Abstract
Hydrochar (HC) produced by the hydrothermal carbonization (HTC) of typically wet biomass is generally considered to be less effective for carbon (C) sequestration in soils compared to biochar (BC) by pyrolysis, due to a higher content of more easily decomposable C. Although the recalcitrance of HC is suggested to improve with increasing HTC production temperature, the way it interacts and becomes associated with soil organic matter (SOM) fractions of different stabilities against decomposition, may also influence its effectiveness for C sequestration in soils. In that respect, this study aimed to verify the potential of HCs from maize silage produced at different HTC temperatures (190, 210 and 230 °C) for C sequestration in a HC-amended sandy loam Podzol. To do this, we conducted a pot trial experiment and traced the fate of HC-derived C (HC-C) within different SOM fractions, namely the free- and occluded particulate organic matter (POMF and POMO, respectively) fractions and that comprising organic matter (OM) bound to clays (OMCl). Approx. 1 year after applying 5% of the different HTC temperature HCs to the soil, the SOM fractions were isolated by density fractionation for each HC treatment (HC190, HC210 and HC230) and the control (absent of HC). All fractions and the HCs were analyzed for organic C (OC) content and isotopic signatures (δ 13C). From the δ 13C signatures, the amount of HC-C and native soil organic carbon (SOC) within each fraction was calculated. Increased C contents and decreased H/C and O/C ratios were observed with increasing HTC production temperatures, which suggests a lower stability for the low temperature HC. After ca. 1 year, a loss of ~ 20–23% of the bulk soil TOC was found in the HC-amended soils. The POMF fraction of the HC-amended soils showed losses of 68–81% HC-C and 52–72% native SOC, which may be due to a positive priming effect caused by HC addition. The POMO and OMCl fractions of the HC-amended soils contained more OC than the control, indicating the integration of HC-C together with SOM within these more stable fractions, while the effect of HTC production temperature on the level of decomposition of the resultant HCs was negligible. In all HC treatments, the OMCl fraction comprised the least amount of HC-C, thus showing the weakest response to C amendment. In conclusion, long(er)-term research on the C net balance that accounts for the observed priming-induced TOC losses and the HC-C enrichment in more stable fractions is required to verify the potential of the different HCs for the purpose of C sequestration in soils.
Graphical Abstract

Highlights
-
Increasing HTC temperature (190, 210 and 230 °C) resulted in physico-chemical and structural differences in the HCs.
-
HC addition to a Podzol potentially resulted in a positive priming effect in the free OM fraction after 1 year.
-
The more stable SOM fractions of the HC-amended Podzol contained more OC than the (HC free) control after 1 year.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Land-use change and poor agricultural practices tend to go hand-in-hand with the transformation of soil organic carbon (SOC) to carbon dioxide (CO2), which thereby increases greenhouse gas (GHG) emissions and reduces soil quality and fertility (Del Galdo et al. 2003; Diochon and Kellman 2009). The role soils play in the global C cycle is well-documented (Batjes 1998; Buyanovsky and Wagner 1998; Lal 2003; 2004; Liu et al. 2003). The content and quality of soil organic matter (SOM), which consists of ca. 50% SOC, is strongly correlated with the ability of a given soil to resist SOM decomposition and mineralization (Trigalet et al. 2017). The fact that these latter processes, which equate to reduced SOC stocks, may be prompted and accelerated by stresses such as land-use change, intensified agriculture and climate change (Del Galdo et al. 2003; Lal 2004), highlights the necessity to protect the SOC pool and improve its resilience to such potential stresses. A popular mitigation strategy aimed at (inter alia) improving the sequestration ability of soils by stabilizing SOM (Fang et al. 2019; Greenberg et al. 2019) and improving soil quality and fertility (Kizito et al. 2019; Kloss et al. 2014) is the application of a C-rich charred material, known as biochar (BC). The production of BC by pyrolysis supports the principles of a circular C economy, whereby ~ 50% of the original C content of the utilized waste (feedstock) material is captured and stored in the final BC product (Lehmann et al. 2006). Furthermore, the recalcitrant nature of BC gives it an enhanced resistance to mineralization and decomposition by microorganisms, which serves to increase SOC stocks and in turn, also improve soil fertility (Steiner et al. 2009; Woolf et al. 2010). However, despite the amelioration effects of BC, its dry and oxygen-limited production process possesses a number of drawbacks (Lehmann et al. 2006; Libra et al. 2011; Malghani et al. 2013).
As such, increasing researches are focused on an alternative biomass conversion technology known as hydrothermal carbonization (HTC) which produces a solid hydrochar (HC), and wastewater. The benefits of HTC include its ability to utilize wet feedstocks (Eibisch et al. 2013) and minimize gaseous yields by operating at lower temperatures compared to pyrolysis, while retaining ≤ 80% of the feedstocks C content, in weight (Basso et al. 2013). Similarly to BC, studies show HC to be an effective soil amendment (Bento et al. 2019; Puccini et al. 2018; Steiner et al. 2009; Woolf et al. 2010). However, a weaker consensus exists regarding its suitability for C sequestration, with conflicting reports either promoting or dismissing its potential there for (Bamminger et al. 2014; Bargmann et al. 2014a; Busch and Glaser 2015; Eibisch et al. 2013; Libra et al. 2011; Malghani et al. 2014). In comparison to high temperature pyrolysis, HTC produces HCs with higher hydrogen (H) and oxygen (O) contents relative to C, making it more susceptible to microbial degradation and consequently, less stable in soils compared to BC (Bai et al. 2013; Malghani et al. 2014). Additionally, the presence of a relatively large, easily degradable labile C fraction in HCs (of various feedstock origins) typically reduces their effectiveness for C sequestration (Dicke et al. 2014; Libra et al. 2011). Nonetheless, diversely focused researches are in general agreement that the stability of HC in soils and therefore its suitability and effectiveness for C sequestration is largely dependent on the HTC process conditions and the feedstock material (Dieguez-Alonso et al. 2018; Schimmelpfennig et al. 2014). In this regard, production temperature claims the greatest degree of control (Kambo and Dutta 2015). It has been suggested that higher HTC production temperatures could improve the recalcitrance of the HC by creating a more condensed C structure (Greenberg et al. 2019; Fang et al. 2015).
