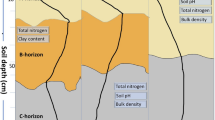
Abstract
There have been many studies on soil quality and crop yield using different biochar application amounts, but few studies have focused on the combination of different methods and amounts of biochar application in moderately degraded Mollisols. In this study, the methods of mixing biochar evenly with the soil of the plough layer (0–20 cm depth) [homogeneous biochar application (HO)] and burying biochar above the soil plow pan (under 20 cm depth) (heterogeneous biochar application (HE)) were used to reveal how biochar application methods influenced soil quality, crop yield and agronomic characteristics in moderately degraded Mollisols (soil organic matter (SOM), 30.33 g kg−1). The biochar application amounts were 0 (control), 10 (level 1), 20 (level 2), and 40 (level 3) t ha−1 in both the HO and HE treatments. The results showed that, compared with control, HO3 significantly increased maize yield in the first year, and HO2, HO3, HE2 and HE3 continuously increased maize yield in the next three years but not significantly. HO1 and HE1 had the lowest maize yield. HO2 tended to delay maize leaf senescence. There was a positive linear relationship between soil quality index (SQI) and biochar application amount in HO. Compared with other treatments, the pH, EC, SOM, available phosphorus, sucrase and catalase activities were highest in HO3. However, the effects of HE on soil quality and crop productivity were limited at first but gradually increased with time. Overall, HO3 was beneficial for improving the soil quality and crop productivity in Mollisols for short-term cultivation (3-year), while HE showed an effect over time.
Graphical Abstract

Highlights
-
Biochar application improved the overall quality of moderate degraded Mollisols but did not have a consistent effect on maize yield.
-
High amounts of homogeneous biochar (HO) tended to delay the maize leaf senescence.
-
Heterogeneous biochar (HE) gradually influenced soil quality and crop productivity with time.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Intensive cultivation without soil protection has caused serious soil degradation in arable land (Guo et al. 2010; Ju et al. 2006) and further degradation of soil quality resulting in low crop yield, and this process has been severely accelerated by soil erosion in slope fields (Huang et al. 2005; Meng and Li, 2009; Zhang et al. 2006). Biochar, as a carbon-rich amendment material, has been widely used to improve soil properties, reduce nutrient loss, and promote agricultural production (Cui et al. 2020). This has attracted much attention in various fields and is considered an effective measure to solve the crisis of soil degradation and sustain soil quality. However, it is still inconsistent that biochar application maintains and improves the soil quality and productivity in arable land of various soil types, especially in soils with different degradation levels (Hardy et al. 2017).
The inherent structural characteristics and physicochemical properties of biochar can effectively improve soil structure (Gul et al. 2015; Kluepfel et al. 2014), directly or indirectly affect water and heat transport and the microecological process in the soil, and further change nutrient transformation, leaching and availability in the soil (Biederman and Harpole 2013). Xie et al. (2020) reported that biochar reduced soil bulk density (BD) by enhancing the formation and stability of soil aggregates, thereby improving soil water-holding capacity. Paz-Ferreiro et al. (2014) found that biochar relied on its uniform and dense pores to form a large number of micropores in the soil, thereby adsorbing and storing substances of different types and components. This provides a sufficient reaction substrate for the microbial community and promotes the activity of soil enzymes. Additionally, biochar application reduced the volatilization of nitrogen and the loss of phosphorus in the soil through the strong adsorption capacity of ammonium ions and phosphate ions and the increase in exchangeable cations (K+, Na+, Ca2+, Mg2+) in the soil, and also reduced toxic elements (such as active aluminum) (Mizuta et al. 2004; Steiner et al. 2007, 2008b; Van Zwieten et al. 2010; Solomon et al. 2016). However, these benefits were rarely seen in the soil with higher fertility in existing publications (Jones et al. 2011).
Most of the above studies have found that the impact of biochar application on soil physical and chemical properties and crop yield is not only affected by the amount and type of biochar application but also closely related to the methods of biochar application (Jeffery et al. 2011; Wu et al. 2018). Currently, most studies mainly focus on the effect of biochar evenly mixed with soil on soil physicochemical properties and crop yield. However, the effects of biochar not mixed with soil (biochar is applied between two layers of soil) are poorly understood. Concentrated application of biochar (biochar is not mixed with soil) can effectively filter a wide range of the contaminants often found in urban stormwater (total suspended solids, nutrients, heavy metals, polycyclic aromatic hydrocarbons, and pathogens) (Reddy et al. 2014). In addition, some studies have shown that the application of biochar mixed with soil is relatively toxic to earthworms, inhibiting growth and reproduction, inducing DNA damage, and even causing death (Zhang et al. 2019). Compared with biochar mixed with soil, biochar not mixed with soil has a small contact area with soil and may have fewer negative effects on soil organisms. Therefore, based on the above study, we suppose that the method of biochar directly buried at a depth of 20 cm above the soil plow pan and then backfilling soil, could effectively filtrate and hold soil water and nutrients and further increase crop yield while reduce the biochar loss during the tillage process. A study of these two application methods may be an important guide to maintain the stability of soil ecosystems.
Mollisols cover a large area of 916 million hectares worldwide and are mainly distributed in regions with native prairie ecosystems and gentle slopes in Northeast China, Southeast Europe, Central North America and South American Pampas (Liu et al. 2012). Mollisols in US Soil Taxonomy (USST) was close to black soil in the Chinese Soil Taxonomy (CST) and to Phaeozems in World Reference Base for Soil Resources (WRB) (Zhang et al. 2016). In Northeast China, Mollisols are distributed over 32 million hectares of area and most of them have been cultivated (Kang et al. 2016a; Wu et al. 2018). The Harbin region is located in the central part of the belt of Mollisols, where the soil organic matter (SOM) was 8–10% before reclamation, while the mean value of the SOM was 4.32% according to an investigation in the 1980s (Zhang et al. 2013). Shen et al. (2006) classified the degree of land degradation in China as slight, moderate, severe and very severe according to soil organic matter declines of < 10, 10–30, 30–50, and > 50%, respectively. In this study, Mollisols with a SOM value of 3.03% were considered moderately degraded soils compared to the SOM of the 1980s. An experiment under farmland conditions was carried out to investigate the effects of different amounts and methods of biochar application on maize productivity and overall soil quality in moderately degraded Mollisols areas by simultaneously measuring soil properties, crop yield and plant agronomic characteristics.
