Abstract
Biochar amendment and substituting chemical fertilizers with organic manure (organic substitution) have been widely reported to increase crop production and decrease reactive nitrogen (Nr) loss including nitrous oxide (N2O), nitric oxide (NO), and ammonia (NH3) emissions, and N runoff and leaching. However, few comprehensive evaluations have been performed on the environmental and economic aspects of biochar amendment or organic substitution. Here, we studied the comprehensive effects of biochar amendment, organic substitution, and biochar amendment combined with organic substitution on crop production, Nr loss, and net ecosystem economic benefit (NEEB) in intensive vegetable production by integrating life-cycle assessment for Nr footprints, empirical models for NH3 volatilization and N runoff and leaching derived from peer-reviewed publications and validated by the current measurements and direct field measurement for N2O and NO emissions during 5 consecutive years of vegetable crop rotations. Five fertilization treatments were applied (SN: synthetic fertilizer application; SNB: SN plus 20 t ha−1 biochar amendment; SNM: substituting 50% of chemical N fertilizer with organic manure; SNMB: SNM plus 20 t ha−1 biochar amendment; and CK: no fertilizer or biochar addition). Compared with the SN, the SNB increased vegetable yield (28.4%, p < 0.05; interannually varying from − 10 to 74.9%) and nitrogen use efficiency (29.2%, interannually varying from − 39.7 to 150.4%), and decreased field Nr loss (45.4%, p < 0.01; interannually varying from − 40.3 to 78.4%), and thus improved NEEB by 7.1%; meanwhile, the SNM increased vegetable yield (11.6%, interannually varying from − 5.4 to 27.1%) and nitrogen use efficiency (45.7%, p < 0.05; interannually varying from 2.3 to 154%), reduced field Nr loss (34.9%, p < 0.01; interannually varying from 8.4–39.0%), and thus improved NEEB by 17.8% (p < 0.05) compared to the SN, being 56.0 × 103 Chinese Yuan (CNY) ha−1 crop−1. Due to the high foreground Nr loss during organic manure production and high input costs of biochar production, the SNMB decreased the NEEB by 8.0% as compared to the SN. Moreover, the SNB and SNM improved vegetable qualities by increasing protein, soluble sugar, and vitamin C contents while decreasing nitrate content (p < 0.05). Therefore, single application of biochar amendment or organic substitution would achieve better NEEB and product quality in vegetable production.
Graphical Abstract

Highlights
-
Both biochar and organic substitution improved vegetable quality, yield and NEEB.
-
Manure production is a hotspot in the foreground Nr loss and N leaching in the field.
-
Organic substitution alone produced best NEEB while least in the combined treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The national vegetable production area in China in 2019 was approximately 20.9 million ha, which accounted for 16.3% of the country’s total arable land (MNR 2021). Vegetable fields receive a high use amount and frequent input of chemical fertilizers and a high multiple cropping index compared to food crop farmland (Zhou et al. 2019), leading to a significant decline in the nitrogen use efficiency (NUE) and soil quality (Mohanty et al. 2020). Reactive nitrogen (Nr) loss in the form of ammonia (NH3), nitrogen oxides (NOX), and nitrous oxide (N2O) accounts for approximately 10% of the nitrogen (N) input, with additional N lost by runoff or leaching in vegetable fields (Tian et al. 2016). All these factors not only aggravate environmental pollution and environmental loss costs (Zhang et al. 2015) but also restrict the sustainable development of the vegetable industry.
As an effective countermeasure, biochar amendment has been widely reported to improve soil quality, NUE and crop yield (Liu et al. 2016; Zhang et al. 2020a, b). Biochar is a stable carbon-rich material produced by the thermochemical transformation of biomass (e.g. crop straw, manure and solid waste) under oxygen-limited or anaerobic conditions (Harter et al. 2016; Osman et al. 2020). Recently, biochar has been extensively studied to determine its ability to enhance nutrient availability in soils (Zhang et al. 2020a, b), promote crop growth (Steinbeiss et al. 2009) and reduce Nr losses (Duan et al. 2018; Xu et al. 2022). Biochar could increase the pH and permeability of the soil (Abel et al. 2013), ease biological and abiotic stresses, and improve microbial activity and biodiversity (Azeem et al. 2020). Although biochar is generally considered to be mostly inert, numerous studies have shown that when applied to soil, it undergoes changes, such as aging and weathering/maturing over time (Yuan et al. 2019; Zhang et al. 2019). A relatively long-term experiment with an annual assessment would illustrate the actual effect of biochar application in vegetable fields.
Meanwhile, substituting some chemical fertilizer with organic manure (organic substitution) has been widely used to reduce the over-reliance on chemical fertilizers and environmental damage (Cai et al. 2018) although complete organic manure cannot meet the immediate demand for crop nutrients (Xia et al. 2017). Organic substitution can regulate the process of soil N transformation (Liu et al. 2014a, b) and provide exogenous carbon by enhancing microbial activity and increasing soil N and organic carbon fixation (Tang et al. 2019), thereby further improving crop yields and NUE and reducing Nr losses (Gu et al. 2016). Previous studies (Zhou et al. 2019) have found that organic substitution may benefit vegetable production. However, integrated studies of the comprehensive effects and mechanisms of biochar amendment combined with organic substitution in vegetable production are lacking.
Obtaining high yield of qualified vegetable products is the primary goal for sustainable vegetable production at minimal environmental damage costs (EDCs) and maximum net economic benefit (NEB). EDCs refer to the costs of degradation of the quality of environmental service functions due to the Nr losses, integratively as N footprint, and greenhouse gases (GHGs) from natural or anthropogenic activities that damage human health and ecosystems (Ying et al. 2017). The net ecosystem economic benefit (NEEB), the difference between the NEB (crop yield benefit minus the costs of agrochemical inputs) and environmental damage cost of crop production, has been widely used to assess the sustainability of development in agricultural production (Liu et al. 2020). In addition to the large amounts of emissions from farmland application, the production and transportation of agrochemical inputs and the associated consumption of energy will indirectly produce Nr and GHGs (Lehmann et al. 2021; Zhang et al. 2013). Therefore, the widely accepted life cycle assessment (LCA) method for Nr release and GHGs should be adopted for comprehensively assessing the EDC (Khoshnevisan et al. 2014) and NEEB in intensive vegetable production.
The objectives of our study were to (1) evaluate the effects of optimal substitution and inter-annual effects on vegetable crop productivity and quality; (2) quantify Nr losses and N footprint using the LCA method; and (3) optimize alternative treatment to improve the feasibility of achieving environmental and economic benefits, including better NEEBs with lower EDCs in intensive vegetable production. Based on the above-mentioned beneficial consequences of amended biochar and substitution of partial synthesized N fertilizer, we hypothesized that biochar amendment and the suitable organic substitution would result in the best NEEB by improving crop production and reducing the Nr losses and GHG (N2O) emissions in a 5-year consecutive vegetable rotation.
2 Materials and methods
2.1 Experimental site description
The test soil was collected from a typical greenhouse vegetable site in Nanjing, Jiangsu Province (32° 01′ N, 118° 52′ E), Yangtze River Delta Alluvial Plain, China, for a 5-year period from November 2017 to November 2021, which is a typical annual rotation cultivation vegetable system for more than 10 years with an average annual temperature and precipitation of 17.5 °C and 1107 mm. The physical and chemical properties of tested soil are shown in Additional file 1: Table S1.