The potential for HC to serve as a C sequestration tool could be significantly increased if, after its addition to soils, it interacted with- and became associated with the primary SOM. Several components of SOM exist in soils, which exhibit different degrees of recalcitrance (Duddigan et al. 2019). These include, in order of increasing protection against degradation (and therefore stability): (1) free (non-occluded) particulate organic matter (POMF); (2) occluded POM contained within aggregates (POMO); and (3) OM association with (bound to) clay particles (OMCl) in organo-mineral complexes (Duddigan et al. 2019; Trigalet et al. 2017). It is important to note that when HC/ BC materials are added to soils, they initially become part of the POMF fraction of the soil, and thereby add stability to this otherwise unstable OM fraction. (Haddix et al. 2016). Due to the recalcitrant nature of BC, it persists as POM particles in soils for thousands of years (Kuzyakov et al. 2009) and is therefore highly effective for C sequestration. However, as previously stated, HC is suggested to be less effective for this purpose than BC. Hence, it is worthwhile to consider whether there are other possibilities to sequester C using HC. A credible approach could involve the interaction and association of the decomposition products of HC with primary SOM, preferentially with the relatively stable SOM fractions, namely POMO and OMCl. The integration of C derived from HC (HC-C) into these fractions implies an enhanced protection of the HC-C against microbial decomposition and losses by erosion and leaching (Lanza et al. 2018). However, up to now, limited knowledge is available regarding the interaction of HC decomposition products with other SOM compounds, in particular, whether they become occluded within aggregates or associate themselves with clay particles. If such interactions do occur, it may be presumed that the way they take place is dependent on the nature of the HC-C.
We hypothesize that HC produced at a low(er) HTC temperature (also referred to as “low-temperature HC”) will show a greater level of decomposition than high(er)-temperature HC, resulting in a more substantial depletion of the HC-C pool in the POMF fraction and vice versa. The need to enhance SOC stocks and C sequestration is particularly prevalent in sandy soils, due to their typically low SOC content and poor soil quality and fertility (Dicke et al. 2014). To gain insight into the respective potentials of different HTC temperature-derived HCs to enhance SOC stocks and C sequestration in low fertility soils, their C input and its subsequent incorporation within the respective SOM fractions need to be examined. As such, this study aims to investigate the degree of decomposition of the HC-C pool within the POMF fraction of a Podzol and the potential of the HC decomposition products released from that (POMF-associated) HC-C pool to become associated with more recalcitrant soil structures (the POMO and OMCl fractions). This then further implies that the HC-C (of maize plant origin) would be considerably more protected against decomposition and mineralization. To achieve this, density fractionation and delta (δ) 13C signatures were employed to determine the amount of HC-C within each SOM component and the potential losses and gains of HC-C within the components.
Accordingly, the specific objectives of this study include to (1) compare the amount of C within the major SOM fractions (POMF, POMO and OMCl) that is derived from the different HTC temperature HCs in order to determine the amount of HC-C that is lost from the POMF fraction and potentially incorporated into the POMO and OMCl fractions of the soil; (2) compare the δ 13C signatures between the three major SOM fractions for the control and each HTC temperature HC (produced at 190, 210 and 230 °C); and (3) evaluate which of the HCs produced at increasing HTC temperatures has a higher C sequestration potential.
This study is justified by the potential economic and environmental advantages offered by HTC over pyrolysis, which necessitates the prioritization of determining the influences of specific HTC conditions on the characteristics of the final HC product, and in such, their suitability and value as a C sequestration tool for soils.
2 Materials and methods
2.1 Hydrochar preparation and characterization
The HC was prepared from a ≤ 1 mm shredded maize silage feedstock. In batches, the feedstock was homogenously mixed with distilled water at a 6:1 ratio (water: biomass), after which it was transferred into an inner Teflon liner and sealed in a stainless steel autoclave (Berghof, Germany). The autoclave was then set to the desired HTC production temperature (190, 210 or 230 °C) for a reaction time of 8 h per batch. After the thermochemical conversion of each batch, the HC and liquid products were separated by vacuum filtration and the HC was washed with distilled water and dried at 65 °C for approximately 48 h to achieve a constant mass. Once the required amount of HC was produced at each HTC temperature (190, 210 and 230 °C), the respective HCs were sieved < 2 mm, producing a fine material. The elemental composition, namely C, H, nitrogen (N) and sulphur (S) of the respective HCs were analyzed directly using a Euro Elemental Analyzer (Eurovector; HEKAtech GmbH). After determining the ash content of the respective HCs according to DIN EN 14775:2010-04, the O content was calculated as the difference between the total CHNS composition and the ash content of the respective HCs, according to the equation:
These results, which were used to calculate the atomic C/N, O/C and H/C ratios, are presented in Table 1. As a matter of interest, a scanning electron microscope (SEM) was used to capture high resolution images at 700 μm and at 2 μm of each HTC temperature HC prior to their addition to the soil to examine their potential structural differences (Fig. 1).
2.2 Soil properties and experimental set-up
The Podzol used in this study was collected from an arable field located in the Bruchhausen-Vilsen Municipality within Lower Saxony, Germany (52° 48′ 35″ N, 8° 59′ 41″ E) in December 2017. The study soils were cultivated with maize in 2017 and 2014. The soil was randomly collected from the upper 30 cm and air-dried in a greenhouse under non-climate-controlled conditions before being sieved ≤ 2 mm. Particle size distribution analysis classified the Podzol as a sandy loam, comprising 66% sand, 22% silt, and 13% clay (de Jager et al. 2020). Carbon and N analysis using a Thermo Fisher Scientific Flash 2000 Elemental Analyzer indicated an average soil C and N content of 18.1 g and 1.5 g per kg−1 soil, respectively. The HCs produced at 190, 210 and 230 °C were respectively homogenously mixed with the Podzol at an application rate of 5% (wt./wt.). It is presumed that the application of HC to the soil initially constituted almost exclusively to the POMF fraction. The soil and HC mixtures are hereinafter referred to as HC190, HC210 and HC230. Seven replicates of each HC-soil mixture and 5 replicates for the control of pure soil (no HC) were placed into respective pots of ~ 1 L volume in August 2018. In order to determine that the HCs were not detrimental to plant growth (a necessary prerequisite for any soil amendment), each pot (HC-amended soils and controls) was then sown with 3 Chinese cabbage seeds and allowed to grow in a climate-controlled greenhouse at a temperature of ~ 22 °C and using supplementary lighting. After a growth period of ca. 3 weeks, the number of plants in each pot was reduced to 1, and all the plants were maintained in the pots for approx. 1 year, with manual irrigation based on plant requirements. At the end of the experiment (September 2019), the soil in each pot was transferred into respective sampling bags and the plants and roots were gently removed from the soil as not to disturb aggregation. The soils were then transported to the laboratory where they were air-dried. The samples from HC190, HC210 and HC230 and the control were then homogenously mixed into respective composite samples prior to being sieved < 2 mm. Further analyses of the soils, as described below, were performed in triplicate.
