Abstract
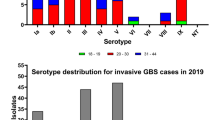
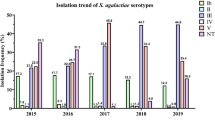
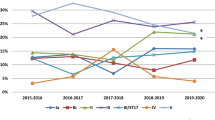
Brazilian data for maternal GBS colonization shows different prevalence rates. This conflicting data may be related to the absence of an official recommendation from the Federal Brazilian Health Authorities describing guidelines and protocols to perform GBS screening in pregnant women, in both public and private clinics. In the present review, we evaluated published reports addressing the prevalence of GBS in different regions of the country, methods used, and, when available, information regarding antibiotic resistance and serological typing of clinical isolates. According to this review, GBS prevalence in pregnant women in Brazil ranged from 4.2 to 28.4%, in the last 10 years. Serotype Ia was the most prevalent. The highest antibiotic resistance rates were found for tetarcycline, although its use to treat GBS infections is not common. Our results also show high resistance rates to clindamycin and erythromycin, which are commonly used as an alternative to penicillin in GBS infecctions. The increased antibiotic resistance, variations in serotype distribution, and high GBS prevalences need to be further investigated. Based on the present situation, recommendations regarding GBS surveillance in the country were raised and may improve our strategies for preventing neonatal infections.


Similar content being viewed by others
References
Edmond KM, Kortsalioudaki C, Scott S, Schrag SJ, Zaidi AKM, Cousens S, Heath PT (2012) Group B streptococcal disease in infants aged younger than 3 months: systematic review and meta-analysis. Lancet. 379(9815):547–556. https://doi.org/10.1016/S0140-6736(11)61651-6
Rosen GH, Randis TM, Desai PV, Sapra KJ, Ma B, Gajer P, Humphrys MS, Ravel J, Gelber SE, Ratner AJ (2017) Group B Streptococcus and the vaginal microbiota. J Infect Dis 216(6):744–751. https://doi.org/10.1093/infdis/jix395
Doran KS, Nizet V (2004) Molecular pathogenesis of neonatal group B streptococcal infection: no longer in its infancy. Mol Microbiol 54(1):23–31. https://doi.org/10.1111/j.1365-2958.2004.04266.x
WHO (2017) Group B Streptococcus infection causes an estimated 150,000 preventable stillbirths and infant deaths every year. Immunization, Vaccines Biol
Centers for Disease Control and Prevention, Verani JR, McGee L, Schrag SJ (2010) Prevention of perinatal Group B Streptococcal disease. Revised Guidelines from CDC, 2010. Morb Mortal Wkly 59(RR-10):1–32 November 19, 2010
Ohlsson A, Shah VS (2016) Intrapartum antibiotics for known maternal Group B streptococcal colonization (Review) Intrapartum antibiotics for known maternal Group B streptococcal colonization. (6):2014–2016. https://doi.org/10.1002/14651858.CD007467.pub4.Copyright
Taminato M, Fram D, Torloni MR, Belasco AGS, Saconato H, Barbosa DA (2011) Screening for group b streptococcus in pregnant women: a systematic review and meta-analysis. Rev Lat Am Enfermagem 19(6):1470–1478. https://doi.org/10.1590/S0104-11692011000600026
Edwards MS, Nizet V, Baker CJ (2011) Group B streptococcal infections. In: Infectious diseases of the fetus and newborn, 6th edn. Elsevier Saunders, Philadelphia, PA, pp 419–469. https://doi.org/10.1016/B978-1-4160-6400-8.00012-2