2.2 Experimental design and field management
Five fertilization treatments with three replicates were established as follows: (1) no fertilizer or biochar addition (CK); (2) synthetic fertilizer application (SN); (3) SN plus 20 t ha−1 biochar amendment (SNB); (4) substituting 50% of chemical N fertilizer with organic manure (SNM); and (5) SNM plus 20 t ha−1 biochar amendment (SNMB). Biochar was added only once in 2016. Additional file 1: Table S1 presents the properties of the biochar. Both organic and synthetic fertilizers were applied as designed for each treatment before sowing in each crop growing season. The N application rate was 240 kg N ha−1 crop−1 as urea; the phosphate (P) application rate was 120 kg P ha−1 crop−1 as superphosphate; the potassium (K) application rate was 240 kg K ha−1 crop−1 as potassium chloride. The organic manure purchased from Nanjing Mingzhu Fertilizer Co., Ltd was applied at 120 kg N ha−1 crop−1 containing 1.1% TN, 0.13% P, 0.83% K and 28.3% organic matter.
Continuous vegetable crop rotations were allocated on adjacent lands. Additional file 1: Table S2 lists the various management dates for the 5-year vegetable cultivation. Vegetable tillage, irrigation and fertilizer application were carried out according to local management practices. The seeds were reasonably sown and mulched to promote seed germination after moderate irrigation. Appropriate irrigation and pesticide spraying were performed during the growing season of vegetables, and plastic greenhouses were installed to ensure the temperature needed for vegetable growth or to withstand adverse weather. There was a short fallow period of about 15–30 days during the vegetable crop rotation. One vegetable crop per year was selected as representative to study the crop yield and Nr losses for 5 consecutive years.
2.3 Sample collection and determination
Fresh soil samples were collected from a depth of 0–20 cm. Three replicate samples were removed of debris such as roots and stones and later were divided into two parts, one air-dried and passed through a 2.0 mm sieve to determine soil pH, electrical conductivity (EC) and TN content, and the other to determine soil nitrate nitrogen (NO3−), ammonium nitrogen (NH4+), dissolved organic carbon (DOC) and dissolved organic nitrogen (DON) contents. Soil TN content was analyzed using an elemental analyzer (Vario EL Cube, Elementar, Germany); soil organic matter (SOC) was determined by the volumetric method using potassium dichromate (K2Cr2O7); soil DOC and DON contents were measured using a multiple N/C analyzer (Jena TOC analyzer, Germany) after extraction with distilled water at a soil to water ratio of 1:4 (w:v). Soil pH, EC, temperature, moisture, NO3− and NH4+ concentrations were measured using methods described in a previous study (Zhang et al. 2020a, b).
After the vegetables were harvested, the yield was calculated based on the total fresh biomass of vegetables in the plot. Samples of fresh vegetables were collected for quality analysis of the protein, vitamin C, soluble sugar and nitrate contents (determined by the standard method of the Quality and Safety Supervision and Testing Center for Agricultural Products and TRANSGENIC Products of the Ministry of Agriculture). The plant samples were first killed at 105 °C and then dried at 70 °C to a constant weight to calculate the moisture and the weight of dry matter. The N uptake in plants was determined by the Kjeldahl method. The NUE was calculated by the N absorption of each treatment and CK and divided by the amount of N applied.
2.4 System boundaries
In this study, the LCA method was used to estimate the N footprint of intensive vegetable production under different fertilizer treatments. The system boundary (Fig. 1) includes the EDC, NEB and NEEB. The EDC consists of N footprint and GHG emissions from agrochemical inputs and energy production. N footprint consists of Nr losses in the foreground interface and the field interface throughout the life cycle. The foreground interface includes the fuel and energy consumption due to agrochemical inputs (inorganic and organic fertilizers, biochar, pesticides, and plastic film) as well as farming operations in production and transportation (Hamedani et al. 2019; Zhou et al. 2019). The field interface includes N2O emissions, NO emissions, NH3 volatilization, N leaching and N runoff during field crop growing seasons. Since all the products are sold as fresh vegetables and are not processed, no consideration is given to the loss of greenhouse gases and natural Nr during food processing. NEB consists of the benefits of vegetable yield and the costs of agrochemical inputs.
2.5 Field monitoring and analysis of Nr losses and GHGs
Gas emissions were collected by the static opaque chamber method every 2 days between 8:00 and 10:00 a.m. for 2 weeks after fertilization and every other week thereafter. Samples were measured with a gas chromatograph (Agilent 7890A, Agilent Ltd., Shanghai, China) and a NOx analyzer Thermo Model 42i (Thermo Environmental Instruments Inc., Franklin, MA, USA) for N2O and NO concentrations, respectively (Zhou et al. 2019). Details of the sampling measurements are given in our previous publications (Fan et al. 2017; Zhou et al. 2019). The emission fluxes of each gas were obtained from the slope of a linear regression of the gas volume ratio of each sample group against the corresponding sampling time, and the cumulative annual emissions were calculated from the average flux weighted by the time interval. The calculation formula of gas emission flux is as follows:
where F represents the N2O and NO emission fluxes (μg N m−2 h−1); ρ represents the density of N2O-N and NO-N under standard conditions, which have values of 1.25 g L−1 and 0.625 g L−1, respectively; V is the volume of the sampling box (m3); A is the surface area of soil in the sampling base (m2); \(\frac{\Delta C}{\Delta t}\) represents the emission rate of gases (the units of N2O and NO are nL L−1 h−1 or ppbv h−1) and the coefficient of the linear regression equation performed between the gas concentration of four or two samples collected each time in each plot, and time, and T is the temperature in the sampling box (°C).
The empirical models used for the calculation of NH3 volatilization, N leaching, N runoff and the change ratio of NH3 volatilization in organic and inorganic fertilizers (Additional file 2: A1) are shown below from 17 peer-reviewed publications on vegetable production in China (Zhou et al. 2019). The change ratio of NH3 volatilization with biochar addition (Additional file 3: A2) is shown below from 17 peer-reviewed publications on vegetable production. We selected one season to determine the NH3 volatilization of vegetable crops for model validation (Additional file 1: Fig. S1). The change ratios of the combined application of organic and inorganic matter and biochar addition were consistent with the measured change ratios in our study, indicating that our estimation model was reasonable. Therefore, the measured change ratios of the combined application of the three factors were used to estimate NH3 volatilization throughout the test period. Among them, the measured value was lower than the model estimated value, which may be due to the low temperature and low TN content in the measurement season, which slows NH3 volatilization. The change ratio of N leaching and N runoff under biochar addition and organic substitution was determined based on a meta-analysis of data from 141 references (Madaline et al. 2021), which is consistent with the result of our empirical model. Due to the lack of N runoff and N leaching loss data for the SNMB, we adopted the average value of the two single treatments to represent the combined treatment. All selected studies met the following criteria. First, NH3 volatilization was measured by the micrometeorological or dynamic chamber method, and N leaching and N runoff were measured by the suction cap or lysimeter method. Second, the means and sample sizes of the control and treatment plots were clarified. Third, the amount of N application, the replacement ratio of organic fertilizer and the amount of biochar addition were clarified.
where the N rate denotes the application rate of N fertilizer; CRM NH3, CRB NH3 and CRMB NH3 denote the relative change rates of soil NH3 volatilization in the SNM, SNB, and SNMB, respectively; RsM denotes the ratio of replacing chemical fertilizer with organic manure; RsB denotes the ratio of added biochar; NM leaching, NB leaching and NMB leaching denote N leaching in the SNM, SNB, and SNMB, respectively; and NM runoff, NB runoff and NMB runoff denote N runoff in the SNM, SNB, and SNMB, respectively.