2 Materials and methods
2.1 Description of the site and experimental materials
The experiment was carried out at the Xiangyang experimental station of Northeast Agricultural University (45°42′ N, 126°36′ E) from October 2017 to October 2020, in Harbin, Heilongjiang Province, China. The region has a typical temperate continental monsoon climate with a mean annual sunshine duration of 2600 h and mean annual precipitation of 500–600 mm. The soil type is Mollisols with a BD of 0.92–0.95 g·cm−3, saturated water content of 60.2%, field water holding capacity of 30.8%, SOM of 30.3 g kg−1, and total nitrogen (TN) of 2.37 g kg−1. Soil particles < 20.00 µm, 2.00-20.00 µm and > 20.00 µm in size were 5.8%, 65.7% and 25.8%, respectively.
The biochar raw material was made up of corn stalks heated at 450 °C in anaerobic conditions, and the retention time of the pyrolysis process was 1 h. Biochar was provided by Liaoning Jin Hefu Agricultural Development Co., Ltd in China. The biochar has a particle size of 1.5–2 mm, total pore volume of 0.0054 ml g−1, microporous pore volume of 0.0006 ml g−1, pH of 9.25, TN of 15.12 g kg−1, total phosphorus (TP) of 7.93 g kg−1, and total potassium (TK) of 16.51 g kg−1.
2.2 Experimental design
The experiment was arranged in a randomized complete block design. Two methods of biochar application were used, including biochar mixed evenly with soil at 0–20 cm depth of the plough layer (HO), the soil at 0–20 cm depth was stripped, the biochar directly buried at a depth of 20 cm above the plow pan, and then the soil was backfilled (HE) in autumn 2017. The biochar application amounts were 0 (control), 10 (level 1), 20 (level 2), and 40 (level 3) t ha−1 in both the HO and HE treatments. There were a total of 7 treatments, and three replicates were adopted for each treatment. The area of each plot was 4 m2 (2 m × 2 m).
Before sowing maize in the autumn of 2017, each replicate plot was separated by polyethylene polypropylene polyester waterproof cloth that was buried vertically at a depth of 1 m and did not disturb the soil in the plot. Subsequently, the biochar was applied uniformly at once (no additional biochar application was used again in the following years) according to different application methods and amounts, and all treatments were completed in the autumn of 2017. The fertilization and seeding in each plot were the same (Additional file 1: Table S1). The spacing of the ridge/furrow was 0.67 m, and the plant spacing was 0.28 m (planting density of 52,000 plants·ha−1). Weeds were controlled by spraying herbicides first in the spring, and then manually removed in the summer. Meteorological data during the maize growth cycle were provided by the China Data Meteorological Network (http://data.cma.cn/) (Additional file 1: Fig. S1).
2.3 Plant growth and development parameters
The agronomic characteristics of maize plants were determined at different growth periods from 2018 to 2020. Three uniform plants were selected from each plot, and the leaf chlorophyll relative content (SPAD) and leaf nitrogen content (leaf N-content) were monitored using a chlorophyll meter (TYS-4 N, Zhejiang, Topu instrument, China). Simultaneously, the plant height was measured with a measuring tape from the ground to the top of the newly emerged and flattened leaves. In addition, in early October of 2018, 2019 and 2020, the aboveground biomass and yield of the plants were measured. All plants were harvested in each plot, and the field weighing method was used to obtain the above-ground biomass. All maize in each plot was exposed to the sun and threshed to measure the yield, which was converted into a hectare yield. The 100-grain weight was measured after the seeds were air-dried into constant weight.
2.4 Soil properties and overall soil quality assessment
Soil samples were collected from the 0–5 and 10–15 cm soil layers using a ring belt with 100 cm3 cylinders for the determination of BD and soil moisture (SM) in 2019–2020 (Bao 2000). A five-point sampling method was adopted to collect soil samples in the 0–15 cm soil layer with soil drills in each plot after the corn harvest in mid-October 2020. The residues and rocks in the soil samples were removed, and the soil was air-dried and then passed through a 2-mm sieve for testing. Parts of the soil samples were used for pH, electrical conductivity (EC), SOM, available nitrogen (AN), TP and available phosphorus (AP) analysis, and the others were used for catalase, phosphatase, urease and sucrase activity analysis.
The soil’s natural moisture content was determined by the drying method. Soil pH and EC (1:5 soil to water ratio) were analyzed using a pH meter (PHS-25, LeiCi, China) and an EC meter (DDSJ-308F, LeiCi, China), respectively. SOM was measured by the dichromate oxidation and titration method (Kalembasa and Jenkinson 1973). AN was measured by the alkaline solution diffusion method (Dorich and Nelson 1984). TP was measured by the HClO4-H2SO4 digestion-molybdenum antimony colorimetric method. AP was measured by the 0.5 mol L−1 NaHCO3 extraction-molybdenum antimony colorimetric method (Bao 2000).
Soil catalase, phosphatase, urease and sucrase were determined by the potassium permanganate titration method (substrate: hydrogen peroxide), disodium phenyl phosphate colorimetric method (substrate: phenyl phosphate), indophenol blue photometry (substrate: urea), and 3, 5-dinitrosalicin acid colorimetric determination (substrate: sucrose), respectively (Guan et al. 1991).
To evaluate the overall quality of the soil, a soil quality index (SQI) was calculated by integrating key soil quality indicators (KSQIs) using the method described by Zhang et al. (2020). Briefly, soil quality-related parameters (i.e. pH, EC, nutrients and enzymes) were selected to establish a minimum dataset (MDS) of soil quality based on Pearson correlation analysis (Pearson) and principal component analysis (PCA) (Gong et al. 2015). The norm was calculated using the equation Nik = \(\sqrt{\sum_{i=1}^{k}({\mu }_{ik}^{2}\cdot {\lambda }_{k})}\), where Nik represents the norm value of the first k-principal components with a characteristic value ≥ 1 for the i-index, μik represents the load of the i-index on the kth principal component, and λk represents the characteristic value of the kth principal component. The SQI was calculated using the equation SQI = \({\sum }_{i=1}^{n}{S}_{i}{W}_{i}\), where Wi and Si are the PC weighting factor and the indicator score for variable i, respectively. It was assumed that a higher SQI indicated better overall soil quality.