2.3 Soil density fractionation and δ 13C analyses
The amount of HC-C fractionated between the three major SOM components (POMF, POMO, and OMCl) was quantified using a density fractionation technique and the stable C isotope, delta (δ) 13C signatures. The method is based on the difference between the δ 13C signature of HC-C (approx. − 13‰ from C4 maize plant feedstock) (Malghani et al. 2014) and original SOM under the growth of C3 plants (approx. − 28‰) (Del Galdo et al. 2003; Malghani et al. 2014). The change in this δ 13C signature of the native SOM was used to determine the amount of HC-C that was lost from the POMF fraction and became integrated into the POMO and OMCl fractions, using mass balance calculations of the δ 13C contents in each fraction.
The density fractionation procedure used to separate the SOM into the individual fractions (POMF, POMO and OMCl), followed the method described in Kalinina et al. (2019), using 13 g of each replicate sample and sodium (Na)-Polytungstate solution with a density of 1.8 g cm−3. Once the POMF, POMO and OMCl fractions were obtained for each sample (of the control and HC-amended soils after 1 year), they were weighed, and their respective weight proportions of the bulk sample were determined (Table 2). Each fraction of each sample was further analyzed for C and N as previously described. Based on the C content (g kg−1 soil, Table 2), the relative weight proportion of each fraction, the accumulated OC and the total OC (TOC) of each fraction for the control and HC-amended soils were determined and are presented in Table 2. The data for the sand and silt fractions were excluded due to their low C contents (≤ 0.52 g kg−1), however they were considered in the determination of the fractionation results. The C contents were also used to calculate the specific target weights required for each fraction (POMF, POMO and OMCl) for each sample for the δ 13C analyses. All samples were prepared according to the guidelines provided by the Centre for Stable Isotope Research and Analysis of the Georg-August-University of Göttingen, Germany. Using Acetanilide as the standard (control) material, the δ 13C analyses for the control, HC-amended soils and the pure HCs, were conducted by the Centre for Stable Isotope Research and Analysis by means of a Flash 2000 Elemental CN Analyzer (Thermo Fisher, Cambridge, United Kingdom) coupled via a Continuous Flow (ConFlo) III Interface to a Delta V Advantage Isotope Ratio Mass Spectrometer (IRMS) (Thermo Electron, Bremen, Germany). The resultant δ 13C signatures [in per mill (‰)] are presented in Fig. 2. The HCs produced at 190, 210 and 230 °C had avg. δ 13C values of − 13.6 ± 0.1, − 13.5 ± 0.1 and − 13.7 ± 0.0, respectively. The potential initial change in the δ 13C signatures of the bulk soil caused by the addition of each HC (at a 5% application rate) was calculated using the δ 13C signatures and C contents of the control and the different HCs. Since the HCs initially constituted as POMF when added to the soils (Haddix et al. 2016), it was expected that this change in δ 13C would only have taken place in the POMF fraction of the HC-amended soils at the beginning of the study. Thus, these calculated initial δ 13C signatures for the HC190, HC210 and HC230 treatments are hereinafter referred to as the initial free particulate organic matter (iPOMF). The measured δ 13C signatures were further used to calculate the percentage of HC-C in all fractions (iPOMF, POMF, POMO and OMCl) of each sample using the adapted mass balance equation (Baronti et al. 2017; Del Galdo et al. 2003):
where, \({C}_{HC-derived }\left[\%\right]\) is the amount of HC-derived C in the sample; \({\delta }^{13}{C}_{sample}\) is the isotopic signature of the sample; \({\delta }^{13}{C}_{control}\) is the isotopic signature of the pure soil before HC addition; and \({\delta }^{13}{C}_{HC}\) is the approximate isotopic signature of the added HC. These results (Online Resource 1) were then used together with the mass of each fraction to calculate the share of HC-C (%) of the bulk soil TOC in each fraction for the control and HC-amended soils. These HC-C values were then normalized according to the respective accumulated OC contents of the bulk soil TOC (Table 2), to represent the share of HC-C and native SOC in each fraction for the control and HC-amended soils. The native SOC content was determined as the difference between the accumulated OC and HC-C. These values are presented in Fig. 3.
2.4 Statistical analyses
The small sample size (n = 3) of this study meant that the critical assumption of a normal distribution could not be determined with certainty; therefore, the Kruskal–Wallis H test, which is considered a non-parametric alternative to the one-way analysis of variance (ANOVA) was applied to all residuals. The Dunn (1964) Pairwise Comparisons post-hoc test was used to determine significant differences in the accumulated OC contents, the δ 13C isotopic signatures, and the HC-derived C contents between groups (p < 0.05). This post-hoc test further applies a Bonferroni correction for multiple comparisons. Where data distributions were similarly shaped, the Kruskal–Wallis H test considered the median values for statistical analyses, and mean ranks where the data distributions were dissimilar in shape (Statistics.laerd.com 2015). Statistical analyses were performed using SPSS version 26.
3 Results and discussion
3.1 Physico-chemical properties of the hydrochars
The potential of thermally converted materials such as BC and HC to sequester C is related to (inter alia) the feedstock material (Eibisch et al. 2013) and the degree of carbonization that occurs during the conversion process, which in turn is largely dependent on the HTC temperature (Reza et al. 2015). These factors result in HCs with variable physico-chemical properties, namely the elemental composition and derived atomic ratios, which hint toward the HCs recalcitrance and resistance to degradation (Bargmann et al. 2014b; Bento et al. 2019; Santín et al. 2017). A relatively low degree of carbonization and an associated increased degradability of HC are supposedly indicated by a high H/C (≥ 0.6) and O/C (≥ 0.4) ratio (Dieguez-Alonso et al. 2018; Eibisch et al. 2013; Gai et al. 2014). Similarly for BC, an H/C ratio > 0.4 and ≤ 0.7 is considered stable and implies that 50% of the BC-C will potentially remain in the soil for at least 100 years (Woolf et al. 2019). Additionally, the C/N ratio is suggested to be indicative of the decomposition of organic compounds (Röhrdanz et al. 2019).
The elemental composition of the HCs shown in Table 1 indicates an increase in the C, N and ash contents, and a decrease in the O content of the HCs with increasing HTC production temperature, which indicates an increased stability of the HCs with increasing HTC production temperature. Except for the ash content, similar results were found by Reza et al. (2015) using a feedstock of wheat straw digestate produced at HTC temperatures of 180, 200 and 220 °C at a residence time of 8 h. It has been stated that a higher degree of carbonization occurs with increasing HTC temperatures, typically resulting in HC with an increased C content and reduced O content, making it more recalcitrant and thus more suitable for the purpose of C sequestration (Reza et al. 2015). The decrease in the elemental H and O contents with increasing HTC temperature is reportedly due to dehydration and decarboxylation processes (Funke et al. 2010; Mohammed et al. 2020), which is coincidental with a higher degree of carbonization and the enhanced formation of aromatic compounds during the HC production process (Dieguez-Alonso et al. 2018). The formation of such aromatic C structures is suggested to promote the stability of SOM and thereby enhance its resistance to degradation (Röhrdanz et al. 2016; Wu et al. 2015).
