Abstract
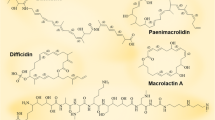
Bacillus sp. has proven to be a goldmine of diverse bioactive lipopeptides, finding wide-range of industrial applications. This review highlights the importance of three major families of lipopeptides (iturin, fengycin, and surfactin) produced by Bacillus sp. and their diverse activities against plant pathogens. This review also emphasizes the role of non-ribosomal peptide synthetases (NRPS) as significant enzymes responsible for synthesizing these lipopeptides, contributing to their peptide diversity. Literature showed that these lipopeptides exhibit potent antifungal activity against various plant pathogens and highlight their specific mechanisms, such as siderophore activity, pore-forming properties, biofilm inhibition, and dislodging activity. The novelty of this review comes from its comprehensive coverage of Bacillus sp. lipopeptides, their production, classification, mechanisms of action, and potential applications in plant protection. It also emphasizes the importance of ongoing research for developing new and enhanced antimicrobial agents. Furthermore, this review article highlights the need for future research to improve the production efficiency of these lipopeptides for commercial applications. It recognizes the potential for these lipopeptides to expand the field of biological pest management for both existing and emerging plant diseases.




Similar content being viewed by others
Data availability
The data associated with this article will be available with corresponding authors and available on request.
References
Raj A, Kumar A (2022) Recent advances in assessment methods and mechanism of microbe-mediated chlorpyrifos remediation. Environ Res 214(Pt 4):114011. https://doi.org/10.1016/j.envres.2022.114011
Malla MA, Dubey A, Kumar A, Yadav S (2023) Unlocking the biotechnological and environmental perspectives of microplastic degradation in soil-ecosystems using metagenomics. Process Saf Environ Prot 170:372–379. https://doi.org/10.1016/j.psep.2022.11.084
Malla MA, Dubey A, Kumar A, Yadav S (2022) Metagenomic analysis displays the potential predictive biodegradation pathways of the persistent pesticides in agricultural soil with a long record of pesticide usage. Microbiol Res 261:127081. https://doi.org/10.1016/j.micres.2022.127081
Dubey A, Malla MA, Khan F et al (2019) Soil microbiome: a key player for conservation of soil health under changing climate. Biodivers Conserv 28(8–9):2405–2429. https://doi.org/10.1007/s10531-019-01760-5
Dubey A, Kumar A, Abd_Allah EF, Hashem A, Khan ML (2019) Growing more with less: Breeding and developing drought resilient soybean to improve food security. Ecol Indic 105(March):425-437. https://doi.org/10.1016/j.ecolind.2018.03.003
Patel S, Sangeeta S (2019) Pesticides as the drivers of neuropsychotic diseases, cancers, and teratogenicity among agro-workers as well as general public. Environ Sci Pollut Res. https://doi.org/10.1007/s11356-018-3642-2
Reichenberger S, Bach M, Skitschak A, Frede HG (2007) Mitigation strategies to reduce pesticide inputs into ground- and surface water and their effectiveness. A review Sci Total Environ 384(1–3):1–35. https://doi.org/10.1016/j.scitotenv.2007.04.046
Kumar A, Dubey A (2020) Rhizosphere microbiome: Engineering bacterial competitiveness for enhancing crop production. J Adv Res 24:337–352. https://doi.org/10.1016/j.jare.2020.04.014