2.5 Statistical analysis
Statistical analysis and graphs were plotted with SPSS 21.0 (SPSS Inc., Chicago, USA) and Origin 2019b. Each data point was summarized by calculating the average value and standard deviation (S.D.). Quantitative data of the soil properties and plant parameters were screened for normal distribution using the Shapiro–Wilk test, and homogeneity of variance was determined by using Levene’s test. One-way analysis of variance (ANOVA) was used to test the differences between the control and biochar treatments. When the ANOVA indicated a significant F value (P ≤ 0.05) for treatments, multiple comparisons using Duncan’s test were performed. The single linear and nonlinear regression analyses were tested via ANOVA at P < 0.05. Multiway ANOVA was used to reveal the effects of biochar application methods (A), application amount (B) and the interaction of A × B on soil physicochemical properties and enzyme activities.
3 Results
3.1 Soil properties and overall soil quality assessment
HO3 had the lowest BD but it was not significant in the 0–5 cm soil layer (Fig. 1a); however, the BD significantly decreased in the 10–15 cm soil layers and SM increased in the 0–5 cm and 10–15 cm soil layers (Fig. 1b–d) in 2019. The linear models can be used to depict SM and BD changes with different biochar application amounts in 2019 (Fig. 2). BD gradually decreased in the 10–15 cm soil layers in HO and HE (Fig. 2a), and SM gradually increased with biochar application (P < 0.05) in the 0–5 cm and 10–15 cm soil layers in HO (Fig. 2b and c). It is worth mentioning that the influence of HO on BD was stronger than that of HE in the 10–15 cm soil layer (R2 = 0.47 vs. R2 = 0.36) (Fig. 2a), and the influence of HO on SM was stronger in the 10–15 cm soil layer than in the 0–5 cm soil layer (R2 = 0.76 vs. R2 = 0.34) (Fig. 2b and c). Additionally, a high biochar content (HO3) significantly increased the average SM in the 10–15 cm soil layers (Fig. 1d), and the average SM at HO increased with biochar application (Fig. 2d) (P < 0.05). Two-way multivariate analysis of variance (MANOVA) for SD and SM in the two cropping seasons (2019 and 2020) is shown in Additional file 1: Table S2. SM was significantly affected by biochar application methods (A) in the 10–15 cm soil layer in 2019. BD and SM were significantly affected by biochar application amount (B) in the 10–15 cm soil layer in 2019. The average SM was significantly affected by B in the 10–15 cm soil layer (P < 0.05).
Soil bulk density and soil moisture in 0–5 cm and 10–15 cm soil layers as affected by biochar application methods and amount in 2019 and 2020. a-b Soil bulk density in 0–5 cm and 10–15 cm soil layers as affect by biochar application methods and amount in 2019 and 2020. c-d Soil moisture in 0–5 cm and 10–15 cm soil layers as affect by biochar application methods and amount in 2019 and 2020. HO, homogeneous application; HE, heterogeneous application; 1, 2 and 3, biochar application was 10, 20 and 40 t ha−1, respectively, in both HO and HE treatments, Control, biochar application was 0. The same lowcase letter over each bar in the same cropping stage represents no significant difference (P > 0.05) between treatments
The relationship between soil bulk, soil moisture and biochar application amount. a The relationship between bulk density in 10–15 cm soil layers and biochar application amount in 2019. b The relationship between moisture in 0–5 cm soil layers and biochar application amount in 2019. c The relationship between soil moisture in 10–15 cm soil layers and biochar application amount in 2019. d The relationship between average soil moisture in 10–15 cm soil layers and biochar application amount in 2019 and 2020. HO, homogeneous application; HE, heterogeneous application
Soil physicochemical properties and soil enzymatic activities under different treatments are shown in Table 1. In general, biochar application improved most soil physicochemical properties and soil enzymatic activities compared to the control. pH, SOM, urease, sucrase, and activities were significantly affected by the A, B and A × B interactions (P < 0.05). HO3 had the highest soil pH and EC (P < 0.05), providing the most suitable environment for crop growth and preventing soil acidification. In addition, compared to control, HO3 had the highest SOM, AP, sucrase and catalase activities (P < 0.05), and HO1 and HE1 significantly decreased AP (P < 0.05). This indicated that high biochar content had more advantages in Mollisols conservation.
To further evaluate the response of overall soil quality to biochar, the SQI was assessed by comprehensively selecting appropriate soil quality indicators. Ten soil quality parameters were used for PCA (Additional file 1: Table S3), and then the correlation between indicators was analyzed (Additional file 1: Table S4). After comparing the correlation coefficients between two indicators in the same group, the minimum dataset (MDS) of soil quality evaluation indicators of sucrase, EC and TN were finally determined in this study. The soil quality and biochar application amount can be depicted as a linear model in HO-related treatments (Fig. 3a) (P < 0.001). Each treatment significantly increased the SQI compared with the control (P < 0.001), and the HO3 treatment had the highest (Fig. 3b). It is worth noting that although HE-related treatments improved the SQI, they did not reach a significant difference (P > 0.05) (Fig. 3b). These results indicated that biochar application can improve soil quality, and HO was better than HE.
Soil quality index (SQI) as affected by biochar application methods and amount. a SQI under different treatments after three continuous cropping years. b The relationship between SQI and biochar application amount. HO, homogeneous application; HE, heterogeneous application; 1, 2 and 3, biochar application was 10, 20 and 40 t ha−1, respectively, in both HO and HE treatments, Control, biochar application was 0. The same lowercase letter represents no significant difference (P > 0.05) between treatments
3.2 Plant height
Biochar application influenced maize plant height differently during growth stages (Fig. 4). Compared with the control, HO1 significantly decreased the plant height at the maturing stage in 2018 (P < 0.05) (Fig. 4a). HE3 significantly increased the plant height at the bell mouth stage in 2019 (P < 0.05) (Fig. 4c). HO2 had the highest constant plant height during most growth stages in 2019 (Fig. 4b and d). Each treatment had no significant effect on plant height at seeding in 2020 (Fig. 4e). HO3 had the highest plant height at the jointing stage (P < 0.001) (Fig. 4f). It was interesting that the plant height decreased with biochar application amount in HE treatments at the maturity stage of 2018 (Fig. 4a) and can be depicted as linear models (Fig. 5a) (P < 0.05), while the plant height increased with biochar application amount at the bell mouth stage in 2019 (Fig. 5b) and jointing stage in 2020 (Fig. 5c) (P < 0.05). Generally, both the HO and HE methods were helpful to accelerate the maize growth, especially HO2 and HO3, which had better performance in all treatments, and HE gradually showed a positive effect over time.
