679
Patel A, Tiwari S, Parihar P, Singh R, Prasad SM (2019) Carbon nanotubes as plant growth regulators: impacts on growth, reproductive system, and soil microbial community. In: Nanomaterials in plants, algae and microorganisms. Academic Press, pp 23–42
Yan S, Zhao L, Li H, Zhang Q, Tan J, Huang M, He S, Li L (2013) Single-walled carbon nanotubes selectively influence maize root tissue development accompanied by the change in the related gene expression. J Hazard Mater 246:110–118
Zhang H, Yue M, Zheng X, Xie C, Zhou H, Li L (2017) Physiological effects of single-and multi-walled carbon nanotubes on rice seedlings. IEEE Trans Nanobiosci 16(7):563–570
Caa JE, Long M, Nations S, Vadan R, Dai L, Luo M, Ambikapathi R, Lee EH, Olszyk D (2008) Effects of functionalized and nonfunctionalized single-walled carbon nanotubes on root elongation of select crop species. Environm Toxicol Chem Int J 27(9):1922–1931
Pourkhaloee A, Haghighi M, Saharkhiz MJ, Jouzi H, Doroodmand MM (2011) Carbon nanotubes can promote seed germination via seed coat penetration. Seed Technol 33(2):155–169
Haghighi M, da Silva JAT (2014) The effect of carbon nanotubes on the seed germination and seedling growth of four vegetable species. J Crop Sci Biotechnol 17(4):201–208
Flores D, Chacón R, Alvarado L, Schmidt A, Alvarado C, Chaves J (2014) Effect of using two different types of carbon nanotubes for blackberry (Rubus adenotrichos) in vitro plant rooting, growth and histology. Am J Plant Sci 5(24):3510
Khodakovskaya MV, De Silva K, Biris AS, Dervishi E, Villagarcia H (2012) Carbon nanotubes induce growth enhancement of tobacco cells. ACS Nano 6(3):2128–2135
Alimohammadi M, Xu Y, Wang D, Biris AS, Khodakovskaya MV (2011) Physiological responses induced in tomato plants by a two-component nanostructural system composed of carbon nanotubes conjugated with quantum dots and its in vivo multimodal detection. Nanotechnology 22(29):295101
Hatami M, Hadian J, Ghorbanpour M (2017) Mechanisms underlying toxicity and stimulatory role of single-walled carbon nanotubes in Hyoscyamus niger during drought stress simulated by polyethylene glycol. J Hazard Mater 324:306–320
Sun W, Shahrajabian MH, Huang Q (2020) Soybean seeds treated with single walled carbon nanotubes (SwCNTs) showed enhanced drought tolerance during germination. Int J Adv Biol Biomed Res (IJABBR) 8:9–16
Corredor E, Testillano PS, Coronado M-J, González-Melendi P, Fernández-Pacheco R, Marquina C, Ibarra MR, de la Fuente JM, Rubiales D, Pérez-de-Luque A (2009) Nanoparticle penetration and transport in living pumpkin plants: in situsubcellular identification. BMC Plant Biol 9(1):45
Wu Y, Phillips JA, Liu H, Yang R, Tan W (2008) Carbon nanotubes protect DNA strands during cellular delivery. ACS Nano 2(10):2023–2028
Martínez-Ballesta MC, Zapata L, Chalbi N, Carvajal M (2016) Multiwalled carbon nanotubes enter broccoli cells enhancing growth and water uptake of plants exposed to salinity. J Nanobiotechnol 14(1):42
Martinez-Ballesta MC, Chelbi N, Lopez-Zaplana A, Carvajal M (2020) Discerning the mechanism of the multiwalled carbon nanotubes effect on root cell water and nutrient transport. Plant Physiol Biochem 146:23–30
Tripathi S, Sonkar SK, Sarkar S (2011) Growth stimulation of gram (Cicer arietinum) plant by water soluble carbon nanotubes. Nanoscale 3(3):1176–1181
Lahiani MH, Dervishi E, Chen J, Nima Z, Gaume A, Biris AS, Khodakovskaya MV (2013) Impact of carbon nanotube exposure to seeds of valuable crops. ACS Appl Mater Interfaces 5(16):7965–7973
Tiwari D, Dasgupta-Schubert N, Villasenor L, Tripathi D, Villegas J (2013) Interaction of carbon nanotubes with mineral nutrients for the promotion of growth of tomato seedlings. Nano Studies 7:87–96
Mondal A, Basu R, Das S, Nandy P (2011) Beneficial role of carbon nanotubes on mustard plant growth: an agricultural prospect. J Nanopart Res 13(10):4519
Taha RA, Hassan MM, Ibrahim EA, Baker NHA, Shaaban EA (2016) Carbon nanotubes impact on date palm in vitro cultures. Plant Cell Tissue Organ Culture (PCTOC) 127(2):525–534
Smirnova E, Gusev A, Zaytseva O, Sheina O, Tkachev A, Kuznetsova E, Lazareva E, Onishchenko G, Feofanov A, Kirpichnikov M (2012) Uptake and accumulation of multiwalled carbon nanotubes change the morphometric and biochemical characteristics of Onobrychis arenaria seedlings. Front Chem Sci Eng 6(2):132–138