Dubey A, Malla MA, Kumar A, Dayanandan S, Khan ML (2020) Plants endophytes: unveiling hidden agenda for bioprospecting toward sustainable agriculture. Crit Rev Biotechnol 40(8):1210–1231. https://doi.org/10.1080/07388551.2020.1808584
Anand G, Bhattacharjee A, Shrivas VL, Dubey S, Sharma S (2021) ACC deaminase positive Enterobacter-mediated mitigation of salinity stress, and plant growth promotion of Cajanus cajan: a lab to field study. Physiol Mol Biol Plants. https://doi.org/10.1007/s12298-021-01031-0
Tripathi YN, Divyanshu K, Kumar S et al (2020) Biopesticides: current status and future prospects in India. In: Keswani C, ed. Bioeconomy for Sustainable Development. Springer Singapore 2020:79–109. https://doi.org/10.1007/978-981-13-9431-7_6
Salazar B, Ortiz A, Keswani C, et al (2022) Bacillus spp. as Bio-factories for antifungal secondary metabolites: innovation beyond whole organism formulations. Microb Ecol 86:1–24 https://doi.org/10.1007/s00248-022-02044-2
Malla MA, Dubey A, Yadav S, Kumar A, Hashem A, Abd Allah EF (2018) Understanding and designing the strategies for the microbe-mediated remediation of environmental contaminants using omics approaches. Front Microbiol 9:1132. https://doi.org/10.3389/fmicb.2018.01132
Dubey A, Kumar A, Khan ML (2020) Role of Biostimulants for enhancing abiotic stress tolerance in Fabaceae Plants. In: Mirza Hasanuzzaman, Susana Araújo SSG, ed. The Plant Family Fabaceae. Springer Singapore 223–236. https://doi.org/10.1007/978-981-15-4752-2_8
Mahapatra S, Yadav R, Ramakrishna W (2022) Bacillus subtilis impact on plant growth, soil health and environment: Dr. Jekyll and Mr. Hyde. J Appl Microbiol 132(5):3543–3562. https://doi.org/10.1111/jam.15480
Wang Z, Liu C, Shi Y, et al (2023) Classification, application, multifarious activities and production improvement of lipopeptides produced by Bacillus. Crit Rev Food Sci Nutr 1–14. https://doi.org/10.1080/10408398.2023.2185588
Li Z, Fernandez KX, Vederas JC, Gänzle MG (2023) Composition and activity of antifungal lipopeptides produced by Bacillus spp. in daqu fermentation. Food Microbiol 111:104211. https://doi.org/10.1016/j.fm.2022.104211
Kaspar F, Neubauer P, Gimpel M (2019) Bioactive secondary metabolites from Bacillus subtilis: a comprehensive review. J Nat Prod 82(7):2038–2053. https://doi.org/10.1021/acs.jnatprod.9b00110
Sreedharan SM, Rishi N, Singh R (2023) Microbial lipopeptides: properties, mechanics and engineering for novel lipopeptides. Microbiol Res 271:127363. https://doi.org/10.1016/j.micres.2023.127363
Mnif I, Rajhi H, Bouallegue A, Trabelsi N, Ghribi D (2022) Characterization of Lipopeptides biosurfactants produced by a newly isolated strain Bacillus subtilis ZNI5: potential environmental application. J Polym Environ 30(6):2378–2391. https://doi.org/10.1007/s10924-021-02361-6
Xiong ZR, Cobo M, Whittal RM, Snyder AB, Worobo RW (2022) Purification and characterization of antifungal lipopeptide produced by Bacillus velezensis isolated from raw honey. PLoS One 17(4):e0266470. https://doi.org/10.1371/journal.pone.0266470
Besson F, Michel G, Claude U, Lyon B (1986) They differ from iturin A by the presence of a free carboxyl group in iturin D and a car- boxymethyl group in iturin E. October XL(4):437–442