Plant height as affected by biochar application methods and amount. a Plant height at maturing stage in 2018. b–d Plant height at jointing, bell mouth and grain filling stage in 2019. e and f Plant height at seeding and jointing stage in 2020. The upper and lower box edges of boxes show the 75th and 25th percentiles, respectively; the ‘●’ shows outliers; ‘○’ shows the average value; the interior horizontal bar marks the median line. HO, homogeneous application; HE, heterogeneous application; 1, 2 and 3, biochar application was 10, 20 and 40 t ha−1, respectively, in both HO and HE treatments, Control, biochar application was 0. The same lowercase letter over each box in the same cropping stage represents no significant difference (P > 0.05) between treatments
The relationship between plant height and biochar application amount. a The relationship between plant height and biochar application amount at maturing stage in 2018. b The relationship between plant height and biochar application amount at bell mouth stage in 2019. c The relationship between plant height and biochar application amount at jointing stage in 2020. HO, homogeneous application; HE, heterogeneous application
3.3 SPAD and leaf-N content
Except for the control, SPAD and leaf-N content showed an upward trend with the biochar application during the grain filling stage of 2019 (Additional file 1: Fig. S2a and c). This indicated that biochar application could delay leaf senescence in the late growth stage of maize and contribute to the accumulation of photosynthetic products, and HO3 and HE3 were the most obvious in delaying leaf senescence. Additionally, except for the control, it could be clearly seen that HO2 and HE2 were more conducive to the growth of plants during the vegetative growth period at the seedling and jointing stages of 2020 (Additional file 1: Fig. S2b and d). Generally, the biochar application amount might greatly influence the leaf photosynthetic index, and the application method had less effect on it.
3.4 Crop productivity
Biochar application contributed to the increase in crop productivity. Compared with the control, the aboveground biomass, air-dried weight of 100 grains and yield of the HO3 treatment were the highest (1.58 t ha−1, 36.33 g and 10.80 t ha−1), and those of the HO1 treatment were the lowest (1.45 t ha−1, 30.67 g, 7.86 t ha−1) in 2020 (Fig. 6a–c). HO3 significantly increased the yield in 2018 (P < 0.05) (Fig. 6c). HE3 had the highest yield in 2019 and 2020, but the difference was not significant (Fig. 6c). Additionally, the relationship between the yield of HO-related and HE-related treatments and biochar application can be described as a quadratic function in 2018 and 2019, respectively (P < 0.05) (Fig. 7a and b). This indicated that regardless of the method of biochar application, higher amounts of biochar application can increase crop productivity, while less biochar application tends to reduce crop productivity.
Crop productivity index as affected by biochar application methods and amount. a Aboveground biomass of crops in 2018–2020. b Air-dried weight of 100 grain of crops in 2018–2020. c The yield of crops in 2018–2020. d Average aboveground biomass of three continuous cropping seasons (CSs). e Average air-dried weight of 100 grain of three CSs. f The total yield of three CSs. HO, homogeneous application; HE, heterogeneous application; 1, 2 and 3, biochar application was 10, 20 and 40 t ha−1, respectively, in both HO and HE treatments, Control, biochar application was 0. The same lowcase letter over each bar in the same cropping stage represents no significant difference (P > 0.05) between treatments
The relationship between crop productivity index and biochar application amount. a The relationship between yield and biochar application amount in 2018. b The relationship between yield and biochar application amount in 2019. c The relationship between average aboveground biomass and biochar application amount in three CSs (2018–2020). d The relationship between total maize yield and biochar application amount in three CSs (2018–2020). CSs, continuous cropping seasons (2018–2020). HO homogeneous application, HE heterogeneous application
In addition, HO1 significantly decreased the average aboveground biomass in the three continuous cropping seasons (CSs) compared with the control (P < 0.05) (Fig. 6d). Each treatment had no significant effect on the average air-dried weight of 100 grains of the three CSs (Fig. 6e). The total yields of the HO3 and HE3 treatments increased by 18.50% and 14.35% compared with the control, respectively, and were significantly higher than those of HO1 and HE1 (P < 0.05) (Fig. 6f). The relationship between the average aboveground biomass of the HO treatments, the total yield of the HE treatments and biochar application amounts can be described by a quadratic function and a linear model (Fig. 7c and d). This indicated that the average aboveground biomass was more sensitive to the increased amount of biochar application under the HO treatment, and the total yield was more sensitive to the increased amount of biochar application under the HE treatment.
4 Discussion
Because of the low inputs, high outputs, and unbalanced fertilization in the farming process, constraining soil degradation and maintaining soil quality have become a research focus worldwide (Guo et al. 2010). The methods to solve soil degradation include straw amendment, application of organic fertilizer and planting green manure; however, biochar as a soil carbon-rich amendment to improve degraded soil is still debated (Hardy et al. 2017). Additionally, the Mollisols in Northeast China, where the experiment in our study was conducted, also have the problem of soil degradation. Soil quality is severely degraded, threatening crop production in Mollisols due to long-term high-intensity cultivation, and the amount of chemical fertilizer application sharply increases without organic fertilizer (Zhou et al. 2016). Our study clearly showed that biochar application methods had different performances, and influenced crop growth, crop yield and soil physicochemical properties.
4.1 Response of soil properties to biochar application after 3 years
Biochar interactions within soils can have a direct or an indirect impact on the plant -soil interface (Lehmann et al. 2015). BD is one of the important indicators to characterize soil physical properties, and it affects the diffusion of nutrients in the soil and the absorption of nutrients by crops (Costa et al. 2013). Similar to a previous report (Zhao et al. 2019), in our study, more biochar application (HO3) tended to decrease the BD while increasing the SM (2019) (Fig. 1). This can be attributed to the fact that, compared with soils, biochar has a lower density and looser structure due to its higher cellulose, pure carbon and carbon compounds (Bird et al. 2008; Spokas et al. 2009; Downie et al. 2012). However, this study also found that the influence of HO treatment on BD was stronger than that of HE treatment in the 10–15 cm soil layer (Fig. 2a), and the influence of HO treatment on SM was stronger in the 10–15 cm soil layer than in the 0–5 cm soil layer (Fig. 2b–d). Therefore, compared with HE, HO was more effective at reversing soil compaction. In addition, the small effect of biochar on the upper SM content could be attributed to the fact that surface soil is susceptible to experiencing evaporation by wind flow, and biochar does not play a dominant role. (Zhao et al. 2021). Thus, in the moderately degraded Mollisols, HO had a better performance than HE in the early stage of maize growth by mediating both BD and SM.