In this study, the atomic ratios (C/N, H/C and O/C) decreased with increasing HTC production temperature (Table 1). A C/N ratio above 30, as found in this study, suggests that net N immobilization may have occurred after HC was applied to the soil (Dieguez-Alonso et al. 2018), which may translate to a negligible or low nutrient supply to plants and microbes. Based on the H/C (≤ 0.6) and O/C (≤ 0.4) ratios that are considered reliable indicators of improved stability (Dieguez-Alonso et al. 2018; Eibisch et al. 2013), our results offer conflicting indications (Table 1). The H/C ratios for all the HTC temperature HCs were above 0.6, thus implying a low stability. Contrastingly, the O/C ratios were all ≤ 0.4, which is indicative of a relatively stable material that is theoretically suitable for C sequestration (Malghani et al. 2014). These concomitantly high H/C ratios and relatively low O/C ratios of the HCs are explained by Basso et al. (2013) as the result of the decarboxylation process that takes place during the HTC production process. The 230 °C HC exhibited the lowest H/C and O/C ratios of all the HCs, which suggests a relationship exists between enhanced stability and higher production temperatures. Basso et al. (2013) provide support for this finding by stating that with increasing HTC temperatures, the processes of dehydration and decarboxylation are enhanced, which lowers the O and H contents in relation to C, and therefore also lowers the H/C and O/C ratios.
The influence of production temperature can also be seen in the basic structure of the HCs, as captured by SEM imaging (Fig. 1).
From the SEM images above (Fig. 1a, d), it is shown that almost no structural disruption occurred in the HC produced at 190 °C, which possesses a coarser texture nearer to the structure of the original feedstock material with larger fibrous remnants (Falco et al. 2011), compared to the HCs produced at 210 °C (Fig. 1b, e) and 230 °C (Fig. 1c, f). According to Libra et al. (2011), more severe HTC process conditions typically result in a higher degree of deformation of the feedstock material. As such, lower HTC temperatures produce HCs with structures similar to the original biomass (Basso et al. 2013). Further support for this is provided by the progressively smaller particle sizes (i.e. finer texture) observed in the higher temperature HCs [210 °C (Fig. 1b) and 230 °C (Fig. 1c)], which signifies a reduction in these materials’ mechanical strength (Falco et al. 2011). As the HTC temperature increases, the fibrous network of the feedstock material is disrupted, and spherical particles begin to form on the surface of the biomass, which increases the surface roughness, as shown in the 210 °C HC (Fig. 1 e) and 230 °C HC (Fig. 1f), however the natural macrostructure of the biomass is maintained (Falco et al. 2011). The thermal decomposition of the fibrous components of the feedstock material is reported to occur at different HTC temperatures (Funke et al. 2010; Libra et al. 2011), which may explain the differential rates of decomposition between the different temperature HCs. At temperatures of ca. 180 °C, the hemicellulose components undergo the initial reaction of hydrolysis (Funke et al. 2010), while lignin is typically readily degraded at temperatures of ca. 200 °C. HTC temperatures above 220 °C are required for the degradation of the cellulosic components of the biomass (Libra et al. 2011), which is illustrated by the enhanced level of degradation evidenced for the 230 °C HC in Fig. 1(c, f). The inherent structure and particle size distribution of the HCs observed in this study may have implications for the interactions and associations that occur with the SOM fractions once the HC is added to soils.
3.2 Carbon contents of the soil organic matter (SOM) fractions
The C sequestration potential of HC in soils is largely dependent on the way it interacts and becomes associated with the major SOM fractions. Therefore, it is important to examine the changes that occur within the SOM fractions after the incorporation of HC into the soil.
The proportions (g per kg−1 soil) of each SOM fraction (POMF, POMO and OMCl) of the bulk soil for the control and HC-amended soils after approx. 1 year are presented in Table 2. The POMF fraction of the control made up ~ 62 g kg−1 of the bulk sample, while the POMO fraction comprised ~ 48 g kg−1 and the OMCl fraction ~ 8 g kg−1. According to Kölbl and Kögel-Knabner (2004), smaller POMO fractions are typical of soils with low clay contents due to decreased macro-aggregate formation, while the POMF fraction is independent of the clay content of the soil. While the HC-amended soils had a smaller proportion of POMF than the control after 1 year of soil incubation, they also comprised more POMO and OMCl, thus revealing a loss of OC from the POMF fraction and a gain of OC in the latter fractions. Furthermore, for the HC-amended soils, the proportion of the POMO fraction (avg. 96.4 ± 18.1 g kg−1) was larger than the POMF fraction (avg. 43.1% ± 11.0 g kg−1), while the OMCl fraction was the smallest (avg. 20.2 ± 3.7 g kg−1). The changes observed in the proportion of the POMO fraction in the HC-amended soils compared to the control are substantiated by Malghani et al. (2014), who observed an increased degree of aggregation in HC-amended soils within 3 months after HC addition, proposedly caused by an enhanced fungal growth or microbial biomass formation, which is often associated with decomposition. George et al. (2012) also noted that the incorporation of HC into aggregates was greater in soils that were subjected to plant- and mycorrhizal fungi growth (such as in this study), compared to incubated soils without these influences.
Furthermore, Libra et al. (2011) reported that very small BC particles were found in micro-aggregates of the Terra Preta de Indio soils of the Amazon Basin. Therefore, aggregation may have also been promoted by the relatively small particle size (Wagai et al. 2009) of the HCs in this study, which was comparable to that of a powder after sieving. The results indicate that after HC addition, the proportion of the less stable POMF fraction and associated labile C decreased and the proportion of the relatively more stable POMO fraction and therewith assumedly more passive C increased, which may be beneficial for the sequestration of C. Compared to the control, the larger amount of accumulated OC content in the POMO and OMCl fractions of the HC-amended soils is due to an increase of their weight proportions. Simultaneously to the positive priming that occurred in the POMF fraction of the HC-amended soils, it is proposed that the likely inclusion of the HC particles within newly formed aggregates, which thus constitute the POMO and OMCl fractions of the HC-amended soils, resulted in the further contribution of C directly from the HC within these fractions. Support hereof is provided by Kalinina et al. (2019), who found that new C additions to the more stable fractions of a Luvisol, namely OM bound to clays and associated organo-mineral complexes, were achieved within 1 to 5 years.