Vornhagen J, Adams KMA, Rajagopal L (2017) Perinatal group B streptococcal infections: virulence factors, immunity, and prevention strategies. Trends Microbiol 25(11):919–931. https://doi.org/10.1016/j.tim.2017.05.013
Burns G, Plumb J (2013) GBS public awareness, advocacy, and prevention-what’s working, what’s not and why we need a maternal GBS vaccine. Vaccine. 31(S4):D58–D65. https://doi.org/10.1016/j.vaccine.2013.02.039
Royal College of Obstetricians and Gynaecologists (2017) Prevention of early-onset neonatal Group B Streptococcal disease: Green-top Guideline No. 36. BJOG An Int J Obstet Gynaecol 124(12):e280–e305. https://doi.org/10.1111/1471-0528.14821
Agência Nacional de Vigilância Sanitária (2013) Microbiologia clínica para o controle de infecção relacionada à assistência à saúde. Agência Nac Vigilância Sanitária - Anvisa 6:1–154 https://spdbcfmusp.files.wordpress.com/2014/09/iras_modulodeteccaobacterias.pdf
Lang S, Palmer M (2003) Characterization of Streptococcus agalactiae CAMP factor as a pore-forming toxin. J Biol Chem 278(40):38167–38173. https://doi.org/10.1074/jbc.M303544200
Costa HdPF (2011) Prevenção Da Doença Perinatal Pelo Estreptococo Do Grupo B 1–18
Júnior AdOF (2014) Protocolo de Assistência Materno Infantil Do Estado Do Rio Grande Do Norte
Kahhale S (2012) Protocolos de Obstetrícia : Descrição, Diagnóstico, Tratamento 1–4. https://doi.org/10.15713/ins.mmj.3
Secretaria de Estado da Saúde (2017) Protocolo de Atenção Ao Pré-Natal: Risco Habitual:1–4. https://doi.org/10.15713/ins.mmj.3
Secretaria Municipal de Saúde do Rio de Janeiro (SMS-Rio) (2016) Atenção ao Pré-Natal Rotinas para gestantes de baixo risco. Coleção Guia Ref Rápida. http://www.rio.rj.gov.br/dlstatic/10112/6552790/4176323/GuiaPrenatal_reunido.pdf
Brasil. Ministério Da Saúde (2012) Atenção Ao Pré-Natal de Baixo Risco. Brasilia: Editora do Ministério da Saúde. http://bvsms.saude.gov.br/bvs/publicacoes/cadernos_atencao_basica_32_prenatal.pdf
Costa AL d R, Lamy Filho F, Chein MB d C, Brito LMO, Lamy ZC, Andrade KL (2008) Prevalência de colonização por estreptococos do grupo B em gestantes atendidas em maternidade pública da região Nordeste do Brasil. Rev Bras Ginecol Obs 30(6):274–280. https://doi.org/10.1590/S0100-72032008000600002
Linhares JJ, Neto PGC, Vasconcelos JLM et al (2011) Prevalência de colonização por Streptococcus agalactiae em gestantes atendidas em maternidade do Ceará, no Brasil, correlacionando com os resultados perinatais. Rev Bras Ginecol e Obs 33(12):395–400. https://doi.org/10.1590/S0100-72032011001200004
Ventura MSM, Rodrigues JLN, Feitosa FE d L, Junior CA d A, Almeida PC (2011) Colonização por Streptococcus do Grupo B em Gestantes com Trabalho de Parto Prematuro e/ou Ruptura Prematura das Membranas. Arq Med 25(2):61–66 http://www.scielo.mec.pt/scielo.php?script=sci_arttext&pid=S0871-34132011000200001&lng=pt&nrm=iso
Oliveira MV, Teles MF, Viana TA (2013) PREVALÊNCIA E FATORES DE RISCO ASSOCIADOS À COLONIZAÇÃO POR Streptococcusagalactiae EM GESTANTES ATENDIDAS NO HOSPITAL MUNICIPAL ESAÚ MATOS EM VITÓRIA DA CONQUISTA – BA. C&D-Revista Eletrônica da Fainor,. 6(1):172–184. http://srv02.fainor.com.br/revista/index.php/memorias/article/view/197%5Cnsrv02.fainor.com.br/revista237/index.php/memorias/article/.../197/146?