Previous studies indicated that 1 t ha−1 of biochar application significantly increased soil pH but did not obviously increase the EC in both nutrient-poor and nutrient-rich soils (Kang et al. 2016b). In this study, more biochar application significantly increased soil pH and EC (Table 1), which provided the most suitable environment for crop growth. This could be due to higher amounts of biochar application containing greater amount of ash and mineral elements such as Na, K, Mg, Ca, etc., which are alkaline after being dissolved in the solutions of soils (Kimetu and Lehmann 2010). Additionally, Mollisols had a relatively higher content of SOM (3.03%) and clay particles (sizes of 2.00–20.00 µm, 65.7%), which could moderate the alkalinity caused by biochar. Thus, the addition of Na, K, Mg, and Ca to ash accelerates the activity of iron in soils. Therefore, in the moderate degradation of Mollisols, biochar application was slightly different from the previous results. The higher amount of biochar application not only significantly increased soil pH, but also significantly increased soil EC, which could balance soil acidity, especially alkalinity.
In the present study, HO3 significantly increased SOM, AP, sucrase and catalase activities (Table 1). This can be explained by the fact that (1) biochar is an additional source of SOM and AP, which can directly increase soil nutrient levels (Antal and Grønli, 2003), and (2) biochar has a high capacity for soil cation exchange and surface area, which can adsorb more substances, especially P with the mode of “deposition cycling” in soils, and increase the soil nutrient levels (Dahlawi et al. 2018; Zhang et al. 2020). In addition, the AP increase is partially explained by the increase in enzyme activities (such as phosphatase, sucrase, and catalase), which increases the transformation of non-available P, especially soil phosphatase, sucrase and catalase, which are specific and crucial to the P and C cycles (Frankenberger and Bingham 1982). Thus, more HO had more obvious effects on improving SOC, available nutrients, and enzyme activities.
Most previous studies on biochar application mainly focused on improving the soil quality of problematic soils. Our present study emphasized the importance of the overall soil in the Mollisols area (Fig. 3). Clearly, the SQI is a valuable index that can reflect the overall quality of the soil covering a wide range of physicochemical and microbial properties (Yan et al. 2021). In the present study, the SQI gradually increased with biochar application in HO, and was significantly higher in both HO2 and HO3 than in the other treatments (Fig. 3a and b). This indicated that HO had a greater impact on soil quality than HE. However, when the amount of biochar application reached a certain level, the soil quality no longer significantly improved. The trends of SQI slowly increasing with biochar application were obvious in HE in all three years, but the results were not significantly different. This may be due to that the amount of biochar application is not enough at present. However, the effects of HE should be focused on in the future because HE has relatively weak negative effects on soil animal diversity and soil ecosystem health compared to HO (Wu et al. 2021; Maurer et al. 2017). Therefore, based on the purpose of production practices, appropriate methods and amounts of biochar application should be considered in farming practices. Interestingly, AN, EC and sucrase were most closely related to soil quality (Additional file 1: Table S3), which indicated that these indicators could be used to coarsely estimate the soil quality after biochar application in Mollisols.
4.2 Response of crop yield and growth to biochar application
Previous studies indicated that biochar application can change the nutrient availability and status in soils, and further influence crop growth (Sohi et al. 2010; Yuan et al. 2016). Plant height is typically considered an indicator reflecting the photosynthesis and respiration capacity of the plant (Guenni et al. 2018). Plants have higher heights, and more therefore branches and leaves can capture more solar radiation and carbon dioxide, further increasing crop yields (Chen et al. 2021). In this study, medium and high amounts of HO and HE promoted crop growth and delayed leaf senescence, and the effects were slightly different in different years. This indicated that in the early stage of biochar application, the influence of HO on crop height was more significant compared with that of HE, while HE showed an effect over time. This can be a possibility due to the fact that the biochar condensing above the plow pan and below the plough layer (HE) can effectively filter the nutrients and hold water and is further beneficial to crop growth in all stages (Reddy et al. 2014). As well, with increasing years, plant roots tend to grow deeper into the biochar layer with high nutrient filtration during the leaching process in HE. This results in the root system absorbing more nutrients from the biochar layer and promotes crop growth (Li et al. 2014).
Furthermore, both HO3 and HE3 increased the leaf SPAD value and leaf-N content (Additional file 1: Fig. S2a and c), delayed leaf senescence during the later stage of maize growth, and thereby extended the grain-filling duration of maize. Additionally, HO2 promoted an increase in the leaf SPAD value and leaf-N content in the seedling stage of 2020 (Additional file 1: Fig. S2b and d), which was beneficial to increasing photosynthate accumulation in the early stages of crop growth. This may be due to the higher content of biochar application promoting the soil nutrient cycling, such as nitrification, through the holding, adsorption and desorption of nutrients in the porous structure of biochar (Joseph et al. 2010). This process increased the soil available nitrogen and promoted plant nitrogen absorption. Thus, both higher amounts of HO and HE tend to promote vegetative growth in the early stage and delay leaf senescence in the later stage of crops to a certain extent. In contrast, lower biochar application reduced the plant height (Fig. 4a) and SPAD (Additional file 1: Fig. S2a, c). It may be due to the fact that lower amounts of biochar application just filled the space of the soil pores, and resulted in acceleration of anoxic conditions which weakened nitrification in the soil. Therefore, low application of biochar (HO1) reduced the uptake of soil AN by crops and further constrained the growth and development of crops (Kammann et al. 2011). In general, both HO and HE had little influence on plant height, SPAD and leaf-N content, and the influence of biochar on maize performance had the dose effects.
Previous studies indicated that different types of biochar mostly increased the production of crops in various climates and soils (Major et al. 2010; Uzoma et al. 2011). This can be attributed to (1) biochar application increasing the abundance of beneficial bacteria in the soils, which improves the function of the soil ecosystem and provides a more friendly environment for crop root growth (Beesley et al. 2010; Steiner et al. 2008a); or (2) biochar improving the physicochemical properties of the soils, especially the high adsorption ability of biochar reducing nutrient loss, while increasing the available nutrient content and promoting crop growth (Wang et al. 2010; Chen et al. 2013); or (3) the adsorption of biochar slowing the release of toxic substances when they are confined to biochar and thus reducing the toxic effects of toxic ions and organic pollutants on plants (Uchimiya et al. 2011). Hussain et al. (2017) found that biochar application enhanced the crop yield in highly degraded or infertile soils based on a summary of previous publications, but it was not significant in fertile and healthy soils. This means that biochar does not always enhance crop yield in cultivated soils. In this study, we found that HO3 only significantly increased maize yield in the first year of biochar application (Fig. 6c). This was due to biochar application improving soil physicochemical properties such as pH, EC, SOM and AP (Table 1). Meanwhile, EC and AP were significantly positively correlated with yield (P < 0.05) (Table 2). In addition, this may be due to biochar application influencing maize yield, which could be severely affected by weather conditions, such as precipitation and temperature, especially in extreme weather conditions. For example, hurricanes made all maize down because maize had a higher height and wider leaves after biochar application than the control, while the soil structure was too loose to sustain the crop upright in August of 2019 and 2020. As well, the ability of biochar to improve soil quality and microecosystems could be weakened with increasing time, although biochar is relatively stable in the soil environment (Spokas et al. 2014; Ippolito et al. 2012). We also found that HE3 had the highest yield in 2019 and 2020 but the difference was not significant (Fig. 6c); this can also be attributed to the biochar layer of HE trapping more nutrients (Hossain et al. 2020). Thus, the effects of the amount and the method of biochar application on maize yield should be continuously studied in the moderately degraded Mollisols in further studies. Generally, the effect of biochar on soil quality and crop yield cannot be negated in the moderately degraded Mollisols, and both the higher HO and HE can effectively promote crop growth and the accumulation of maize yield to a certain extent, although the effect is determined by various environmental conditions.