Wan C, Fan X, Lou Z, Wang H, Olatunde A, Rengasamy KRR (2021) Iturin: cyclic lipopeptide with multifunction biological potential. Crit Rev Food Sci Nutr 1–13. https://doi.org/10.1080/10408398.2021.1922355
de Souza Freitas F, de Assis Coelho, Lage T, Ayupe BAL, de Paula Siqueira T, de Barros M, Tótola MR (2020) Bacillus subtilis TR47II as a source of bioactive lipopeptides against Gram-negative pathogens causing nosocomial infections. 3 Biotech 10(11):474. https://doi.org/10.1007/s13205-020-02459-z
Etchegaray A, De Castro BC, De Melo IS et al (2008) Effect of a highly concentrated lipopeptide extract of Bacillus subtilis on fungal and bacterial cells. Arch Microbiol 190(6):611–622. https://doi.org/10.1007/s00203-008-0409-z
Abbo AS, Osman Idris M, Hammad AM (2014) The antifungal effects of four tomato rhizosphere Bacillus spp. against Alternaria alternata. Int J Sci Res 3 (7):1324–1328 https://www.ijsr.net/archive/v3i7/MDIwMTQxMTQ1.pdf
Gond SK, Bergen MS, Torres MS, White JF (2015) Endophytic Bacillus spp. produce antifungal lipopeptides and induce host defence gene expression in maize. Microbiol Res 172:79–87. https://doi.org/10.1016/j.micres.2014.11.004
Vanittanakom N, Loeffler W, Koch U, Jung G (1986) Fengycin–a novel antifungal lipopeptide antibiotic produced by Bacillus subtilis F-29-3. J Antibiot (Tokyo) 39(7):888–901. https://doi.org/10.7164/antibiotics.39.888
Liu Y, Lu J, Sun J et al (2019) C16-Fengycin A affect the growth of Candida albicans by destroying its cell wall and accumulating reactive oxygen species. Appl Microbiol Biotechnol 103(21–22):8963–8975. https://doi.org/10.1007/s00253-019-10117-5
Chitarra GS, Breeuwer P, Nout MJR, Van Aelst AC, Rombouts FM, Abee T (2003) An antifungal compound produced by Bacillus subtilis YM 10–20 inhibits germination of Penicillium roqueforti conidiospores. J Appl Microbiol 94(2):159–166. https://doi.org/10.1046/j.1365-2672.2003.01819.x
Théatre A, Hoste ACR, Rigolet A et al (2022) Bacillus sp: a remarkable source of bioactive lipopeptides. Adv Biochem Eng Biotechnol 181:123–179. https://doi.org/10.1007/10_2021_182
Hanif A, Zhang F, Li P et al (2019) Fengycin Produced by Bacillus amyloliquefaciens FZB42 Inhibits Fusarium graminearum Growth and Mycotoxins Biosynthesis. Toxins (Basel) 11(5):295. https://doi.org/10.3390/toxins11050295
Ait Kaki A, Smargiasso N, Ongena M et al (2020) Characterization of New Fengycin Cyclic Lipopeptide Variants Produced by Bacillus amyloliquefaciens (ET) Originating from a Salt Lake of Eastern Algeria. Curr Microbiol 77(3):443–451. https://doi.org/10.1007/s00284-019-01855-w
Kisil OV, Trefilov VS, Sadykova VS, Zvereva ME, Kubareva EA (2023) Surfactin: its biological activity and possibility of application in agriculture. Appl Biochem Microbiol 59(1):1–13. https://doi.org/10.1134/S0003683823010027
Fenibo EO, Ijoma GN, Selvarajan R, Chikere CB (2019) Microbial surfactants: The next generation multifunctional biomolecules for applications in the petroleum industry and its associated environmental remediation. Microorganisms 7(11):581. https://doi.org/10.3390/microorganisms7110581
Perez KJ, Viana JDS, Lopes FC et al (2017) Bacillus spp. Isolated from Puba as a Source of Biosurfactants and Antimicrobial Lipopeptides. Front Microbiol 8:61. https://doi.org/10.3389/fmicb.2017.00061
Wang C, Cao Y, Wang Y, Sun L, Song H (2019) Enhancing surfactin production by using systematic CRISPRi repression to screen amino acid biosynthesis genes in Bacillus subtilis. Microb Cell Fact 18(1):90. https://doi.org/10.1186/s12934-019-1139-4