Nomura ML, Passini Júnior R, Oliveira UM, Calil R (2009) Colonização materna e neonatal por estreptococo do grupo B em situações de ruptura pré-termo de membranas e no trabalho de parto prematuro. Rev Bras Ginecol e Obs. 31(8):397–403. https://doi.org/10.1590/S0100-72032009000800005
Rezende C, Azeredo A, Silveira DG, Malta RCG, de Castro V d CO, Miziara RC (2010) PESQUISA DE Streptococcus agalactiae NA SECREÇÃO VAGINAL E ANAL DE GESTANTES DE UM MUNICÍPIO DO NOROESTE PAULISTA. Rev Uniara 13(2):194–201
Martins BL, Jacob T, De Oliveira C (2017) Prevalência de Streptococcus agalactiae em secreção vaginal de gestantes atendidas em um laboratório de análises clínicas do interior do estado de são Paulo. SALUSVITA 36(3):695–707
Marconi C, Rocchetti TT, Rall VLM, De Carvalho LR, Borges VTM, Da Silva MG (2010) Detection of Streptococcus agalactiae colonization in pregnant women by using combined swab cultures: cross-sectional prevalence study. Sao Paulo Med J 128(2):60–62. https://doi.org/10.1590/S1516-31802010000200003
Rocchetti TT, Marconi C, Rall VLM, Borges VTM, Corrente JE, Da Silva MG (2010) Group B streptococci colonization in pregnant women: risk factors and evaluation of the vaginal flora. Arch Gynecol Obstet 283(4):717–721. https://doi.org/10.1007/s00404-010-1439-8
Lajos GJ, Passini Junior R, Nomura ML, Amaral E, Pereira BG, Milanez H, Parpinelli MÂ (2008) Colonização bacteriana do canal cervical em gestantes com trabalho de parto prematuro ou ruptura prematura de membranas. Rev Bras Ginecol e Obs. 30(8):393–399. https://doi.org/10.1590/S0100-72032008000800004
Higashi AB, da Silva IR, Goldman RE (2016) Prevalence of Streptococcus of Group B in pregnant women and the relation with neonatal infection. Rev Enferm e Atenção à Saúde 5(1):24–36
Função JM, Narchi NZ (2013) PESQUISA DO ESTREPTOCOCO DO GRUPO B EM GESTANTES DA ZONA LESTE DE SÃO PAULO. Rev da Esc Enferm da USP 47(1):22–29
Botelho ACN, Oliveira JG, Damasco AP, Santos KTB, Ferreira AFM, Rocha GT, Marinho PS, Bornia RBG, Pinto TCA, Américo MA, Fracalanzza SEL, Teixeira LM (2018) Streptococcus agalactiae carriage among pregnant women living in Rio de Janeiro, Brazil, over a period of eight years. PLoS One 13(5):e0196925. https://doi.org/10.1371/journal.pone.0196925
Castellano-Filho DS, da Silva VL, Nascimento TC, Vieira M d T, Diniz CG (2010) Detection of group B Streptococcus in Brazilian pregnant women and antimicrobial susceptibility patterns. Braz J Microbiol 41(4):1047–1055. https://doi.org/10.1590/S1517-83822010000400024
Atkins KL, Atkinson RM, Shanks A, Parvin CA, Dunne WM, Gross G (2006) Evaluation of polymerase chain reaction for group B streptococcus detection using an improved culture method. Obstet Gynecol 108(3 Pt 1):488–491. https://doi.org/10.1097/01.AOG.0000228961.42272.31
Bastos AN, Bastos RV, Dias VC, Bastos LQ d A, Souza RC d, Bastos VQ d A (2012) Streptococcus agalactiae em gestantes : incidência em laboratório clínico de Juiz de Fora ( MG ) - 2007 a 2009. HU Rev 38(2):45–50
Barbosa NG, de Brito DVD, dos Reis H et al (2016) Colonização Materna Por Estreptococos Do Grupo B : Prevalência E Suscetibilidade Aos Antimicrobianos. Rev Pesqui em Saúde 17(1):13–16
de Melo SCCS, Balandis Costa A, Teixeira F et al (2018) Prevalence of Streptococcus agalactiae colonization in pregnant women from the 18th Health Region of Paraná State. Rev Inst Med Trop Sao Paulo 60(2):2–7. https://doi.org/10.1590/S1678-9946201860002
de Melo SCCS, Santos NC d S, Oliveira M d et al (2016) Antimicrobial susceptibility of Streptococcus agalactiae isolated from pregnant women. Rev Inst Med Trop Sao Paulo 58(1):83. https://doi.org/10.1590/S1678-9946201658083