The TOC content of the bulk soil (sum of all fractions) and the accumulated OC content of each fraction for the control and HC-amended soils are also presented in Table 2. The addition of 5% HC containing 579 (190 °C HC), 607 (210 °C HC) and 705 (230 °C HC) g C kg−1 soil (Table 1) to the Podzol with 18.1 g C kg−1 soil at the beginning of the study would have likely increased the TOC content of the HC-amended (bulk) soils to approx. 47.1 (HC190), 48.5 (HC210) and 53.4 (HC230) g C kg−1 soil. Therefore, when comparing these values to those presented in Table 2, it is evident that during the 1 year study duration, the TOC content of the HC-amended soils decreased by ~ 20–23%. These C losses in the HC-amended soils are likely due to a positive priming effect, and the consequent mineralization of relatively easily decomposed labile C compounds of the HCs and the native SOC stocks, due to microbial stimulation (Gronwald et al. 2015; Lanza et al. 2018; Woolf et al. 2019). Since the HC-C is generally more recalcitrant than native SOC, a preferential mineralization of the latter typically occurs (Bargmann et al. 2014b; Dieguez-Alonso et al. 2018; Dodor et al. 2018).
This rather undesired effect was also reported in a meta-analysis by Wang et al. (2016), whereby the addition of BC to sandy soils with low fertility and clay contents < 10%, induced a significant positive priming effect. Bamminger et al. (2014) also found that after adding 40% HC (produced from maize silage at 220 °C) to an arable soil, only 15% of the observed C mineralization was that of the HC-derived C. Thus, the remaining C-mineralization was due to positive priming of the native SOC caused by HC addition. It is proposed that the HC provided a source of C and other nutrients to microorganisms, thereby reducing the limitations that previously prevented the mineralization of the native SOC (Bamminger et al. 2014).
3.3 The dynamics of hydrochar-derived carbon in the soil organic matter (SOM) fractions
3.3.1 δ 13C isotopic signatures of the SOM fractions
The use of the natural abundance δ 13C isotope allows for the differentiation between C inputs into the soil, based on the distinct δ 13C values of different plant tissues (Connin et al. 1996). In this study, the evolution of the δ 13C isotopic signatures between the SOM previously covered with C3 vegetation (approx. − 28‰) and the added HC produced from a (C4) maize silage feedstock (approx. − 13‰), were used to trace the fate of the HC-derived C within the SOM fractions.
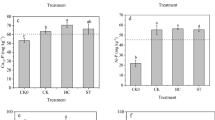
The δ 13C values of the SOM fractions of the control (avg. − 28.5‰) agree with the typical δ 13C value of soils under C3 vegetation (ca. − 28‰) (Del Galdo et al. 2003). However, an increase in the δ 13C values of the SOM fractions in the control was observed in the order of POMF < POMO < OMCl (Fig. 2). The statistically significantly higher δ 13C value of the OMCl fraction compared to the POMF fraction (p = 0.034) may be due to a prior period of C4 (i.e. maize) cultivation at the study site in 2014 and 2017, as noted in Sect. 2.2. The δ 13C traces of this earlier maize cultivation seem to have already been restored to the δ 13C value of the original SOM in the POMF and POMO fractions (− 29.0‰ ± 0.4 and − 28.7‰ ± 0.1, respectively). However, evidence there of is still visible in the slowest reacting OMCl fraction (Greenberg et al. 2019), which exhibits the highest δ 13C value (− 27.9‰ ± 0.1) of the bulk control sample. Shortly after adding the different HTC temperature HCs to the soil, the iPOMF δ 13C values increased compared to the control, resulting in δ 13C values of − 19.0‰ ± 0.1, − 18.7‰ ± 0.0 and − 18.4‰ ± 0.0 for the HC190, HC210 and HC230 treatments, respectively. Hence, the iPOMF fraction is the actual initial value for comparing the development of the δ 13C values of the POMF fraction over time.
The average δ 13C signatures (‰) of the major soil organic matter (SOM) fractions for the control and hydrochar (HC)-amended soils. The average calculated initial δ 13C signatures (‰) of each HC-amended soil is presented as iPOMF, which is the POM fraction just after adding HC. Error bars represent standard deviation of the means. Different letters indicate significant differences (p < 0.05) in means between treatments
After approx. 1 year of incubation (exactly 394 days from the beginning to the end of the pot trials), the δ 13C values of the HC190 and HC210 POMF fractions decreased to − 21.0‰ ± 0.2 and − 20.2‰ ± 0.4, respectively, compared to the iPOMF (approx. − 19.0‰). This indicates a loss of the maize-derived material from this fraction and simultaneously, a development towards the δ 13C signature of the original SOM (Fig. 2). The δ 13C values of the POMO and OMCl fractions in all HC-amended soils increased compared to the control, indicating the incorporation of HC. However, the OMCl fraction had the lowest δ 13C values of all the fractions for all the HC-amended soils (ranging between − 23.4‰ ± 0.8 and − 23.8‰ ± 0.8), which indicates that this fraction had the lowest input of HC-C compared to the POMF and POMO fractions. It is therefore evident that the interactions and associations that occur between HC and clay-bound OM in soils are relatively slowly acting, while in the POMF and POMO fractions, these processes likely occur at a faster rate, resulting in more rapid transformations (Christensen 2001; Greenberg et al. 2019).
The lower δ 13C values in all SOM fractions of the HC190 treatment compared to the other HC-amended soils might indicate a greater degree of decomposition, resulting in a more advanced development towards the original SOM status and a lower stability of the 190 °C HC, which confirms the results provided in Sect. 3.1. The HC230 POMF fraction maintained a δ 13C value (− 17.9‰ ± 0.0) similar to that of the iPOMF δ 13C value after HC addition (− 18.4‰ ± 0.0) (Fig. 2), and furthermore, exhibited a higher δ 13C value in the POMO fraction compared to the other HC-amended soils. This suggests that the HC produced at 230 °C may have a greater recalcitrance and was not as easily decomposed as the lower-temperature HCs, thereby allowing more of the HC-derived C to remain in these fractions (POMF and POMO). Fang et al. (2015) reported a similarly strong positive correlation between the recalcitrance of a HC and increasing production temperatures.
3.3.2 Hydrochar-derived carbon content of the SOM fractions
The determination of the amount of C in the different SOM fractions that is derived directly from the added HCs provides insight into the fate of the HC within the soils, specifically regarding its decomposition and/or interactions and associations of the resultant transformation products with the SOM fractions.