Diallo MM, Vural C, Şahar U, Ozdemir G (2021) Kurstakin molecules facilitate diesel oil assimilation by Acinetobacter haemolyticus strain 2SA through overexpression of alkane hydroxylase genes. Environ Technol (United Kingdom) 42(13):2031–2045. https://doi.org/10.1080/09593330.2019.1689301
Luo C, Chen Y, Liu X et al (2019) Engineered biosynthesis of cyclic lipopeptide locillomycins in surrogate host Bacillus velezensis FZB42 and derivative strains enhance antibacterial activity. Appl Microbiol Biotechnol 103(11):4467–4481. https://doi.org/10.1007/s00253-019-09784-1
Dang Y, Zhao F, Liu X et al (2019) Enhanced production of antifungal lipopeptide iturin A by Bacillus amyloliquefaciens LL3 through metabolic engineering and culture conditions optimization. Microb Cell Fact 18(1):68. https://doi.org/10.1186/s12934-019-1121-1
Kim YT, Kim SE, Lee WJ et al (2020) Isolation and characterization of a high iturin yielding Bacillus velezensis UV mutant with improved antifungal activity. Gupta V, ed. PLoS One 15(12):e0234177. https://doi.org/10.1371/journal.pone.0234177
Baltz RH (2008) Biosynthesis and genetic engineering of lipopeptide antibiotics related to daptomycin. Curr Top Med Chem 8(8):618–638. https://doi.org/10.2174/156802608784221497
Ma Z, Zhang S, Zhang S et al (2020) Isolation and characterization of a new cyclic lipopeptide surfactin from a marine-derived Bacillus velezensis SH-B74. J Antibiot (Tokyo). https://doi.org/10.1038/s41429-020-0347-9
Naik S, Palys S, Di Falco M et al (2021) Isolation and Characterization of Bacillus velezensis EB14, an Endophytic Bacterial Strain Antagonistic to Poplar Stem Canker Pathogen Sphaerulina musiva and Its Interactions with the Endophytic Fungal Microbiome in Poplar. PhytoFrontiers™ 1(3):229–238. https://doi.org/10.1094/PHYTOFR-10-20-0023-R
Chen H, Wang L, Su CX, Gong GH, Wang P, Yu ZL (2008) Isolation and characterization of lipopeptide antibiotics produced by Bacillus subtilis. Lett Appl Microbiol. https://doi.org/10.1111/j.1472-765X.2008.02412.x
Inès M, Dhouha G (2015) Lipopeptide surfactants: Production, recovery and pore forming capacity. Peptides 71:100–112. https://doi.org/10.1016/j.peptides.2015.07.006
Jiao S, Li X, Yu H, Yang H, Li X, Shen Z (2017) In situ enhancement of surfactin biosynthesis in Bacillus subtilis using novel artificial inducible promoters. Biotechnol Bioeng 114(4):832–842. https://doi.org/10.1002/bit.26197
Malfanova N, Kamilova F, Validov S et al (2011) Characterization of Bacillus subtilis HC8, a novel plant-beneficial endophytic strain from giant hogweed. Microb Biotechnol 4(4):523–532. https://doi.org/10.1111/j.1751-7915.2011.00253.x
Li Y, Héloir M-C, Zhang X et al (2019) Surfactin and fengycin contribute to the protection of a Bacillus subtilis strain against grape downy mildew by both direct effect and defence stimulation. Mol Plant Pathol 20(8):1037–1050. https://doi.org/10.1111/mpp.12809
Lahlali R, Ezrari S, Radouane N et al (2022) Biological control of plant pathogens: a global perspective. Microorganisms 10(3):596. https://doi.org/10.3390/microorganisms10030596
Dubey A, Saiyam D, Kumar A, Hashem A, Abduallah EF, Khan ML (2021) Bacterial root endophytes: Characterization of their competence and plant growth promotion in soybean (glycine max (L.) merr.) under drought stress. Int J Environ Res Public Health 18(3):1–20. https://doi.org/10.3390/ijerph18030931