2.6 EDC and NEEB
In this study, the NEEB, NEB and the EDC of each treatment were estimated as follows:
where Ya (kg ha−1) represents the yield of vegetable “a”; Pa (¥ kg−1) represents the market price of vegetable “a”; Rm (kg ha−1) represents the use of agrochemical inputs and energy “m”; and Pm (kg−1) represents the actual purchase prices of agrochemical inputs and energy “m”. The detailed price is shown in Additional file 1: Table S3.
The EDC is defined mainly as the damage costs caused by the foreground and field Nr losses to the ecosystem and human health and N2O as a GHG to climate warming, and is evaluated by the following equation.
where NriA (kg N ha−1) represents the total emission of reactive nitrogen “i”; Pi (¥ kg−1 N) represents the cost of environmental damage per unit mass of reactive nitrogen “i”; N2OA (kg N ha−1) represents greenhouse gas emissions (N2O); and PN2O (¥ kg−1 N) represents the greenhouse effect caused by greenhouse gas emissions (expressed in international trading prices). Detailed EDCs are shown in Additional file 1: Table S3.
2.7 Uncertainty analysis
Considering that the foreground Nr loss parameters were obtained from our published articles and the emission factors were extracted from those widely used studies, we should recognize the inaccuracy of the results caused by the uncertainty of the evaluation criteria in this study. A separate empirical model was established using corrected R2 values to limit the uncertainty, and the relevant parameters cited have been given their standard errors. Still, the variations can be large due to differences in seasons, crop species, and unit damage prices. Furthermore, biochar relies heavily on the empirical analysis of market participants and researchers due to differences in feedstock and preparation processes and prices (Sun and Huang 2020; Hao and Tian 2020). Therefore, the overall effects of biochar amendment and organic substitution on the ecosystem may have been underestimated or overestimated to some extent.
2.8 Data analysis and statistics
Data calculation and statistical analyses were performed by Microsoft Excel and SPSS (IBM Co., Armonk, NY, USA) and visualized by Origin (Version 9.0, USA) and R software. One-way ANOVAs were performed to analyze the responses of crop production and Nr losses to the different treatments and inter-annual variation. Two-factor ANOVAs were performed to evaluate the interaction between the treatment and year. The Tukey test was used for multiple comparisons of whether the difference between the means of each variable was significant (p < 0.05).
3 Results
3.1 Soil physical and chemical properties
As shown in Fig. 2, compared with the SN, the SNB increased the pH (5.8%, p < 0.01), the contents of DON (57.3%, p < 0.05) and NO3−-N (18.3%, p < 0.05); the SNM and SNMB increased the SOC (25.4 and 28.4%, respectively; p < 0.01) and TN (11.2 and 13.6%, respectively; p < 0.05), thus all improving the soil quality (Additional file 1: Fig. S2a). In the SNB, the content of NO3−-N (158.1–433.1 mg kg−1) was 116.3% (p < 0.01) and 65.4% (p < 0.01) higher than that in the SNM (29.9–237.9 mg kg−1) and SNMB (63.0–256.9 mg kg−1), respectively, while the content of NH4+-N (46.9–71.0 mg kg−1) was decreased by 18.8% (p < 0.05) and 9.7%, respectively. Therefore, biochar amendment had a positive effect on the transformation of N to a state that is more easily absorbed and utilized by crops, and the effect is better than that of the SNMB. The inter-annual variation trends of soil physical and chemical properties in all treatments were basically similar, although the soil NO3−-N showed a decreasing trend for 4 consecutive years (Additional file 1: Table S4).
The average soil pH (a), EC (b), NH4+ (c), NO3− (d), DOC (e), DON (f), SOC (g), and TN (h) under different treatments over a 5-year period of intensive vegetable production. Different letters above the bars indicate significant differences among the different treatments (p < 0.05). Bars indicate the standard deviation
3.2 Crop productivity and quality
Across the five seasons, the largest vegetable yield was from the SNB, which was 1.3 times greater than that from the SN (Fig. 3a). The average vegetable yields varied at 1020.0–2149.6 kg ha−1 crop−1 for all treatments (Fig. 3b). On average, the SNB and the SNM increased the vegetable yield by 28.4% (p < 0.05; interannually varying from − 10 to 74.9%) and 11.6% (interannually varying from − 5.4 to 27.1%) while the SNMB decreased the vegetable yield by 4.4% (interannually varying from − 12.3 to 19.7%) compared with the SN. After biochar amendment, the vegetable yields showed an increasing trend from 2017 to 2019, at 2.21 t ha−1, 2.26 t ha−1, and 2.49 t ha−1, although the effect of biochar gradually decreased after approximately 4 to 5 years of observation.
Productivity and nitrogen use efficiency under different treatments in intensive vegetable production. The vegetable dry biomass (a), nitrogen uptake (c) and nitrogen use efficiency (e) under different treatments in intensive vegetable production, and (b), (d), (f) the average biomass, nitrogen uptake and nitrogen use efficiency of the treatments over a 5-year period. Different letters above the bars indicate significant differences among the different treatments (p < 0.05). Bars indicate the standard deviation. Two-way ANOVA results for vegetable biomass and nitrogen uptake in plants of year, treatment and their interactions (year × treatment) are also shown in the figure. *, ** and *** indicate significant differences at the p < 0.05, p < 0.01 and p < 0.001 levels, respectively
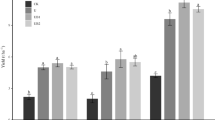
Compared with the SN (66.4 kg ha−1 crop−1), the SNM (80.0 kg ha−1 crop−1) increased the N uptake by 20.5% (p < 0.05; interannually varying from 1.1 to 44.5%), while the SNB (75.1 kg ha−1 crop−1) and SNMB (72.6 kg ha−1 crop−1) increased the N uptake by an average of 13.1% and 9.5%, respectively (Fig. 3c, d). The SNM and SNB improved the NUE by an average of 45.7% (p < 0.05; interannually varying from 2.3 to 154%) and 29.2% (interannually varying from − 39.7 to 150.4%) compared to the SN (Fig. 3e, f).
Compared with the SNM and SNMB, the contents of the protein and soluble sugar were increased by 13.4% and 18.1%, 6.7% and 17.8%, while the nitrate content was decreased by 7% and 13% under the SNB, respectively (Fig. 4a–d). The average product contents of protein, soluble sugar, and vitamin C over the 5 years under the SNB were increased by 22.6%, 25.9%, and 28.8%, respectively as compared to the SN (p < 0.05), while the corresponding values were insignificantly increased by 11.1%, 12.6%, and 13.8% under the SNM (Fig. 4e). Compared to the SN, the content of nitrate under the SNB was lower by 27.2% (p < 0.05) while the value was 25.7% for the SNM. Hence, biochar amendment is the optimal fertilization for improving crop quality (Additional file 1: Fig. S2b).