From Fig. 3, it is evident that at the beginning of the study (iPOMF), the HCs constituted between ca. 65–70% of the TOC content of the bulk soil. After approx. 1 year of soil incubation, 68–81% HC-derived C and 51–72% native SOC were lost from the POMF fraction in the HC-amended soils, due to positive priming which is in line with the low stability inferred by the H/C ratios (Sect. 3.1). It is also clear from Fig. 3 that the POMO fractions of all the HC-amended soils contained more HC-derived C than the POMF fractions. Furthermore, the native SOC content in the POMO fractions of all the HC-amended soils also increased considerably compared to the POMF fractions, resulting in almost equal proportions of HC-C and SOC-C in the POMO fractions. This improved HC-C and native SOC content suggests that the microbial decomposition of the labile C fractions of the different temperature HCs and of the native SOC, as evidenced by the difference between the iPOMF and POMF fractions, was only temporary and that the HC-derived C and native SOC became associated and incorporated within aggregates during the 1 year study. Similarly, Keith et al. (2011) reported observations of stabilization of labile OM into organo-mineral fractions via rapid interaction processes in a BC-amended soil. Our findings are substantiated by numerous studies (Keith et al. 2011; Wang et al. 2016; Woolf et al. 2019), which stated that the initially rapid mineralization of BC/HC-C was transient and was followed by a significantly lower rate of mineralization once the more labile C fraction had been decomposed by microorganisms. A study by Lanza et al. (2018) proposed that the labile C fraction of a HC produced from maize silage at 210 °C for 8 h, showed a rapid rate of decomposition within the first year after soil incorporation, followed by a significant reduction in mineralization due to the recalcitrance of a more stable form of C.
The average percent (%) carbon (C) derived from the different temperature hydrochars (HC-C) relative to the native soil organic carbon (SOC) content within the major soil organic matter (SOM) fractions at the beginning of the study (as iPOMF), and after approx. 1 year of soil incubation (as POMF, POMO and OMCl). Error bars represent standard deviation of the means. Different letters indicate significant differences (p < 0.05) in means between HC-C and native SOC within fractions. An asterisk (*) indicates significant differences (p < 0.05) in means between HC treatments
The results in Fig. 3 also indicate that the OMCl fractions of the HC-amended soils contained the least HC-derived C compared to the other fractions. Furthermore, this organo-mineral associated fraction also comprised more native SOC than HC-derived C. This finding highlights the slow reactivity of this fraction to changes such as organic amendment additions, compared to the POMO fraction (Christensen 2001; Greenberg et al. 2019).
4 Conclusion
The results from this study showed that the addition of maize-derive HCs produced at different HTC temperatures induced a positive priming effect which resulted in substantial losses of both HC-C and native SOC from the soil. The higher OC content of the POMO and OMCl fractions of the HC-amended soils suggests that HC-C was able to interact- and become associated with the SOM in these more stable fractions. However, this kind of C stabilization may not be enough to counteract or balance the losses observed from the less stable POMF fraction. While the effect of HTC temperature was evident in the physico-chemical- and structural properties of the HCs, no significant differences were observed in the overall losses and gains of C that occurred in the different HC treatments. Despite the large contribution of C to the soil by HC addition, the induced priming effect may counteract the overall aim of long-term C sequestration. Therefore, to verify the effectiveness of the different HTC temperature HCs for long-term C sequestration in soils, long(er)-term research on the C net balance that accounts for the observed priming-induced TOC losses and the observed HC-C enrichment in more stable fractions is required. For this purpose, the applied C fractionation coupled with stable C isotope measurements has proven a promising tool in this study.
Availability of data and materials
The data analyzed during this study which support its findings are available on request from the corresponding author.
Code availability
Not applicable.
References
Bai M, Wilske B, Buegger F, Esperschütz J, Kammann CI, Eckhardt C, Koestler M, Kraft P, Bach M, Frede H-G, Breuer L (2013) Degradation kinetics of biochar from pyrolysis and hydrothermal carbonization in temperate soils. Plant Soil 372:375–387
Bamminger C, Marschner B, Jüschke E (2014) An incubation study on the stability and biological effects of pyrogenic and hydrothermal biochar in two soils. Eur J Soil Sci 65:72–82. https://doi.org/10.1111/ejss.12074
Bargmann I, Martens R, Rillig MC, Kruse A, Kücke M (2014a) Hydrochar amendment promotes microbial immobilization of mineral nitrogen. J Plant Nutr Soil Sci 177:59–67. https://doi.org/10.1002/jpln.201300154
Bargmann I, Rillig MC, Kruse A, Greef JM, Kücke M (2014b) Effects of hydrochar application on the dynamics of soluble nitrogen in soils and on plant availability. J Plant Nutr Soil Sci 177:48–58. https://doi.org/10.1002/jpln.201300069
Baronti S, Alberti G, Camin F, Criscuoli I, Genesio L, Mass R, Vaccari FP, Ziller L, Miglietta F (2017) Hydrochar enhances growth of poplar for bioenergy while marginally contributing to direct soil carbon sequestration. GCB Bioenergy 9:1618–1626
Basso D, Castello D, Baratieri M, Fiori L (2013) Hydrothermal carbonization of waste biomass: progress report and prospects. In: 21st European Biomass Conference and Exhibition, 3-7 June, Copenhagen, Denmark, pp 1478–1487
Batjes NH (1998) Mitigation of atmospheric CO2 concentrations by increased carbon sequestration in the soil. Biol Fertil Soils 27:230–235. https://doi.org/10.1007/s003740050425
Bento LR, Castro AJR, Moreira AB, Ferreira OP, Bisinoti MC, Melo CA (2019) Release of nutrients and organic carbon in different soil types from hydrochar obtained using sugarcane bagasse and vinasse. Geoderma 334:24–32. https://doi.org/10.1016/j.geoderma.2018.07.034
Busch D, Glaser B (2015) Stability of co-composted hydrochar and biochar under field conditions in a temperate soil. Soil Use Manag 31:251–258. https://doi.org/10.1111/sum.12180
Buyanovsky GA, Wagner GH (1998) Carbon cycling in cultivated land and its global significance. Global Change Biol 4:131–141. https://doi.org/10.1046/j.1365-2486.1998.00130.x