Malviya D, Sahu PK, Singh UB et al (2020) Lesson from ecotoxicity: revisiting the microbial lipopeptides for the management of emerging diseases for crop protection. Int J Environ Res Public Health. https://doi.org/10.3390/ijerph17041434
Ben Abdallah D, Tounsi S, Gharsallah H, Hammami A, Frikha-Gargouri O (2018) Lipopeptides from Bacillus amyloliquefaciens strain 32a as promising biocontrol compounds against the plant pathogen Agrobacterium tumefaciens. Environ Sci Pollut Res Int 25(36):36518–36529. https://doi.org/10.1007/s11356-018-3570-1
Nguyen NH, Trotel-Aziz P, Villaume S et al (2020) Bacillus subtilis and Pseudomonas fluorescens Trigger Common and Distinct Systemic Immune Responses in Arabidopsis thaliana Depending on the Pathogen Lifestyle. Vaccines 8(3). https://doi.org/10.3390/vaccines8030503
Lin R, Zhang Q, Yin L et al (2022) Isolation and characterization of a mycosubtilin homologue antagonizing Verticillium dahliae produced by Bacillus subtilis strain Z15. PLoS One 17(6):e0269861. https://doi.org/10.1371/journal.pone.0269861
Vlot AC, Sales JH, Lenk M et al (2021) Systemic propagation of immunity in plants. New Phytol 229(3):1234–1250. https://doi.org/10.1111/nph.16953
Kumar A, Dubey A, Malla MA, Dames J (2021) Pyrosequencing and phenotypic microarray to decipher bacterial community variation in Sorghum bicolor (L.) Moench rhizosphere. Curr Res Microb Sci 2(2020):100025. https://doi.org/10.1016/j.crmicr.2021.100025
Lam VB, Meyer T, Arias AA, Ongena M, Oni FE, Höfte M (2021) Bacillus Cyclic Lipopeptides Iturin and Fengycin Control Rice Blast Caused by Pyricularia oryzae in Potting and Acid Sulfate Soils by Direct Antagonism and Induced Systemic Resistance. Microorganisms 9(7):1441. https://doi.org/10.3390/microorganisms9071441
Zhang L, Sun C. Fengycins (2018) Cyclic Lipopeptides from Marine Bacillus subtilis Strains, Kill the Plant-Pathogenic Fungus Magnaporthe grisea by Inducing Reactive Oxygen Species Production and Chromatin Condensation. Master ER, ed. Appl Environ Microbiol 84(18). https://doi.org/10.1128/AEM.00445-18
He Y, Zhu M, Huang J, Hsiang T, Zheng L (2019) Biocontrol potential of a Bacillus subtilis strain BJ-1 against the rice blast fungus Magnaporthe oryzae. Can J Plant Pathol 41(1):47–59. https://doi.org/10.1080/07060661.2018.1564792
El-Gendi H, Al-Askar AA, Király L, Samy MA, Moawad H, Abdelkhalek A (2022) Foliar Applications of Bacillus subtilis HA1 Culture Filtrate Enhance Tomato Growth and Induce Systemic Resistance against Tobacco mosaic virus Infection. Horticulturae 8(4). https://doi.org/10.3390/horticulturae8040301
Guo S, Zhang J, Dong L et al (2019) Fengycin produced by Bacillus subtilis NCD-2 is involved in suppression of clubroot on Chinese cabbage. Biol Control 136:104001. https://doi.org/10.1016/j.biocontrol.2019.104001
Li S, Xu J, Fu L et al (2022) Biocontrol of wheat crown rot using Bacillus halotolerans QTH8. Pathog (Basel, Switzerland) 11(5). https://doi.org/10.3390/pathogens11050595
Chishti Z, Ahmad Z, Zhang X, Jha SK (2021) Optimization of biotic and abiotic factors liable for biodegradation of chlorpyrifos and their modeling using neural network approaches. Appl Soil Ecol 166:103990