Quality (protein, soluble sugar, vitamin C, and nitrate) of vegetables under different treatments and years in intensive vegetable production. Different letters above the bars indicate significant differences among the different treatments at p < 0.05. Bars indicate the standard deviation (mean ± SD, n = 3)
3.3 N footprint and EDC
During the whole vegetable rotation period, the dynamic changes in N2O fluxes were varying with soil temperature and soil water-filled porosity (Additional file 1: Fig. S3a). N2O emissions were higher under 65–80% WHC and 30 °C than those under 60% WHC and 25 °C. For the first 3 years from 2017 to 2019, N2O fluxes ranged from 9.66 to 2890.26 µg N m−2 h−1, and its peak was detected within 1 week after fertilization (Additional file 1: Fig. S3c), with the largest N2O flux occurring in the SN (on average 1787.73 µg N m−2 h−1) which was 57.4% higher than the biochar amendment and 53.6% higher than the organic substitution (p < 0.01). For the next 2 years during 2020 and 2021, the largest N2O flux became on average 2036.92 µg N m−2 h−1 and occurred for the SNB.
The NO fluxes presented similar seasonal dynamics to those of N2O fluxes (Additional file 1: Fig. S3b). Cumulative N2O emissions varied from 0.20 to 3.76 kg N ha−1 crop−1 and the cumulative NO emissions ranged from 0.04 to 0.45 kg N ha−1 crop−1 across all (Table 1). Compared with the SN, the organic substitution reduced N2O emissions and NO emissions by 18.9–27.4% and 35.9–39.5% (p < 0.05), respectively, and no significant differences occurred among these treatments. The SNB increased NH3 volatilization by 13.3% and 6.3%, but decreased N leaching and N runoff by 5.9–11.2% compared with the organic substitution.
The foreground interface N footprint ranged from 2.8 to 35.1 g N kg−1 yield (Fig. 5a). The production and transport of organic manure dominated the foreground interface N footprint by 84.9–91.4%, which eventually led to an increase in the foreground N footprint under the SNM (28.0 g N kg−1 yield) and SNMB (35.1 g N kg−1 yield) compared with the SNB (4.9 g N kg−1 yield) (p < 0.01). The field N footprint ranged from 0.3 to 24.2 g N kg−1 yield (Fig. 5b). N leaching dominated the N footprint, accounting for an average of 46.8%, whereas NH3 volatilization and N runoff were secondary contributors. Compared with the SN, the SNB, SNM, and SNMB reduced the field Nr loss by 45.4%, 34.9% and 25.3% (p < 0.01; interannually varying from − 40.3 to 78.4%, 8.4 to 39.0% and − 22.0 to 49.5%, respectively). When considering the foreground interface and the field interface together, the SNB (18.2 g N kg−1 yield) reduced the N footprint by 58.4 and 65.8% compared with the SNM (43.7 g N kg−1 yield) and SNMB (53.2 g N kg−1 yield) (p < 0.01), respectively.
Contributions of different sources to the foreground N footprint (a), field N footprint (b), and environmental damage costs (c) under different treatments in intensive vegetable production. Others refer to the manufacture and transport of potassium, phosphorus, insecticides and plastic film. Energy refers to fuel and electricity consumption. The lowercase letters represent significant differences according to Tukey’s multiple range test (p < 0.05)
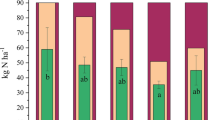
The EDC associated with Nr losses and GHG emissions ranged from 0.16 × 103 CNY ha−1 crop−1 for CK to 2.75 × 103 CNY ha−1 crop−1 for the SNMB (Fig. 5c). Foreground Nr losses were the main contributor to EDC in the organic substitution, with an average of 74.8%, resulting in EDC increasing by 130% in the SNM (2.57 × 103 CNY ha−1 crop−1) and 145% in the SNMB (2.75 × 103 CNY ha−1 crop−1) compared to the SN (p < 0.01). In contrast, field Nr losses were the main contributor to EDC under the SNB at 40.1%, and the EDC of SNB (1.11 × 103 CNY ha−1 crop−1) was 1.28% lower than that under the SN.
3.4 NEEB
The NEEB is comprised of yield gains minus agrochemical input costs and EDCs (Fig. 6). The NEEBs of four fertilization treatments ranged from 51.6 × 103 CNY ha−1 crop−1 to 66.0 × 103 CNY ha−1 crop−1, 33.2–70.7% higher than that of the CK (38.6 × 103 CNY ha−1 crop−1). Among them, the SNM achieved the optimal NEEB (66.0 × 103 CNY ha−1 crop−1) and increased by 17.8% (p < 0.01) compared to the SN (56.1 × 103 CNY ha−1 crop−1). The NEEB of the SNB (60.0 × 103 CNY ha−1 crop−1) was 7.1% higher than that of the SN, but 10.0% lower than that of the SNM, mainly due to the cost of agrochemical inputs. The SNMB decreased the NEEB by 14.1% compared to the SNB (p < 0.05), and even an 8% decrease compared to the SN.
4 Discussion
4.1 Crop production and NUE as affected by biochar and organic substitution
The findings showed that N fertilizer significantly increased vegetable yields and N uptake compared with the plots without N fertilizer, which was consistent with the results reported by previous studies (Zhang et al. 2011). Among the different treatments for adding N fertilizer, biochar amendment significantly increased crop production and N uptake, serving as the optimal fertilization treatment (Fig. 3), which supported the findings of previous meta-analyses (Jeffery et al. 2011). The contents of soil DON and NO3−-N, which proved to be important factors limiting vegetable yield (Fig. 7), were improved in the SNB, whereas the soil pH and SOC were not affected, indicating that the increase in vegetable yield with biochar is unlikely to be a result of the lime effect. We found that vegetable growth and N assimilation were enhanced by the low NH4+/NO3− ratio (Fig. 2), which supported the previous observations (Hu et al. 2021). In biochar-amended soils, due to its higher surface area and porosity, the higher N bioavailability promoted soil microbial activity (Agegnehu et al. 2016) and accelerated nutrient cycling, further increasing crop productivity and NUE (Bailey et al. 2011). However, soils with biochar amended for more than 4 years failed to improve the N bioavailability or soil nitrification. Organic substitution dramatically increased NUE and N uptake compared with traditional N management, which was consistent with the previous report (Zhang et al. 2020a, b). Reasonably, the rapid supply of N by chemical fertilizers early in the crop growing season and a continuous supply of N can promote crop growth at the later stage due to the long-lasting effect of organic manures (Liang et al. 2019).
We also observed the improvement of vegetable qualities by biochar amendment in this study. The SNB significantly increased the contents of soluble protein, vitamin C, and soluble sugar in vegetables and significantly reduced the nitrate content compared with the SN (Fig. 4e). Probably due to the slow-release effect of the porous structure of biochar on the adsorption of fertilizer nutrients (Lehmann et al. 2003), biochar amendment may facilitate the proper supply of N and the conversion rate of NH4+ to NO3−, avoiding the accumulation of plant NO3−. Moreover, biochar may improve the quality by promoting the synthesis of related enzymes in the plant (Liu et al. 2014a, b) with its abundant trace elements (Additional file 1: Table S1). The SNM improved vegetable quality to some extent without significant differences as compared to the SN (Fig. 4e). Organic fertilizers improved SOC and soil structure (Fig. 2g), and enhanced the performance of microorganisms related to soil aggregate structure, thus promoting the production of aggregates and reducing the amount of NH4+ converted to NO3− (Luan et al. 2019). However, the alteration of soil microbial and enzyme activities by biochar amendment and organic substitution should be further clarified for improving vegetable qualities.