Kiss FS, Rossato J d S, Graudenz MS, Gutierrez LLP (2013) Prevalência da colonização por Streptococcus agalactiae em uma amostra de mulheres grávidas e não grávidas de Porto Alegre, estado do Rio Grande do Sul. Sci Med (Porto Alegre) 23(3):169–174
Senger FR, Alves IA, Pellegrini D d CP, Prestes DC, Souza EF d, Corte ED (2015) Prevalência da colonização por Streptococcus agalactiae em gestantes atendidas na rede pública de saúde de Santo Ângelo/RS. Rev Epidemiol e Control Infecção 6(1):1–5. https://doi.org/10.17058/reci.v6i1.6272
Veit AR, Roehrs MCSM, Mayer LE, Santos SO, Martini R, Tizotti MK, Kempfer CB, Martins VR, Fronza L, Cunha MDC, Colpo PR, Konopka CK, Horner R (2011) Colonization prevalence and susceptibility of Streptococcus agalactiae in pregnant women at HUSM. Saúde (Santa Maria) 36(1):9–14. https://doi.org/10.5902/223658342391
Kapatai G, Patel D, Efstratiou A, Chalker VJ (2017) Comparison of molecular serotyping approaches of Streptococcus agalactiae from genomic sequences. BMC Genomics 18(1):1–11. https://doi.org/10.1186/s12864-017-3820-5
Imperi M, Pataracchia M, Alfarone G, Baldassarri L, Orefici G, Creti R (2010) A multiplex PCR assay for the direct identification of the capsular type (Ia to IX) of Streptococcus agalactiae. J Microbiol Methods 80(2):212–214. https://doi.org/10.1016/j.mimet.2009.11.010
Slotved HC, Kong F, Lambertsen L, Sauer S, Gilbert GL (2007) Serotype IX, a proposed new Streptococcus agalactiae serotype. J Clin Microbiol 45(9):2929–2936. https://doi.org/10.1128/JCM.00117-07
Skoff TH, Farley MM, Petit S, Craig AS, Schaffner W, Gershman K, Harrison LH, Lynfield R, Mohle-Boetani J, Zansky S, Albanese BA, Stefonek K, Zell ER, Jackson D, Thompson T, Schrag SJ (2009) Increasing burden of invasive group B streptococcal disease in nonpregnant adults, 1990–2007. Clin Infect Dis 49(1):85–92. https://doi.org/10.1086/599369
Nuccitelli A, Rinaudo CD, Maione D (2015) Group B Streptococcus vaccine: state of the art. Ther Adv Vaccines 3(3):76–90. https://doi.org/10.1177/2051013615579869
Phares CR, Lynfield R, Farley MM et al (2008) Epidemiology of invasive group B streptococcal disease in the United States, 1999-2005. JAMA. 2008(17):2056–2065. https://doi.org/10.1016/S0084-3873(08)79076-X
Tettelin H, Masignani V, Cieslewicz MJ, Eisen JA, Peterson S, Wessels MR, Paulsen IT, Nelson KE, Margarit I, Read TD, Madoff LC, Wolf AM, Beanan MJ, Brinkac LM, Daugherty SC, DeBoy RT, Durkin AS, Kolonay JF, Madupu R, Lewis MR, Radune D, Fedorova NB, Scanlan D, Khouri H, Mulligan S, Carty HA, Cline RT, van Aken SE, Gill J, Scarselli M, Mora M, Iacobini ET, Brettoni C, Galli G, Mariani M, Vegni F, Maione D, Rinaudo D, Rappuoli R, Telford JL, Kasper DL, Grandi G, Fraser CM (2002) Complete genome sequence and comparative genomic analysis of an emerging human pathogen, serotype V Streptococcus agalactiae. Proc Natl Acad Sci U S A 99(19):12391–12396. https://doi.org/10.1073/pnas.182380799
Schuchat A (1998) Epidemiology of group B streptococcal disease in the United States: shifting paradigms. Clin Microbiol Rev 11(3):497–513. https://doi.org/10.1128/CMR.11.3.497
Le Doare K, Faal A, Jaiteh M et al (2017) Association between functional antibody against Group B Streptococcus and maternal and infant colonization in a Gambian cohort. Vaccine. 35(22):2970–2978. https://doi.org/10.1016/j.vaccine.2017.04.013
Simoes JA, Alves VMN, Fracalanzza SEL, Camargo RPS, Mathias L, Milanez HMBP, Brolazo EM (2007) Phenotypical characteristics of group B streptococcus in parturients. Braz J Infect Dis 11(2):261–266. https://doi.org/10.1590/S1413-86702007000200019
Soares GCT, Alviano DS, da Silva Santos G, Alviano CS, Mattos-Guaraldi AL, Nagao PE (2013) Prevalence of Group B Streptococcus serotypes III and V in pregnant women of Rio de Janeiro, Brazil. Braz J Microbiol 44(3):869–872. https://doi.org/10.1590/S1517-83822013000300032