5 Conclusions
The results of the three-year study suggested that the application of biochar can improve the overall quality of moderately degraded Mollisols to varying degrees. The improvement of overall soil quality was conducive to the growth and development of crops (e.g. delayed leaf senescence), which can increase crop productivity (e.g. crop yield and aboveground biomass of crops). Higher homogeneous and heterogeneous amounts of biochar application could promote vegetative growth in the early stage, delay leaf senescence in the later stage of crops to a certain extent and improve crop productivity. In general, HO3 was the best soil carbon-rich amendment for improving overall soil quality and crop productivity for short-term cultivation (3-year), while HE showed an effect over time.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Code availability
Not applicable.
References
Antal MJ, Grønli M (2003) The art, science, and technology of charcoal production. Ind Eng Chem Res 42:1619–1640. https://doi.org/10.1021/ie0207919
Bao SD (2000) Soil Agrochemical Analysis (third edition). China Agriculture Press, Beijing, pp 14–21
Beesley L, Moreno-Jiménez E, Gomez-Eyles JL (2010) Effects of biochar and greenwaste compost amendments on mobility, bioavailability and toxicity of inorganic and organic contaminants in a multi-element polluted soil. Environ Pollut 158:2282–2287. https://doi.org/10.1016/j.envpol.2010.02.003
Biederman LA, Harpole WS (2013) Biochar and its effects on plant productivity and nutrient cycling: a meta-analysis. GCB Bioenergy 5:202–214. https://doi.org/10.1111/gcbb.12037
Bird MI, Ascough PL, Young IM, Wood CV, Scott AC (2008) X-ray microtomographic imaging of charcoal. J Archaeol Sci 35:2698–2706. https://doi.org/10.1016/j.jas.2008.04.018
Chen WF, Zhang WM, Meng J (2013) Advances and prospects in research of biochar utilization in agriculture. Sci Agric Sin 46:3324–3333
Chen Y, Wang L, Bai YL, Lu YL, Ni L, Wang YH, Xu MZ (2021) Quantitative relationship between effective accumulated temperature and plant height & leaf area index of summer maize under different nitrogen, phosphorus and potassium levels. Sci Agric Sin 54:4761–4777
Costa JC, Borges JAR, Pires LF (2013) Soil bulk density evaluated by gamma-ray attenuation: analysis of system geometry. Soil Tillage Res 129:23–31. https://doi.org/10.1016/j.still.2013.01.003
Cui BJ, Cui EP, Hu C, Fan XY, Gao F (2020) Effects of selected biochars application on the microbial community structures and diversities in the rhizosphere of water spinach (Ipomoea aquatica Forssk.) irrigated with reclaimed water. Environ Sci 41:5636–5647. https://doi.org/10.13227/j.hjkx.202006087
Dahlawi S, Naeem A, Rengel Z, Naidu R (2018) Biochar application for the remediation of salt-affected soils: challenges and opportunities. Sci Total Environ 625:320–335. https://doi.org/10.1016/j.scitotenv.2017.12.257
Dorich R, Nelson D (1984) Evaluation of manual cadmium reduction methods for determination of nitrate in potassium chloride extracts of soils. Soil Sci Soc Am J 48:72–75. https://doi.org/10.2136/sssaj1984.03615995004800010013x
Downie A, Crosky A, Munroe P (2012) Physical properties of biochar, biochar for environmental management. Routledge, pp 45–64
Frankenberger JW, Bingham F (1982) Influence of salinity on soil enzyme activities. Soil Sci Soc of Am J 46:1173–1177. https://doi.org/10.2136/sssaj1982.03615995004600060011x
Gong L, Zhang X, Ran Q (2015) Quality assessment of oasis soil in the upper reaches of Tarim River based on minimum data set. Acta Pedofil Sin 52:682–689
Guan SY, Zhang DS, Zhang ZM (1991) Methods of soil enzyme activities analysis. Agriculture Press, Beijing, pp 263–271
Guenni O, Romero E, Guedez Y, de Guenni LB, Pittermann J (2018) Influence of low light intensity on growth and biomass allocation, leaf photosynthesis and canopy radiation interception and use in two forage species of Centrosema (DC.) Benth. Grass Forage Sci 73:967–978. https://doi.org/10.1111/gfs.12368
Gul S, Whalen JK, Thomas BW, Sachdeva V, Deng H (2015) Physico-chemical properties and microbial responses in biochar-amended soils: mechanisms and future directions. Agric Ecosyst Environ 206:46–59. https://doi.org/10.1016/j.agee.2015.03.015
Guo JH, Liu XJ, Zhang Y, Shen JL, Han WX, Zhang WF, Christie P, Goulding K, Vitousek PM, Zhang F (2010) Significant acidification in major Chinese croplands. Science 327:1008–1010. https://doi.org/10.1126/science.1182570
Hardy B, Cornelis JT, Houben D, Leifeld J, Lambert R, Dufey J (2017) Evaluation of the long-term effect of biochar on properties of temperate agricultural soil at pre-industrial charcoal kiln sites in Wallonia, Belgium. Eur J Soil Sci 68:80–89. https://doi.org/10.1111/ejss.12395
Hossain MZ, Bahar MM, Sarkar B, Donne SW, Ok YS, Palansooriya KN, Kirkham MB, Chowdhury S, Bolan N (2020) Biochar and its importance on nutrient dynamics in soil and plant. Biochar 2:379–420. https://doi.org/10.1007/s42773-020-00065-z
Huang J, Zhang HL, Fu WY, Ma B, Li X, Wang AW (2005) Analysis of the changing characteristics of the soil fertilities in the black soil area of Northeast China. Chin J Soil Sci 36:21–25
Hussain M, Farooq M, Nawaz A, Al-Sadi AM, Solaiman ZM, Alghamdi SS, Ammara U, Ok YS, Siddique KHM (2017) Biochar for crop production: potential benefits and risks. J Soils Sediments 17:685–716. https://doi.org/10.1007/s11368-016-1360-2