Christensen BT (2001) Physical fractionation of soil and structural and functional complexity in organic matter turnover. Eur J Soil Sci 52:345–353. https://doi.org/10.1046/j.1365-2389.2001.00417.x
de Jager M, Röhrdanz M, Giani L (2020) The influence of hydrochar from biogas digestate on soil improvement and plant growth aspects. Biochar 2:177–194. https://doi.org/10.1007/s42773-020-00054-2
Del Galdo I, Six J, Peressotti A, Cotrufo MF (2003) Assessing the impact of land-use change on soil C sequestration in agricultural soils by means of organic matter fractionation and stable C isotopes. Global Change Biol 9:1204–1213. https://doi.org/10.1046/j.1365-2486.2003.00657.x
Dicke C, Lanza G, Mumme J, Ellerbrock R, Kern J (2014) Effect of hydrothermally carbonized char application on trace gas emissions from two sandy soil horizons. J Environ Qual 43:1790–1798. https://doi.org/10.2134/jeq2013.12.0513
Dieguez-Alonso A, Funke A, Anca-Couce A, Rombolà AG, Ojeda G, Bachmann J, Behrendt F (2018) Towards biochar and hydrochar engineering-influence of process conditions on surface physical and chemical properties, thermal stability, nutrient availability, toxicity and wettability. Energies 11:496. https://doi.org/10.3390/en11030496
Diochon AC, Kellman L (2009) Physical fractionation of soil organic matter: destabilization of deep soil carbon following harvesting of a temperate coniferous forest. J Geophys Res Biogeosci 114:1–9. https://doi.org/10.1029/2008JG000844
Dodor DE, Amanor YJ, Attor FT, Adjadeh TA, Neina D, Miyittah M (2018) Co-application of biochar and cattle manure counteract positive priming of carbon mineralization in a sandy soil. Environ Syst Res 7:5. https://doi.org/10.1186/s40068-018-0108-y
Duddigan S, Shaw L, Alexander P, Collins C (2019) A comparison of physical soil organic matter fractionation methods. Appl Environ Soil Sci 2019:1–12. https://doi.org/10.1155/2019/3831241
Eibisch N, Helfrich M, Don A, Mikutta R, Kruse A, Ellerbrock R, Flessa H (2013) Properties and degradability of hydrothermal carbonization products. J Environ Qual 42:1565–1573. https://doi.org/10.2134/jeq2013.02.0045
Falco C, Baccile N, Titirici MM (2011) Morphological and structural differences between glucose, cellulose and lignocellulosic biomass derived hydrothermal carbons. Green Chem 13:3273. https://doi.org/10.1039/c1gc15742f
Fang J, Gao B, Chen J, Zimmerman AR (2015) Hydrochars derived from plant biomass under various conditions: Characterization and potential applications and impacts. Chem Eng J 267:253–259
Fang Y, Singh BP, Nazaries L, Keith A, Tavakkoli E, Wilson N, Singh B (2019) Interactive carbon priming, microbial response and biochar persistence in a Vertisol with varied inputs of biochar and labile organic matter. Eur J Soil Sci 70:960–974. https://doi.org/10.1111/ejss.12808
Federal Ministry for the Environment, Nature Conservation, Building and Nuclear Safety (BMUB) (2016) Climate Action Plan 2050: Principles and goals of the German government’s climate policy. BMUB Public Relations Division, Berlin, pp 91. Available online: https://www.bmu.de/fileadmin/Daten_BMU/Pools/Broschueren/klimaschutzplan_2050_en_bf.pdf. Accessed 07 October 2020
Funke A, Ziegler F (2010) Hydrothermal carbonization of biomass: A summary and discussion of chemical mechanisms for process engineering. Biofuel Bioprod Biorefin 4:160–177. https://doi.org/10.1002/bbb.198
Gai X, Wang H, Liu J, Zhai L, Liu S, Ren T, Liu H (2014) Effects of feedstock and pyrolysis temperature on biochar adsorption of ammonium and nitrate. PLoS ONE 9(12):e113888
George C, Wagner M, Kücke M, Rillig MC (2012) Divergent consequences of hydrochar in the plant-soil system: Arbuscular mycorrhiza, nodulation, plant growth and soil aggregation effects. Appl Soil Ecol 59:68–72. https://doi.org/10.1016/j.apsoil.2012.02.021
Greenberg I, Kaiser M, Gunina A, Ledesma P, Polifka S, Wiedner K, Mueller CW, Glaser B, Ludwig B (2019) Substitution of mineral fertilizers with biogas digestate plus biochar increases physically stabilized soil carbon but not crop biomass in a field trial. Sci Total Environ 680:181–189. https://doi.org/10.1016/j.scitotenv.2019.05.051
Gronwald M, Don A, Tiemeyer B, Halfrich M (2015) Effects of fresh and aged chars from pyrolysis and hydrothermal carbonization on nutrient sorption in agricultural soils. Soil 1:475–489
Haddix ML, Paul EA, Cotrufo MF (2016) Dual, differential isotope labelling shows the preferential movement of labile plant constituents into mineral-bonded soil organic matter. Glob Chang Biol 22:2301–2312. https://doi.org/10.1111/gcb.13237
Kalinina O, Cherkinsky A, Chertov O, Goryachkin S, Kurganova I, Lopes de Gerenyu V, Lyuri D, Kuzyakov Y, Giani L (2019) Post-agricultural restoration: implications for dynamics of soil organic matter pools. CATENA 181:104096. https://doi.org/10.1016/j.catena.2019.104096
Kambo HS, Dutta A (2015) A comparative review of biochar and hydrochar in terms of production, physico-chemical properties and applications. Renew Sust Energ Rev 45:359–378. https://doi.org/10.1016/j.rser.2015.01.050
Keith A, Singh B, Singh BP (2011) Interactive priming of biochar and labile organic matter mineralization in a smectitie-rich soil. Environ Sci Technol 45:9611–9618
Kizito S, Luo H, Lu J, Bah H, Dong R, Wu S (2019) Role of nutrient-enriched biochar as a soil amendment during maize growth: Exploring practical alternatives to recycle agricultural residuals and to reduce chemical fertilizer demand. Sustainability 11:3211. https://doi.org/10.3390/su11113211
Kloss S, Zehetner F, Wimmer B, Buecker J, Rempt F, Soja G (2014) Biochar application to temperate soils: effects on soil fertility and crop growth under greenhouse conditions. J Plant Nutr Soil Sci 177:3–15. https://doi.org/10.1002/jpln.201200282
Kölbl A, Kögel-Knabner I (2004) Content and composition of free and occluded particulate organic matter in a differently textured arable Cambisol as revealed by solid-state 13C NMR spectroscopy. J Plant Nutr Soil Sci 167:45–53. https://doi.org/10.1002/jpln.200321185
Kuzyakov Y, Subbotina I, Chen H, Bogomolova I, Xu X (2009) Black carbon decomposition and incorporation into soil microbial biomass estimated by 14C labeling. Soil Biol Biochem 41:210–219. https://doi.org/10.1016/j.soilbio.2008.10.016
Lal R (2003) Soil erosion and the global carbon budget. Environ Int 29:437–450. https://doi.org/10.1016/S0160-4120(02)00192-7
Lal R (2004) Soil carbon sequestration to mitigate climate change. Geoderma 123:1–22. https://doi.org/10.1016/j.geoderma.2004.01.032
Lanza G, Stang A, Kern J, Wirth S, Gessler A (2018) Degradability of raw and post-processed chars in a two-year field experiment. Sci Total Environ 628–629:1600–1608. https://doi.org/10.1016/j.scitotenv.2018.02.164