4.2 N footprint and EDC as affected by biochar and organic substitution
Different fertilization treatments significantly increased the N footprint in vegetable production (Fig. 5). When considering the Nr losses caused by the foreground and field interfaces, the total Nr loss range of each treatment during the experimental period was 3.1–85.2 kg N ha−1 crop−1, which was much larger than that in other farmland ecosystems, mainly due to the frequent large amount of N input.
The foreground N footprint of organic manure substitution increased significantly by approximately five to eight times that of the other fertilization treatments, because of the large amount of NH3 volatilization in the production process of organic manure. This finding is generally consistent with meta-analyses showing that NH3 volatilization dominates Nr losses during the process of producing organic manure (Hou et al. 2015). Conversely, biochar amendment greatly reduced the N footprint, which was not significantly different from the CK and SN. Thus, the preparation of biochar using pyrolytic coking and carbon-rich plant waste of raw materials would serve as a green sustainable development measure that can alleviate the Nr losses in vegetable production.
The results of the field interface also showed that fertilization significantly increased the N footprint, which is consistent with previous studies (Cui et al. 2013; Zhou et al. 2019). First, the high N cycling pattern leads to serious N leaching in vegetable systems. Second, a multiple cropping index and frequent irrigation lead to a significant ratio of NH3 volatilization and N runoff (Xia et al. 2016). Meanwhile, this study confirmed that biochar amendment and organic substitution significantly reduced the N footprint, and the differences among them were not significant. Not surprisingly, there was a negative correlation between the N footprint and NUE in the field (Cui et al. 2013). Biochar or organic manure promotes microbial activity with the increase of available carbon supplies (Mandal et al. 2016), facilitates the conversion and fixation of mineral N into organic N and DON, and reduces the leaching of N (Fig. 7) (Zhou et al. 2019). The direct application of biochar to soil may be an effective method of increasing soil C and N storage (Tan et al. 2018).
The EDC caused by the intermediate product of nitrification and denitrification could not be ignored, although the emission of N2O and NO accounted for a very small proportion of the Nr losses at the field interface (Henckel and Conrad 1998). In the present study, biochar amendment and organic substitution were found to mitigate Nr losses by altering NH4+ and DOC and thereby affected emissions during nitrification (Fig. 7). NH4+ was concentrated in the reduced soil layer and susceptible to slow diffusion to the soil surface (Kapoor et al. 2008). In addition, readily decomposable organic carbon sources were both C and N substrates for nitrification (Zhou et al. 2019). Biochar amendment reduced soil N effectiveness for N2O production and promoted the further reduction of N2O to N2 in denitrification with increased electron transfer (Xu et al. 2022). However, biochar amendment increased nitrification associated N2O emissions 2–3 years after fertilization, which is consistent with findings by Edwards et al. (2018), but reduced cumulative N2O emissions compared with the SN throughout the 5 years experimental period (Table 1).
4.3 NEEB as affected by biochar and organic substitution
Assessments of NEEBs, which show the relationship between agronomic productivity and environmental sustainability, can provide a scientific basis for improving agronomic management in terms of costs and encourage farmers to adopt management that benefits C (Zhang et al. 2015). Previous studies have reported the effects of biochar amendment (Li et al. 2015) and organic manure (Zhou et al. 2019) on crop yields and NEEBs, although little attention has been focused on the influence of different coordinated application treatments, which directly affect farmers’ motivation. Combining the economic and environmental costs, the SNM generated the highest NEEBs in the current study. This was attributed to the reduced cost of agrochemical inputs due to organic farming. This result represents a substantial change and encourages farmers to adopt a rational strategy of combining organic and inorganic fertilizers. However, it is worth noting that the SNM caused a significantly higher EDC than the other treatments due to the excessive loss of Nr in the early composting process, which emphasizes that reducing the foreground N footprint of the compost production process should be the focus of developing mitigation measurements. Studies have pointed out that NH3 volatilization can be attenuated by adopting appropriate organic management measures, such as processing organic waste before incorporation to produce biochar (Khan et al. 2013; Tang et al. 2019; Yao et al. 2018). On the one hand, converting agricultural organic waste into organic manure is a resource-saving approach that can reduce the pressure of environmental pollution to some extent (Zhou et al. 2019); on the other hand, it can improve soil quality and soil environment and reduce global warming (Zhang et al. 2020a, b). It should be noted that the SNMB reduced the NEEBs of the ecosystem compared with the SN and did not achieve the expected results and its disadvantages were highlighted; therefore, it is not recommended.
Although not significantly different in NEEB from the SN, the SNB may still be an appropriate agricultural approach for providing high productivity and environmental benefits in vegetable systems. First, nutrients and trace elements from biochar replenish soil fertility, provide space for microorganisms to survive and reproduce, and improve the structure and abundance of microbial communities (Ducey et al. 2013). Second, the nutrients in biochar are mainly in organic form with the slow nutrient release, which ensures nutrient availability throughout the growing season and improves carbon and nitrogen retention (Xiang et al. 2017). Although biochar is an environmentally friendly soil amendment with high adoption potential for farmers, few studies have performed comprehensive analyses to support this claim (Häring et al. 2017). Our study showed that the impact of biochar addition on the NEEBs was not significant compared to that of the SN mainly because biochar application is not competitive with other alternatives for soil amendment due to labor and purchase costs, which is consistent with previous studies (Petelina et al. 2014). Even with significant increases in vegetable productivity after biochar application, production and usage on small and medium-sized farms remain a significant challenge (Strassburg et al. 2016). This study suggests that possible low-cost options include modifying the preparation process to produce biochar at low cost, using biochar in low quantities (depending on whether productivity increases as a result), or using biochar for more beneficial land uses.
However, the SNMB reduced the NEEBs compared to the SN, mainly due to the EDC of organic compost and agrochemical inputs of biochar were too large to cover the yield gains.
4.4 Inter-annual effects of biochar and organic substitution
Generally, biochar amendment can sequester carbon and reduce Nr emissions; the temporal evolution pattern of biochar in situ cannot be ignored. Studies have reported that after many years of application, biochar loses the ability to increase crop yields and reduce Nr losses (Aller et al. 2018) and may even reduce soil microbial activity or abundance (Ameloot et al. 2014), while others have found that aged biochar could still improve soil fertility and promote crop growth (Qin et al. 2016). Through temperature and precipitation changes, anthropogenic tillage and microbial activities can lead to physical fragmentation or chemical oxidation after mixing biochar into the soil, which decreases its effectiveness in storing carbon in the subsurface, but improves soil fertility and reduces pollutants (Wang et al. 2020). The properties of biochar, such as pH, ash, and functional groups, changed with aging due to constant exposure (Aller et al. 2018). Therefore, relatively long-term observations may indicate changes in the effectiveness of our treatments. This is confirmed by our findings that vegetable yield decreased in the 4th to the 5th year of the trial period in the SNB and SNMB and the production effect of the SNMB was lower than the effect of both alone and sometimes even lower than the SN. The long-term high C/N of biochar and organic application stimulated microbial colonization, which led to N fixation in the soil, which is not beneficial for the current crop utilization and growth.