Palmeiro JK, Dalla-Costa LM, Fracalanzza SEL, Botelho ACN, da Silva Nogueira K, Scheffer MC, de Almeida Torres RSL, de Carvalho NS, Cogo LL, Madeira HMF (2010) Phenotypic and genotypic characterization of group B streptococcal isolates in southern Brazil. J Clin Microbiol 48(12):4397–4403. https://doi.org/10.1128/JCM.00419-10
Andrade PD, Russo J d S, Gouveia JB et al (2017) Molecular characterization of group B Streptococcus serotypes by multiplex polymerase chain reaction. Med Express 4(4):6–8. https://doi.org/10.5935/MedicalExpress.2017.04.06
Nagano N, Nagano Y, Toyama M, Kimura K, Tamura T, Shibayama K, Arakawa Y (2012) Nosocomial spread of multidrug-resistant group B streptococci with reduced penicillin susceptibility belonging to clonal complex 1. J Antimicrob Chemother 67(4):849–856. https://doi.org/10.1093/jac/dkr546
Easmon CSF, Hastings MJG, Clare AJ et al (1981) Nosocomial transmission of group B streptococci. Br Med J (Clin Res Ed) 283(6289):459–461. https://doi.org/10.1136/bmj.283.6289.459
Dutra VG, Alves VMN, Olendzki AN, Dias CAG, de Bastos AFA, Santos GO, de Amorin ELT, Sousa MÂB, Santos R, Ribeiro PCS, Fontes CF, Andrey M, Magalhães K, Araujo AA, Paffadore LF, Marconi C, Murta EFC, Fernandes Jr PC, Raddi MSG, Marinho PS, Bornia RBG, Palmeiro JK, Dalla-Costa LM, Pinto TCA, Botelho ACN, Teixeira LM, Fracalanzza SEL (2014) Streptococcus agalactie in Brazil: serotype distribution, virulence determinants and antimicrobiol suscetibility. BMC Infect Dis 14(1):323–330
Pinto TCA, Costa NS, Vianna Souza AR, da Silva LG, Corrêa ABA, Fernandes FG, Oliveira ICM, de Mattos MC, Rosado AS, Benchetrit LC (2013) Distribution of serotypes and evaluation of antimicrobial susceptibility among human and bovine Streptococcus agalactiae strains isolated in Brazil between 1980 and 2006. Braz J Infect Dis. 17(2):131–136. https://doi.org/10.1016/j.bjid.2012.09.006
Kwatra G, Cunnington MC, Merrall E, Adrian PV, Ip M, Klugman KP, Tam WH, Madhi SA (2016) Prevalence of maternal colonisation with group B streptococcus: a systematic review and meta-analysis. Lancet Infect Dis 16(9):1076–1084. https://doi.org/10.1016/S1473-3099(16)30055-X
Barbosa NG, dos Reis H, Mantese OC, Mussi-Pinhata MM, Abdallah VOS, Gontijo Filho PP (2016) Early-onset neonatal sepsis by Group B Streptococcus in a Brazilian public hospital. Braz J Infect Dis. 20(6):647–648. https://doi.org/10.1016/j.bjid.2016.07.013
Evangelista MLB, de Mello Freitas FT (2015) Group B streptococcus neonatal infection in an intensive care unit in Brazil: high fatality and missed opportunities for antibiotic prophylaxis. Braz J Infect Dis 19(1):98–99. https://doi.org/10.1016/j.bjid.2014.06.007
Centers for Disease Control and Prevention (2010) Prevention of perinatal Group B Streptococcal disease. Morb Mortal Wkly. https://doi.org/10.1097/01.EDE.0000032431.83648.8D
Costa NDVL, De Carvalho M, Pone SM, Saint CG (2010) Gestantes colonizadas pelo Streptococcus do grupo B e seus recémnascidos: Análise crítica da conduta adotada no Instituto Fernandes Figueira, Fundação Oswaldo Cruz. Rev Paul Pediatr 28(2):155–161. https://doi.org/10.1590/S0103-05822010000200005
da Silva FA, Vidal CF d L, Araújo EC d (2015) Validation of the content of the prevention protocol for early sepsis caused by Streptococcus agalactiaein newborns. Rev Lat Am Enfermagem. 23(4):635–641. https://doi.org/10.1590/0104-1169.0179.2598
Silva JM, Stein AT, Schünemann HJ, Bordin R, Kuchenbecker R, de Lourdes Drachler M (2013) Academic detailing and adherence to guidelines for Group B streptococci prenatal screening: a randomized controlled trial. BMC Pregnancy Childbirth 13(68):1–5. https://doi.org/10.1186/1471-2393-13-68