Jiang M, Pang X, Liu H et al (2021) Iturin A induces resistance and improves the quality and safety of harvested cherry tomato. Molecules 26(22). https://doi.org/10.3390/molecules26226905
Liu W, Sun C (2021) C17-fengycin B, produced by deep-sea-derived Bacillus subtilis, possessing a strong antifungal activity against Fusarium solani. J Oceanol Limnol 39(5):1938–1947. https://doi.org/10.1007/s00343-020-0215-2
Jiang C, Li Z, Shi Y et al (2020) Bacillus subtilis inhibits Aspergillus carbonarius by producing iturin A, which disturbs the transport, energy metabolism, and osmotic pressure of fungal cells as revealed by transcriptomics analysis. Int J Food Microbiol 330:108783. https://doi.org/10.1016/j.ijfoodmicro.2020.108783
Xu Y, Cai D, Zhang H et al (2020) Enhanced production of iturin A in Bacillus amyloliquefaciens by genetic engineering and medium optimization. Process Biochem 90:50–57. https://doi.org/10.1016/j.procbio.2019.11.017
Desmyttere H, Deweer C, Muchembled J et al (2019) Antifungal activities of Bacillus subtilis Lipopeptides to Two Venturia inaequalis strains possessing different Tebuconazole sensitivity. Front Microbiol 10(OCT):1–10
Chandler S, Van Hese N, Coutte F, Jacques P, Höfte M, De Vleesschauwer D (2015) Role of cyclic lipopeptides produced by Bacillus subtilis in mounting induced immunity in rice (Oryza sativa L.). Physiol Mol Plant Pathol 91:20–30. https://doi.org/10.1016/j.pmpp.2015.05.010
Vu HNT, Nguyen DT, Nguyen HQ et al (2018) Antimicrobial and cytotoxic properties of bioactive metabolites produced by Streptomyces cavourensis YBQ59 Isolated from Cinnamomum cassia Prels in Yen Bai Province of Vietnam. Curr Microbiol. https://doi.org/10.1007/s00284-018-1517-x
Dimopoulou A, Theologidis I, Varympopi A et al (2021) Shifting Perspectives of Translational Research in Bio-Bactericides: Reviewing the Bacillus amyloliquefaciens Paradigm. Biology (Basel) 10(11):1202. https://doi.org/10.3390/biology10111202
Yi Y, Luan P, Liu S et al (2022) Efficacy of Bacillus subtilis XZ18–3 as a biocontrol agent against Rhizoctonia cerealis on Wheat. Agriculture 12(2). https://doi.org/10.3390/agriculture12020258
Chen S, Deng Y, Chang C et al (2015) Pathway and kinetics of cyhalothrin biodegradation by Bacillus thuringiensis strain ZS-19. Sci Rep 5(1):8784. https://doi.org/10.1038/srep08784
Korangi Alleluya V, Argüelles Arias A, Ribeiro B et al (2023) Bacillus lipopeptide-mediated biocontrol of peanut stem rot caused by Athelia rolfsii. Front Plant Sci 14. https://doi.org/10.3389/fpls.2023.1069971
Etesami H, Jeong BR, Glick BR (2023) Biocontrol of plant diseases by Bacillus spp. Physiol Mol Plant Pathol 126:102048. https://doi.org/10.1016/j.pmpp.2023.102048
Acknowledgements
AK gratefully acknowledges DST-SERB for financial support obtained through the project grant of (CRG/2021/003696), New Delhi, Govt of India.
Author information
Authors and Affiliations
Contributions
DS and AD prepared the draft of the manuscript. The manuscript was overseen and revised by MAM and AK.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Luis Augusto Nero
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Saiyam, D., Dubey, A., Malla, M.A. et al. Lipopeptides from Bacillus: unveiling biotechnological prospects—sources, properties, and diverse applications. Braz J Microbiol 55, 281–295 (2024). https://doi.org/10.1007/s42770-023-01228-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42770-023-01228-3