In addition, the Nr losses of the biochar amendment strategies significantly increased in the 4th to 5th years (Additional file 1: Fig. S3), resulting in a lower overall effect of SNMB in reducing field Nr losses (N2O, NO, NH3) than the SNM. This ultimately leads to a decrease in the NEEBs of the SNMB is thus not recommended, probably because the long-term tillage of the soil reduced the electron transfer ability and led to the loss of the ability to reduce N2O (Yuan et al. 2019), which is not conducive to the stability of the combined dispensing. Although the aging effect of biochar negated the initially observed effects on Nr losses, we cannot conclude that biochar is inevitably losing its capacity for mitigating soil Nr losses due to the large interannual variations observed (Fig. 5). It should be noted that the beneficial effects of biochar on soil properties by providing more favorable conditions for microbial growth are long lasting, and the focus should be on a comprehensive assessment of the long-term effects of biochar applications in various agroecosystems.
5 Conclusions
Our 5-year observations demonstrate that the SNM and SNB improved crop yield, quality, and NUE by reducing Nr losses and promoting SOC sequestration, and they ultimately increased the NEEBs compared to the SN, while the SNM produced the best NEEB. However, the SNMB reduced the NEEBs compared to the SN mainly because of the high costs of agrochemical inputs from biochar and EDC from manure production. Overall, the SNM is a win–win approach that not only provides substantial economic benefits to farmers but also has a low upfront cost. The expected NEEBs can be improved by optimizing the biochar production process and organic manure substituting rates. The combined effects of fertilizer, biochar and organic manure need to be further explored in various geographic settings and ecosystem types.
Availability of data and materials
Data has been included as additional files and will be made available on reasonable request.
References
Abel S, Peters A, Trinks S, Schonsky H, Facklam M, Wessolek G (2013) Impact of biochar and hydrochar addition on water retention and water repellency of sandy soil. Geoderma 202:183–191. https://doi.org/10.1016/j.geoderma.2013.03.003
Agegnehu G, Nelson PN, Bird MI (2016) The effects of biochar, compost and their mixture and nitrogen fertilizer on yield and nitrogen use efficiency of barley grown on a Nitisol in the highlands of Ethiopia. Sci Total Environ 569–570:869–879. https://doi.org/10.1016/j.scitotenv.2016.05.033
Aller DM, Archontoulis SV, Zhang W, Sawadgo W, Laird DA, Moore K (2018) Long term biochar effects on corn yield, soil quality and profitability in the US Midwest. Field Crops Res 227:30–40. https://doi.org/10.1016/j.fcr.2018.07.012
Ameloot N, Sleutel S, Case SD, Alberti G, Mcnamara NP, Zavalloni C, Vervisch B, Delle G, De NS (2014) C mineralization and microbial activity in four biochar field experiments several years after incorporation. Soil Biol Biochem 78:195–203. https://doi.org/10.1016/j.soilbio.2014.08.004
Azeem M, Sun D, Crowley D, Hayat R, Hussain Q, Ali A, Tahir MI, Jeyasundar PG, Rinklebe J, Zhang Z (2020) Crop types have stronger effects on soil microbial communities and functionalities than biochar or fertilizer during two cycles of legume-cereal rotations of dry land. Sci Total Environ 715:136958. https://doi.org/10.1016/j.scitotenv.2020.136958
Bailey VL, Fansler SJ, Smith JL, Bolton HJ (2011) Reconciling apparent variability in effects of biochar amendment on soil enzyme activities by assay optimization. Soil Biol Biochem 43(2):296–301. https://doi.org/10.1016/j.soilbio.2010.10.014
Cai S, Pittelkow CM, Zhao X, Wang S (2018) Winter legume-rice rotations can reduce nitrogen pollution and carbon footprint while maintaining net ecosystem economic benefits. J Clean Prod 195:289–300. https://doi.org/10.1016/j.jclepro.2018.05.115
Cui Z, Yue S, Wang G, Zhang F, Chen X (2013) In-season root-zone N management for mitigating greenhouse gas emission and reactive N losses in intensive wheat production. Environ Sci Technol 47(11):6015–6022. https://doi.org/10.1021/es4003026
Duan P, Zhang X, Zhang Q, Wu Z, Xiong Z (2018) Field-aged biochar stimulated N2O production from greenhouse vegetable production soils by nitrification and denitrification. Sci Total Environ 642:1303–1310. https://doi.org/10.1016/j.scitotenv.2018.06.166
Ducey TF, Ippolito JA, Cantrell KB, Novak JM, Lentz RD (2013) Addition of activated switchgrass biochar to an aridic subsoil increases microbial nitrogen cycling gene abundances. Appl Soil Ecol 65:65–72. https://doi.org/10.1016/j.apsoil.2013.01.006
Edwards JD, Pittelkow CM, Kent AD, Yang W (2018) Dynamic biochar effects on soil nitrous oxide emissions and underlying microbial processes during the maize growing season. Soil Biol Biochem 122:81–90
Fan C, Li B, Xiong Z (2017) Nitrification inhibitors mitigated reactive gaseous nitrogen intensity in intensive vegetable soils from China. Sci Total Environ 612:480–489. https://doi.org/10.1016/j.scitotenv.2017.08.159
Gu L, Liu T, Wang J, Liu P, Dong S, Zhao B, Hwat S, Zhang J, Zhao B, Li J (2016) Lysimeter study of nitrogen losses and nitrogen use efficiency of northern Chinese wheat. Field Crop Res 188:82–95. https://doi.org/10.1016/j.fcr.2015.10.014
Hamedani SR, Kuppens T, Malina R, Bocci E, Colantoni A, Villarini M (2019) Life cycle assessment and environmental valuation of biochar production: two case studies in Belgium. Energies 12(11):2166. https://doi.org/10.3390/en12112166
Hao Y, Tian CS (2020) A hybrid framework for carbon trading price forecasting: the role of multiple influence factor. J Clean Prod 262:120378. https://doi.org/10.1016/j.jclepro.2020.120378
Häring V, Manka’abusi D, Akoto-Danso EK, Werner S, Atiah K, Steiner C, Lompo DJP, Adiku S, Buerkert A, Marschner B (2017) Effects of biochar, waste water irrigation and fertilization on soil properties in West African urban agriculture. Sci Rep 7:10738. https://doi.org/10.1038/s41598-017-10718-y
Harter J, Weigold P, El-Hadidi M, Huson DH, Kappler A, Behrens S (2016) Soil biochar amendment shapes the composition of N2O-reducing microbial communities. Sci Total Environ 562:379–390. https://doi.org/10.1016/j.scitotenv.2016.03.220
Henckel T, Conrad R (1998) Characterization of microbial NO production, N2O production and CH4 oxidation initiated by aeration of anoxic rice field soil. Biogeochemistry 40:17–36. https://doi.org/10.1023/A:1005819013950
Hou Y, Velthof GL, Oenema O (2015) Mitigation of ammonia, nitrous oxide and methane emissions from manure management chains: a meta-analysis and integrated assessment. Glob Chang Biol 21(3):1293–1312. https://doi.org/10.1111/gcb.12767