Ippolito JA, Laird DA, Busscher WJ (2012) Environmental benefits of biochar. J Environ Qual 41:967–972. https://doi.org/10.2134/jeq2012.0151
Jeffery S, Verheijen FGA, van der Velde M, Bastos AC (2011) A quantitative review of the effects of biochar application to soils on crop productivity using meta-analysis. Agric Ecosyst Environ 144:175–187. https://doi.org/10.1016/j.agee.2011.08.015
Jones DL, Edwards-Jones G, Murphy DV (2011) Biochar mediated alterations in herbicide breakdown and leaching in soil. Soil Biol Biochem 43:804–813. https://doi.org/10.1016/j.soilbio.2010.12.015
Joseph SD, Camps-Arbestain M, Lin Y, Munroe P, Chia C, Hook J, Van Zwieten L, Kimber S, Cowie A, Singh B (2010) An investigation into the reactions of biochar in soil. Soil Res 48:501–515. https://doi.org/10.1071/SR10009
Ju XT, Kou CL, Zhang FS, Christie P (2006) Nitrogen balance and groundwater nitrate contamination: comparison among three intensive cropping systems on the North China Plain. Environ Pollut 143:117–125. https://doi.org/10.1016/j.envpol.2005.11.005
Kalembasa SJ, Jenkinson DS (1973) A comparative study of titrimetric and gravimetric methods for the determination of organic carbon in soil. J Sci Food Agric 24:1085–1090. https://doi.org/10.1002/jsfa.2740240910
Kammann CI, Linsel S, Goessling JW, Koyro HW (2011) Influence of biochar on drought tolerance of Chenopodium quinoa Willd and on soil-plant relations. Plant Soil 345:195–210. https://doi.org/10.1007/s11104-011-0771-5
Kang RH, Ren Y, Wu HJ, Zhang SX (2016a) Changes in the nutrients and fertility of black soil over 26 years in Northeast China. Sci Agric Sin 49:2113–2125
Kang SW, Park JW, Seo DC, Ok YS, Park KD, Choi IW, Cho JS (2016b) Effect of biochar application on rice yield and greenhouse gas emission under different nutrient conditions from paddy soil. J Environ Eng. https://doi.org/10.1061/(ASCE)EE.1943-7870.0001083
Kimetu JM, Lehmann J (2010) Stability and stabilisation of biochar and green manure in soil with different organic carbon contents. Aust J Soil Res 48:577–586. https://doi.org/10.1071/SR10036
Kluepfel L, Keiluweit M, Kleber M, Sander M (2014) Redox properties of plant biomass-derived black carbon (Biochar). Environ Sci Technol 48:5601–5611. https://doi.org/10.1021/es500906d
Lehmann J, Kuzyakov Y, Pan G, Ok YS (2015) Biochars and the plant-soil interface. Plant Soil 395:1–5. https://doi.org/10.1007/s11104-015-2658-3
Li HB, Ma QH, Li HG, Zhang FS, Rengel Z, Shen JB (2014) Root morphological responses to localized nutrient supply differ among crop species with contrasting root traits. Plant Soil 376:151–163. https://doi.org/10.1007/s11104-013-1965-9
Liu XB, Burras CL, Kravchenko YS, Duran A, Huffman T, Morras H, Studdert G, Zhang XY, Cruse RM, Yuan XH (2012) Overview of Mollisols in the world: distribution, land use and management. Can J Soil Sci 92(3):383–402. https://doi.org/10.4141/cjss2010-058
Major J, Rondon M, Molina D, Riha SJ, Lehmann J (2010) Maize yield and nutrition during 4 years after biochar application to a Colombian savanna oxisol. Plant Soil 333:117–128. https://doi.org/10.1007/s11104-010-0327-0
Meng LQ, Li Y (2009) The mechanism of gully development on sloping farmland in black soil area, Northeast China. J Soil Water Conserv 23(7–11):44
Mizuta K, Matsumoto T, Hatate Y, Nishihara K, Nakanishi T (2004) Removal of nitrate-nitrogen from drinking water using bamboo powder charcoal. Bioresour Technol 95:255–257. https://doi.org/10.1016/j.biortech.2004.02.015
Paz-Ferreiro J, Fu S, Mendez A, Gasco G (2014) Interactive effects of biochar and the earthworm Pontoscolex corethrurus on plant productivity and soil enzyme activities. J Soils Sediments 14:483–494. https://doi.org/10.1007/s11368-013-0806-z
Reddy KR, Xie T, Dastgheibi S (2014) Evaluation of biochar as a potential filter media for the removal of mixed contaminants from urban storm water runoff. J Environ Eng. https://doi.org/10.1061/(ASCE)EE.1943-7870.0000872
Shen WS, Cao XZ, Shen FY (2006) Classification and grading of land degradation in China. J Ecol Rural Environ 22:88–93
Sohi SP, Krull E, Lopez-Capel E, Bol R (2010) A review of biochar and its use and function in soil. Adv Agron 105:47–82. https://doi.org/10.1016/S0065-2113(10)05002-9
Solomon D, Lehmann J, Fraser JA, Leach M, Amanor K, Frausin V, Kristiansen SM, Millimouno D, Fairhead J (2016) Indigenous African soil enrichment as a climate-smart sustainable agriculture alternative. Front Ecol Environ 14:71–76. https://doi.org/10.1002/fee.1226
Spokas K, Koskinen W, Baker J, Reicosky D (2009) Impacts of woodchip biochar additions on greenhouse gas production and sorption/degradation of two herbicides in a Minnesota soil. Chemosphere 77:574–581. https://doi.org/10.1016/j.chemosphere.2009.06.053
Spokas KA, Novak JM, Masiello CA, Johnson MG, Colosky EC, Ippolito JA, Trigo C (2014) Physical disintegration of biochar: an overlooked process. Environ Sci Technol Lett 1:326–332. https://doi.org/10.1021/ez500199t
Steiner C, Teixeira WG, Lehmann J, Nehls T, de Macedo JLV, Blum WEH, Zech W (2007) Long term effects of manure, charcoal and mineral fertilization on crop production and fertility on a highly weathered Central Amazonian upland soil. Plant Soil 291:275–290. https://doi.org/10.1007/s11104-007-9193-9
Steiner C, Das KC, Garcia M, Förster B, Zech W (2008a) Charcoal and smoke extract stimulate the soil microbial community in a highly weathered xanthic Ferralsol. Pedobiologia 51:359–366. https://doi.org/10.1016/j.pedobi.2007.08.002