De Mello DS, Tsunechiro MA, Mendelski CA, Pierre SA, Silva AR, Padoveze MC (2015) Group B Streptococcus: compliance with the information in prenatal card records and knowledge of pregnant women. Am J Infect Control 43(4):400–401. https://doi.org/10.1016/j.ajic.2014.12.026
Madhi SA, Dangor Z, Heath PT, Schrag S, Izu A, Sobanjo-ter Meulen A, Dull PM (2013) Considerations for a phase-III trial to evaluate a group B Streptococcus polysaccharide-protein conjugate vaccine in pregnant women for the prevention of early- and late-onset invasive disease in young-infants. Vaccine. 31(S4):D52–D57. https://doi.org/10.1016/j.vaccine.2013.02.029
Teatero S, Ferrieri P, Martin I, Demczuk W, McGeer A, Fittipaldi N (2017) Serotype distribution, population structure, and antimicrobial resistance of Group B Streptococcus strains recovered from colonized pregnant women. Burnham C-AD, ed. J Clin Microbiol 55(2):412–422. https://doi.org/10.1128/JCM.01615-16
Da Cunha V, Davies MR, Douarre PE et al (2014) Streptococcus agalactiae clones infecting humans were selected and fixed through the extensive use of tetracycline. Nat Commun 5:1–12. https://doi.org/10.1038/ncomms5544
Amundson NR, Flores AE, Hillier SL, Baker CJ, Ferrieri P (2005) DNA macrorestriction analysis of nontypeable group b streptococcal isolates: clonal evolution of nontypeable and type v isolates. J Clin Microbiol 43(2):572–576. https://doi.org/10.1128/JCM.43.2.572
Centers for Disease Control and Prevention (2013) Antibiotic resistance threats in the United States. Atlanta, Georgia; 2013. http://www.cdc.gov/drugresistance/threat-report-2013/index.html
Glezen WP, Alpers M (1999) Maternal immunization. Clin Infect Dis 28(2):219–224. https://doi.org/10.1086/515122
Bolukaoto JY, Monyama CM, Chukwu MO, Lekala SM, Nchabeleng M, Maloba MRB, Mavenyengwa RT, Lebelo SL, Monokoane ST, Tshepuwane C, Moyo SR (2015) Antibiotic resistance of Streptococcus agalactiae isolated from pregnant women in Garankuwa, South Africa. BMC Res Notes 8(1):6–12. https://doi.org/10.1186/s13104-015-1328-0
Corrrêa AB d A, da Silva LG, Pinto T d CA et al (2011) The genetic diversity and phenotypic characterization of Streptococcus agalactiae isolates from Rio de Janeiro, Brazil. Mem Inst Oswaldo Cruz 106(8):1002–1006
Nakamura PAM, Schuab RBB, Neves FPG, Pereira CFA, de Paula GR, Barros RR (2011) Antimicrobial resistance profiles and genetic characterisation of macrolide resistant isolates of Streptococcus agalactiae. Mem Inst Oswaldo Cruz 106(2):119–122. https://doi.org/10.1590/S0074-02762011000200001
Dahesh S, Hensler ME, Van Sorge NM et al (2008) Point mutation in the group B streptococcal pbp2x gene conferring decreased susceptibility to β-lactam antibiotics. Antimicrob Agents Chemother 52(8):2915–2918. https://doi.org/10.1128/AAC.00461-08
Acknowledgments
We would like to thank Luis Carlos Ferreira for his helpful revision and comments.
Author information
Authors and Affiliations
Contributions
CSN conducted the literature research and wrote the manuscript. NFBS help to collected data and helped with manuscript writing. RCCF helped with the idea design and critically reviewed the manuscript. CRT designed the idea, followed the literature research and wrote the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible Editor: Agnes Figueiredo
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
do Nascimento, C.S., dos Santos, N.F.B., Ferreira, R.C.C. et al. Streptococcus agalactiae in pregnant women in Brazil: prevalence, serotypes, and antibiotic resistance. Braz J Microbiol 50, 943–952 (2019). https://doi.org/10.1007/s42770-019-00129-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42770-019-00129-8