Hu X, Wang X, Abbas T, Fang T, Miao D, Li Y, Chang S, Li Y (2021) Higher ammonium-to-nitrate ratio shapes distinct soil nitrifying community and favors the growth of Moso bamboo in contrast to broadleaf tree species. Biol Fertil Soils 57:1171–1182
Jeffery S, Verheijen FGA, van der Velde M, Bastos AC (2011) A quantitative review of the effects of biochar application to soils on crop productivity using meta-analysis. Agric Ecosyst Environ 144(1):175–187. https://doi.org/10.1016/j.agee.2011.08.015
Kapoor V, Singh U, Patil SK, Magre H, Shrivastava LK, Mishra VN, Das RO, Samadhiya VK, Sanabria J, Diamond R (2008) Rice growth, grain yield, and floodwater nutrient dynamics as affected by nutrient placement method and rate. Agron J 100(3):526–536. https://doi.org/10.2134/agronj2007.0007
Khan S, Chao C, Waqas M, Arp HP, Zhu YG (2013) Sewage sludge biochar influence upon rice (Oryza sativa L) yield, metal bioaccumulation and greenhouse gas emissions from acidic paddy soil. Environ Sci Technol 47:8624–8632. https://doi.org/10.1021/es400554x
Khoshnevisan B, Rafiee S, Omid M, Mousazadeh H, Clark S (2014) Environmental impact assessment of tomato and cucumber cultivation in greenhouses using life cycle assessment and adaptive neuro-fuzzy inference system. J Clean Prod 73:183–192. https://doi.org/10.1016/j.jclepro.2013.09.057
Lehmann J, Silva JP, Steiner C, Nehls T, Zech W, Glaser B (2003) Nutrient availability and leaching in an archaeological Anthrosol and a Ferralsol of the Central Amazon basin: fertilizer, manure and charcoal amendments. Plant Soil 249:343–357. https://doi.org/10.1023/A:1022833116184
Lehmann J, Cowie A, Masiello CA, Kammann C, Woolf D, Amonette JE, Cayuela ML, Camps-Arbestain M, Whitman T (2021) Biochar in climate change mitigation. Nat Geosci 14:883–892. https://doi.org/10.1038/s41561-021-00852-8
Li B, Fan C, Zhang H, Chen Z, Sun L, Xiong Z (2015) Combined effects of nitrogen fertilization and biochar on the net global warming potential, greenhouse gas intensity and net ecosystem economic budget in intensive vegetable agriculture in Southeastern China. Atmos Environ 100:10–19. https://doi.org/10.1016/j.atmosenv.2014.10.034
Liang L, Ridoutt BG, Lal R, Wang D, Wu W, Peng P, Hang S, Wang L, Zhao G (2019) Nitrogen footprint and nitrogen use efficiency of greenhouse tomato production in North China. J Clean Prod 208:285–296. https://doi.org/10.1016/j.jclepro.2018.10.149
Liu C, Lu M, Cui J, Li B, Fang CM (2014a) Effects of straw carbon input on carbon dynamics in agricultural soils: a meta-analysis. Glob Chang Biol 20(5):1366–1381. https://doi.org/10.1111/gcb.12517
Liu X, Li L, Bian R, Chen D, Qu J, Kibue GW, Pan G, Zhang X, Zheng J, Zheng J (2014b) Effect of biochar amendment on soil-silicon availability and rice uptake. J Plant Nutr Soil 177(1):91–96. https://doi.org/10.1002/jpln.201200582
Liu X, Zheng J, Zhang D, Cheng K, Zhou H, Zhang A, Li L, Joseph S, Smith P, Crowley D, Kuzyakov Y, Pan G (2016) Biochar has no effect on soil respiration across Chinese agricultural soils. Sci Total Environ 554–555:259–265. https://doi.org/10.1016/j.scitotenv.2016.02.179
Liu TQ, Li SH, Guo LG, Cao CG, Li CF, Zhai ZB, Zhou JY, Mei YM, Ke HJ (2020) Advantages of nitrogen fertilizer deep placement in greenhouse gas emissions and net ecosystem economic benefits from no-tillage paddy fields. J Clean Prod 263:121322. https://doi.org/10.1016/j.jclepro.2020.121322
Luan H, Gao W, Huang S, Tang J, Li M, Zhang H, Chen X (2019) Partial substitution of chemical fertilizer with organic amendments affects soil organic carbon composition and stability in a greenhouse vegetable production system. Soil till Res 191:1859–2196. https://doi.org/10.1016/j.still.2019.04.009
Madaline DY, Gerard HR, Wim V (2021) Impacts of agronomic measures on crop, soil, and environmental indicators: a review and synthesis of meta-analysis. Agric Ecosyst Environ 319:107551. https://doi.org/10.1016/j.agee.2021.107551
Mandal S, Sarkar B, Bolan N, Novak J, Ok YS, Zwieten LV, Singh BP, Kirkham MB, Choppala G, Spokas K, Naidu R (2016) Designing advanced biochar products for maximizing greenhouse gas mitigation potential. Crit Rev Environ Sci Technol 46(17):1367–1401. https://doi.org/10.1080/10643389.2016.1239975
Ministry of Natural Resources (MNR) (2021) Major data bulletin of the third National Land Survey. Ministry of Natural Resources of the People's Republic of China. http://www.mnr.gov.cn/dt/ywbb/202108/t20210826_2678340.html
Mohanty S, Nayak AK, Swain CK, Dhal BR, Kumar A, Kumar U, Tripathi R, Shahid M, Behera KK (2020) Impact of integrated nutrient management options on GHG emission, N loss and N use efficiency of low land rice. Soil till Res 200:104616. https://doi.org/10.1016/j.still.2020.104616
Osman AI, Hefny M, Maksoud MA, Elgarahy AM, Rooney DW (2020) Recent advances in carbon capture storage and utilisation technologies: a review. Environ Chem Lett 19:797–849. https://doi.org/10.1007/s10311-020-01133-3
Petelina E, Sanscartier D, Macwilliam S, Ridsdale R (2014) Environmental, social, and economic benefits of biochar application for land reclamation purposes. In: British Columbia Mine reclamation symposium: University of British Columbia. http://hdl.handle.net/2429/51133
Qin X, Wang H, Liu C, Li J, Wan Y, Gao Q, Fan F, Liao Y (2016) Long-term effect of biochar application on yield-scaled greenhouse gas emissions in a rice paddy cropping system: a four-year case study in south China. Sci Total Environ 569–570:1390–1401. https://doi.org/10.1016/j.scitotenv.2016.06.222
Steinbeiss S, Gleixner G, Antonietti M (2009) Effect of biochar amendment on soil carbon balance and soil microbial activity. Soil Biol Biochem 41(6):1301–1310. https://doi.org/10.1016/j.soilbio.2009.03.016
Strassburg BBN, Barros FSM, Crouzeilles R, Iribarrem A, Santos JS, Silva D, Sansevero JBB, Alves-Pinto HN, Feltran-Barbieri R, Latawiec AE (2016) The role of natural regeneration to ecosystem services provision and habitat availability: a case study in the Brazilian Atlantic Forest. Biotropica 48(6):890–899. https://doi.org/10.1111/btp.12393
Sun W, Huang C (2020) A novel carbon price prediction model combines the secondary decomposition algorithm and the long short-term memory network. Energy 207:118294. https://doi.org/10.1016/j.energy.2020.118294
Tan G, Wang H, Xu N, Liu H, Zhai L (2018) Biochar amendment with fertilizers increases peanut N uptake, alleviates soil N2O emissions without affecting NH3 volatilization in field experiments. Environ Sci Pollut Res 25:8817–8826. https://doi.org/10.1007/s11356-017-1116-6