Larue C, Pinault M, Czarny B, Georgin D, Jaillard D, Bendiab N, Mayne-L’Hermite M, Taran F, Dive V, Carrière M (2012) Quantitative evaluation of multi-walled carbon nanotube uptake in wheat and rapeseed. J Hazard Mater 227:155–163
Pilevar ZT, Mahmoodzadeh H, Eshaghi A (2015) Impact of multi-walled carbon nanotubes on seed germination and seedling growth of Cichorium intybus L. L J Biol Environ Sci 6(1):438–445
Keller AA, Wang H, Zhou D, Lenihan HS, Cherr G, Cardinale BJ, Miller R, Ji Z (2010) Stability and aggregation of metal oxide nanoparticles in natural aqueous matrices. Environ Sci Technol 44(6):1962–1967
Dasgupta-Schubert N, Tiwari D, Francis ER, Torres PM, Villaseñor L, Mora CV (2017) Plant responses to nano and micro structured carbon allotropes: Water imbibition by maize seeds upon exposure to multiwalled carbon nanotubes and activated carbon. Adv Nano Res 5(3):245
Pereira MM, Mouton L, Yéprémian C, Couté A, Lo J, Marconcini JM, Ladeira LO, Raposo NR, Brandão HM, Brayner R (2014) Ecotoxicological effects of carbon nanotubes and cellulose nanofibers in Chlorella vulgaris. J Nanobiotechnol 12(1):15
Ferguson PL, Chandler GT, Templeton RC, DeMarco A, Scrivens WA, Englehart BA (2008) Influence of sediment− amendment with single-walled carbon nanotubes and diesel soot on bioaccumulation of hydrophobic organic contaminants by benthic invertebrates. Environ Sci Technol 42(10):3879–3885
Petersen EJ, Huang Q, Weber J, Walter J (2008) Bioaccumulation of radio-labeled carbon nanotubes by Eisenia foetida. Environ Sci Technol 42(8):3090–3095
Bullard-Dillard R, Creek KE, Scrivens WA, Tour JM (1996) Tissue Sites of Uptake of14C-Labeled C60. Bioorg Chem 24(4):376–385
Wang C, Liu H, Chen J, Tian Y, Shi J, Li D, Guo C, Ma Q (2014) Carboxylated multi-walled carbon nanotubes aggravated biochemical and subcellular damages in leaves of broad bean (Vicia faba L.) seedlings under combined stress of lead and cadmium. J Hazard Mater 274:404–412
Lin S, Reppert J, Hu Q, Hudson JS, Reid ML, Ratnikova TA, Rao AM, Luo H, Ke PC (2009) Uptake, translocation, and transmission of carbon nanomaterials in rice plants. Small 5(10):1128–1132
Stampoulis D, Sinha SK, White JC (2009) Assay-dependent phytotoxicity of nanoparticles to plants. Environ Sci Technol 43(24):9473–9479
Lin D, Xing B (2007) Phytotoxicity of nanoparticles: inhibition of seed germination and root growth. Environ Pollut 150(2):243–250
Ghosh M, Chakraborty A, Bandyopadhyay M, Mukherjee A (2011) Multi-walled carbon nanotubes (MWCNT): induction of DNA damage in plant and mammalian cells. J Hazard Mater 197:327–336
Zhai G, Gutowski SM, Walters KS, Yan B, Schnoor JL (2015) Charge, size, and cellular selectivity for multiwall carbon nanotubes by maize and soybean. Environ Sci Technol 49(12):7380–7390
Chen G, Qiu J, Liu Y, Jiang R, Cai S, Liu Y, Zhu F, Zeng F, Luan T, Ouyang G (2015) Carbon nanotubes act as contaminant carriers and translocate within plants. Scientific Rep 5:15682
Miralles P, Johnson E, Church TL, Harris AT (2012) Multiwalled carbon nanotubes in alfalfa and wheat: toxicology and uptake. J R Soc Interface 9(77):3514–3527
Tan X-m, Lin C, Fugetsu B (2009) Studies on toxicity of multi-walled carbon nanotubes on suspension rice cells. Carbon 47(15):3479–3487
Tan X-m, Fugetsu B (2007) Multi-walled carbon nanotubes interact with cultured rice cells: evidence of a self-defense response. J Biomed Nanotechnol 3(3):285–288
Ghodake G, Seo YD, Park D, Lee DS (2010) Phytotoxicity of carbon nanotubes assessed by Brassica juncea and Phaseolus mungo. J Nanoelectron Optoelectron 5(2):157–160
Giraldo JP, Landry MP, Faltermeier SM, McNicholas TP, Iverson NM, Boghossian AA, Reuel NF, Hilmer AJ, Sen F, Brew JA (2014) Plant nanobionics approach to augment photosynthesis and biochemical sensing. Nat Mater 13(4):400–408
Acknowledgement
Authors are highly grateful for the CONACYT Basic Science project (A1-S-47641), DST FIST Laboratory, Vimala College (Autonomous), Thrissur and DBT STAR College Scheme, Department of Biotechnology, Govt. of India.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no competing interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mathew, S., Tiwari, D.K. & Tripathi, D. Interaction of carbon nanotubes with plant system: a review. Carbon Lett. 31, 167–176 (2021). https://doi.org/10.1007/s42823-020-00195-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42823-020-00195-1