Lehmann J, Gaunt J, Rondon M (2006) Bio-char sequestration in terrestrial ecosystems—a review. Mitigation Adapt Strateg Glob Change 11:403–427. https://doi.org/10.1007/s11027-005-9006-5
Libra JA, Ro KS, Kammann C, Funke A, Berge ND, Neubauer Y, Titirici M-M, Fühner C, Bens O, Kern J, Emmerich K-H (2011) Hydrothermal carbonisation of biomass residuals: a comparative review of the chemistry, processes and applications of wet and dry pyrolysis. BioFuels 2:71–106
Liu S, Bliss N, Sundquist E, Huntington TG (2003) Modeling carbon dynamics in vegetation and soil under the impact of soil erosion and deposition. Global Biogeochem Cycles 17(2):1074. https://doi.org/10.1029/2002GB002010
Malghani S, Gleixner G, Trumbore SE (2013) Chars produced by slow pyrolysis and hydrothermal carbonization vary in carbon sequestration potential and greenhouse gases emissions. Soil Biol Biochem 62:137–146. https://doi.org/10.1016/j.soilbio.2013.03.013
Malghani S, Jüschke E, Baumert J, Thuille A, Antonietti M, Trumbore S, Gleixner G (2014) Carbon sequestration potential of hydrothermal carbonization char (hydrochar) in two contrasting soils; results of a 1-year field study. Biol Fertil Soils 51:123–134. https://doi.org/10.1007/s00374-014-0980-1
Mohammed IS, Na R, Kushima K, Shimizu N (2020) Investigating the effect of processing parameters on the products of hydrothermal carbonization of corn stover. Sustainability 12(12):5100. https://doi.org/10.3390/su12125100
Puccini M, Ceccarini L, Antichi D, Seggiani M, Tavarini S, Latorre MH, Vitolo S (2018) Hydrothermal carbonization of municipal woody and herbaceous prunings: hydrochar valorisation as soil amendment and growth medium for horticulture. Sustainability 10:846. https://doi.org/10.3390/su10030846
Reza MT, Mumme J, Ebert A (2015) Characterization of hydrochar obtained from hydrothermal carbonization of wheat straw digestate. Biomass Convers Biorefin 5:425–435. https://doi.org/10.1007/s13399-015-0163-9
Röhrdanz M, Rebling T, Ohlert J, Jasper J, Greve T, Buchwald R, von Frieling P, Wark M (2016) Hydrothermal carbonization of biomass from landscape management—influence of process parameters on soil properties of hydrochars. J Environ Manage 173:72–78. https://doi.org/10.1016/j.jenvman.2016.03.006
Röhrdanz M, Greve T, de Jager M, Buchwald R, Wark R (2019) Co-composted hydrochar substrates as growing media for horticultural crops. Sci Hortic 252:96–103
Santín C, Doerr SH, Merino A, Bucheli TD, Bryant R, Ascough P, Gao X, Masiello CA (2017) Carbon sequestration potential and physicochemical properties differ between wildfire charcoals and slow-pyrolysis biochars. Sci Rep 7:11233
Schimmelpfennig S, Müller C, Grünhage L, Koch C, Kammann C (2014) Biochar, hydrochar and uncarbonized feedstock application to permanent grassland-Effects on greenhouse gas emissions and plant growth. Agric Ecosyst Environ 191:39–52. https://doi.org/10.1016/j.agee.2014.03.027
Statistics.laerd.com (2015) Kruskal-Wallis H Test. Available online: https://statistics.laerd.com/premium/spss/kwht/kruskal-wallis-test-in-spss.php. Accessed 12 January 2021
Steiner C, Teixeira WG, Woods WI, Zech W (2009) Indigenous knowledge about terra preta formation. In: Woods WI, Teixeira WG, Lehmann J, Steiner C, WinklerPrins A, Rebellato L (eds) Amazonian dark earths: Wim Sombroek’s vision. Springer, Dordrecht, pp 193–204
Trigalet S, Chartin C, Krüger I, Carnol M, Van Oost K, Van Wesemael B (2017) Soil organic carbon fractionation for improving agricultural soil quality assessment—a case study in Southern Belgium (Wallonia). Biotechnol Agron Soc Environ 21(3):191–200. https://doi.org/10.25518/1780-4507.13422
Wagai R, Mayer LM, Kitayama K (2009) Nature of the “occluded” low-density fraction in soil organic matter studies: A critical review. Soil Sci Plant Nutr 55(1):13–25. https://doi.org/10.1111/j.1747-0765.2008.00356.x
Wang J, Xiong Z, Kuzyakov Y (2016) Biochar stability in soil: meta-analysis of decomposition and priming effects. GCB Bioenergy 8:512–523. https://doi.org/10.1111/gcbb.12266
Woolf D, Amonette JE, Street-Perrott FA, Lehmann J, Joseph S (2010) Sustainable biochar to mitigate global climate change. Nat Commun 1:1–9. https://doi.org/10.1038/ncomms1053
Woolf D, Lehmann J, Cowie A, Cayuela ML, Whitman T, Sohi S (2019) Biochar for climate change mitigation: navigating from science to evidence-based policy. In: Lal R, Stewart BA (eds) Soil and climate. CRC Press Taylor & Francis Group, Florida, pp 219–348
Wu M, Feng Q, Sun X, Wang H, Gielen G, Wu W (2015) Rice (Oryza sativa L) plantation affects the stability of biochar in paddy soil. Scientific Reports 5:1–10. https://doi.org/10.1038/srep10001
Acknowledgements
We would like to express our gratitude to the European Union Interreg North Sea Region Project for their sponsorship. Secondly, a sincere thank you to Joel Bröring for his assistance in the laboratory work, and to Olga Kalinina for her valuable advice and discussions. Thirdly, an earnest thank you is owed to my husband, Dr. Thomas Pollmann, for his continued support and encouragement.
Funding
This study was conducted under the auspices of the ‘BIOCAS, circular BIOmass CAScade to 100%’ project, which is funded by the European Union Interreg North Sea Region Project 38-2-4-17. The funders played no role in the design of the study, the collection and analyses of data or the preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
MdJ and LG conceptualized and designed the study. Hydrochar production was performed by MdJ together with FS. The chemical- and SEM analyses of the hydrochars were performed by FS under the supervision of MW. Data collection and analyses were performed by MdJ. All versions of the manuscript were written by MdJ. Sample collection and the revision and editing of all manuscript versions were performed by LG. All authors have read and approved the final manuscript for submission.
Corresponding author
Ethics declarations
Competing interests
The authors have no conflicts of interest to disclose, financial or otherwise.
Supplementary information
Additional file 1.
Percentage (%) carbon in the major soil organic matter fractions derived from the different HTC temperature hydrochars.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
de Jager, M., Schröter, F., Wark, M. et al. The stability of carbon from a maize-derived hydrochar as a function of fractionation and hydrothermal carbonization temperature in a Podzol. Biochar 4, 52 (2022). https://doi.org/10.1007/s42773-022-00175-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42773-022-00175-w