Tang Q, Ti C, Xia L, Xia Y, Wei Z, Yan X (2019) Ecosystem services of partial organic substitution for chemical fertilizer in a peri-urban zone in China. J Clean Prod 224:779–788. https://doi.org/10.1016/j.jclepro.2019.03.201
Tian H, Lu C, Ciais P, Michalak AM, Canadl JG, Saikawa E, Huntzinger DN, Gumey KR, Sich S, Zhang B (2016) The terrestrial biosphere as a net source of greenhouse gases to the atmosphere. Nature 531:225–228. https://doi.org/10.1038/nature16946
Wang L, O’Connor D, Rinklebe J, Ok YS, Tsang DCW, Shen Z, Hou D (2020) Biochar aging: mechanisms, physicochemical changes, assessment, and implications for field applications. Environ Sci Technol 54(23):14797–14814. https://doi.org/10.1021/acs.est.0c04033
Xia L, Lam S, Chen D, Wang J, Tang Q, Yan X (2016) Can knowledge-based n management produce more staple grain with lower greenhouse gas emission and reactive nitrogen pollution? a meta-analysis. Glob Chang Biol 23(5):1917–1925. https://doi.org/10.1111/gcb.13455
Xia L, Lam SK, Yan X, Chen D (2017) How does recycling of livestock manure in agroecosystems affect crop productivity, reactive nitrogen losses and soil carbon balance? Environ Sci Technol 51(13):7450–7457. https://doi.org/10.1021/acs.est.6b06470
Xiang Y, Qi D, Duan H, Ying G (2017) Effects of biochar application on root traits: a meta-analysis. GCB Bioenergy 9(10):1563–1572. https://doi.org/10.1111/gcbb.12449
Xu X, Yuan X, Zhang Q, Wei Q, Liu X, Deng W, Wang J, Yang W, Deng B, Zhang L (2022) Biochar derived from spent mushroom substrate reduced N2O emissions with lower water content but increased CH4 emissions under flooded condition from fertilized soils in Camellia oleifera plantations. Chemosphere 287:132110. https://doi.org/10.1016/j.chemosphere.2021.132110
Yao Y, Zhang M, Tian Y, Zhao M, Zhang B, Zhao M, Zeng K, Yin B (2018) Urea deep placement for minimizing NH3 loss in an intensive rice cropping system. Field Crop Res 218:254–266. https://doi.org/10.1016/j.fcr.2017.03.013
Ying H, Ye Y, Cui Z, Chen X (2017) Managing nitrogen for sustainable wheat production. J Clean Prod 162:1308–1316. https://doi.org/10.1016/j.jclepro.2017.05.196
Yuan H, Zhang Z, Li M, Clough T, Wrage-Mönnig N, Qin S, Ge T, Liao H, Zhou S (2019) Biochar’s role as an electron shuttle for mediating soil N2O emissions. Soil Biol Biochem 133:94–96. https://doi.org/10.1016/j.soilbio.2019.03.002
Zhang F, Cui Z, Fan M, Zhang W, Chen X, Jiang R (2011) Integrated soil-crop system management: reducing environmental risk while increasing crop productivity and improving nutrient use efficiency in China. J Environ Qual 40(4):1051–1057. https://doi.org/10.2134/jeq2010.0292
Zhang W, Dou Z, He P, Ju X, Powlson D, Chadwick D, Norse D, Lu Y, Zhang Y, Wu L, Chen X, Cassman KG, Zhang F (2013) New technologies reduce greenhouse gas emissions from nitrogenous fertilizer in China. Proc Natl Acad Sci 110(21):8375–8380. https://doi.org/10.1073/pnas.1210447110
Zhang X, Davidson EA, Mauzerall DL, Searchinger TD, Dumas P, Shen Y (2015) Managing nitrogen for sustainable development. Nature 528:51–59. https://doi.org/10.1038/nature15743
Zhang X, Duan P, Wu Z, Xiong Z (2019) Aged biochar stimulated ammonia-oxidizing archaea and bacteria-derived N2O and NO production in an acidic vegetable soil. Sci Total Environ 687:433–440. https://doi.org/10.1016/j.scitotenv.2019.06.128
Zhang Q, Song Y, Wu Z, Yan X, Gunina A, Kuzyakov Y, Xiong Z (2020a) Effects of six-year biochar amendment on soil aggregation, crop growth, and nitrogen and phosphorus use efficiencies in a rice-wheat rotation. J Clean Prod 242:118435. https://doi.org/10.1016/j.jclepro.2019.118435
Zhang Z, Dong X, Wang S, Pu X (2020b) Benefits of organic manure combined with biochar amendments to cotton root growth and yield under continuous cropping systems in Xinjiang, China. Sci Rep 10:4718. https://doi.org/10.1038/s41598-020-61118-8
Zhou J, Li B, Xia L, Fan C, Xiong Z (2019) Organic-substitute strategies reduced carbon and reactive nitrogen footprints and gained net ecosystem economic benefit for intensive vegetable production. J Clean Prod 225:984–994. https://doi.org/10.1016/j.jclepro.2019.03.191
Acknowledgements
We appreciate editors and three anonymous reviewers for their valuable comments and critical evaluation on this manuscript.
Funding
This work was jointly supported by the National Natural Science Foundation of China (41977078, 32001213) and the Postgraduate Research & Practice Innovation Program of Jiangsu Province, China (KYCX21_0618, KYCX20_0591).
Author information
Authors and Affiliations
Contributions
RB: conceptualization, software, writing; QZ: supervision, software; LZ: lab measurement; XX: methodology; XZ: data analysis; YD: project administration; XY: conceptualization, supervision; ZX: funding acquisition, supervision, conceptualization, writing and revision. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Supplementary Information
Additional file 1: Table S1.
The properties of soil and biochar; Table S2. Cultivation and fertilization management practices in intensive vegetable production from 2017-2021 at Nanjing, China; Table S3. Components of NEB (´103 CNY ha-1 5yr-1) and EDC (´103 CNY ha-1 5yr-1) under different strategies in intensive vegetable production; Table S4. Soil physical and chemical properties under different treatments in intensive vegetable production; Fig S1. NH3 fluxes and cumulative NH3 emissions during the first vegetable crop season in 2020; Fig S2. The evaluation system of soil quality (WFPS, DOC, DON, SOC, TN, N2O) and crop quality (Biomass, N content, Protein, Soluble sugar, Vitamin C, Nitrate) under different treatments in intensive vegetable production; Fig S3. Temporal variation of mean soil temperature, WFPS, (a) NO fluxes (b) and N2O fluxes (c) during the experimental period. Error bars indicate the standard error of the mean of each treatment.
Additional file 2: Supplement A1.
The 17 peer-reviewed publications on vegetable production in China to derive the calculation of NH3 volatilization, N leaching, N runoff parameters and the change ratio of NH3 volatilization in organic and inorganic fertilizers used for the current study’s empirical models.
Additional file 3: Supplement A2.
The 17 peer-reviewed publications on vegetable production to derive the change ratio of NH3 volatilization with biochar addition used for the current study’s empirical models.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bi, R., Zhang, Q., Zhan, L. et al. Biochar and organic substitution improved net ecosystem economic benefit in intensive vegetable production. Biochar 4, 46 (2022). https://doi.org/10.1007/s42773-022-00168-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42773-022-00168-9