Steiner C, Glaser B, Teixeira WG, Lehmann J, Blum WEH, Zech W (2008b) Nitrogen retention and plant uptake on a highly weathered central Amazonian Ferralsol amended with compost and charcoal. J Plant Nutr Soil Sci 171:893–899. https://doi.org/10.1002/jpln.200625199
Uchimiya M, Wartelle LH, Klasson KT, Fortier CA, Lima IM (2011) Influence of pyrolysis temperature on biochar property and function as a heavy metal sorbent in soil. J Agric Food Chem 59:2501–2510. https://doi.org/10.1021/jf104206c
Uzoma K, Inoue M, Andry H, Fujimaki H, Zahoor A, Nishihara E (2011) Effect of cow manure biochar on maize productivity under sandy soil condition. Soil Use Manage 27:205–212. https://doi.org/10.1111/j.1475-2743.2011.00340.x
Van Zwieten L, Kimber S, Morris S, Chan KY, Downie A, Rust J, Joseph S, Cowie A (2010) Effects of biochar from slow pyrolysis of papermill waste on agronomic performance and soil fertility. Plant Soil 327:235–246. https://doi.org/10.1007/s11104-009-0050-x
Wang H, Lin K, Hou Z, Richardson B, Gan J (2010) Sorption of the herbicide terbuthylazine in two New Zealand forest soils amended with biosolids and biochars. J Soils Sediments 10:283–289. https://doi.org/10.1007/s11368-009-0111-z
Wu HL, Wang SC, Huai SC, Yan ZH, Ma CB, Xue YD, Xu MG, Lu CA (2018) Evolutionary characteristics of fertility and productivity of typical black soil in recent 30 years. J Plant Nutr Fertil 24:1456–1464
Xie W, Chen Q, Wu L, Yang H, Xu J, Zhang Y (2020) Coastal saline soil aggregate formation and salt distribution are affected by straw and nitrogen application: a 4-year field study. Soil Tillage Res. https://doi.org/10.1016/j.still.2019.104535
Yan SH, Gao YM, Tian MJ, Tian YQ, Li JS (2021) Comprehensive evaluation of effects of various carbon-rich amendments on tomato production under continuous saline water irrigation: overall soil quality, plant nutrient uptake, crop yields and fruit quality. Agric Water Manage 255:106995. https://doi.org/10.1016/j.agwat.2021.106995
Yuan H, Lu T, Wang Y, Chen Y, Lei T (2016) Sewage sludge biochar: nutrient composition and its effect on the leaching of soil nutrients. Geoderma 267:17–23. https://doi.org/10.1016/j.geoderma.2015.12.020
Zhang XP, Liang AZ, Shen Y, Li WF, Zhang XL, Wang YX, Xie YJ, Liu FF, Yang XM (2006) Erosion characteristics of black soils in Northeast China. Scientia Geographica Sinica 26:687–692
Zhang X, Sui Y, Song C (2013) Degradation process of arable mollisols. Soil Crop 2:1–6
Zhang SL, Jiang LL, Liu XB, Zhang XY, Fu SC, Dai L (2016) Soil nutrient variance by slope position in a Mollisol farmland area of Northeast China. Chin Geogra Sci 26(4):508–517. https://doi.org/10.1007/s11769-015-0737-2
Zhang QM, Saleem M, Wang CX (2019) Effects of biochar on the earthworm (Eisenia foetida) in soil contaminated with and/or without pesticide mesotrione. Sci Total Environ 671:52–58. https://doi.org/10.1016/j.scitotenv.2019.03.364
Zhang X, Qu J, Li H, La S, Tian Y, Gao L (2020) Biochar addition combined with daily fertigation improves overall soil quality and enhances water-fertilizer productivity of cucumber in alkaline soils of a semi-arid region. Geoderma 363:114170. https://doi.org/10.1016/j.geoderma.2019.114170
Zhao LL, Li LS, Cai HJ, Shi XH, Xue SP (2019) Comprehensive effects of organic materials incorporation on soil hydraulic conductivity and air permeability. Sci Agric Sin 52:1045–1057
Zhao W, Liu Y, Li Z, Bao Z (2021) Effects of biochar on infiltration and evaporation of soil water with sand mulching. J Biobased Mater Bioenergy 15:369–373. https://doi.org/10.1166/jbmb.2021.2058
Zhou J, Jiang X, Zhou B, Zhao B, Ma M, Guan D, Li J, Chen S, Cao F, Shen D (2016) Thirty four years of nitrogen fertilization decreases fungal diversity and alters fungal community composition in black soil in northeast China. Soil Biol Biochem 95:135–143. https://doi.org/10.1016/j.soilbio.2015.12.012
Acknowledgements
Thanks for the editor professor Ayesha Siddiqka and the anonymous reviewers' comments. Thanks to Haijun Zhang, Jiping Huo, Hao Wang, Weitao Xu, Jiani Xue, Ziliang Xiao, Bing Xu, Wan Wang, Xinhua Hao, Jiuqi Wang, Yao Wang, Qingsong Shen, Xinrui Wang, Yu Li, Xu Liu, Xueshan Wang, Fengjuan Qu who helped us in building experimental plots, investigations, and laboratory analyses.
Funding
This work was sponsored by the National Key Research and Development Program of China (2021YFD1500801) and the National Natural Science Foundation of China (42177321).
Author information
Authors and Affiliations
Contributions
SY: Investigation, collected data, validation, data analysis, writing-original draft. SZ: Conceived ideas, investigation, conceptualization, supervision, writing review and editing, funding acquisition. PY: Investigation, data collection, comments. MA: Language editing, comments. All of the authors participated in the study and have agreed to the content of the manuscript. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Supplementary Information
Additional file 1.
Supplementary figures and tables.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yan, S., Zhang, S., Yan, P. et al. Effect of biochar application method and amount on the soil quality and maize yield in Mollisols of Northeast China. Biochar 4, 56 (2022). https://doi.org/10.1007/s42773-022-00180-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42773-022-00180-z