Abstract
Cardiomyopathies, neuropathies, cancer and accelerated ageing are unequivocally distinct diseases, yet they also show overlapping pathological hallmarks, including a gradual loss of genomic integrity and proteotoxic stress. Recent lines of evidence suggest that this overlap could be the result of remarkably interconnected molecular cascades between nuclear genomic instability and a loss of protein homeostasis. In this review, we discuss these complex connections, as well as their possible impact on disease. We focus in particular on the inherent ability of a wide range of genomic alterations to challenge protein homeostasis. In doing so, we provide evidence suggesting that a loss of protein homeostasis could be a far more prevalent consequence of genomic instability than generally believed. In certain cases, such as aneuploidy, a loss of protein homeostasis appears to be a crucial mechanism for pathology, which indicates that enhancing protein quality control systems could be a promising therapeutic strategy in diseases associated with genomic instability.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
To safeguard nuclear genome integrity, cells rely on an extensive network of cell cycle checkpoints, DNA repair pathways and damage-induced signaling cascades, collectively referred to as the DNA Damage Response (DDR) (reviewed in Giglia-Mari et al. 2011). Although the DDR successfully deals with DNA damage and prevents them from becoming ‘locked-in’ genomic alterations, DNA lesions can occasionally be repaired improperly, resulting in a tendency of any genome to accumulate changes over time, a phenomenon referred to as genomic instability (Niedernhofer et al. 2018). For example, point mutations (i.e. base substitutions) are the result of stochastic replication errors or DNA lesions that are improperly detected or repaired (Aguilera and Gómez-González 2008). Larger, structural variants—so named because they require a disruption of the DNA sugar backbone—are caused by various mutational processes during DNA recombination, replication or repair (reviewed in Carvalho and Lupski 2016). These different types of ‘locked-in’ genomic alterations can be inherited, but due the constant pressures of DNA damage and the inherent stochasticity of genome replication and maintenance, they can also occur de novo (i.e. in the germline of the parent). Additionally, they can arise somatically (i.e. acquired during development and life), resulting in distinct and unique genomic alterations in each individual cell (Shendure and Akey 2015). Lastly, genomic instability can also be considered to include the accumulation of unrepaired, persistent DNA lesions, although many of these are thought to eventually result in mutations or chromosomal rearrangements as well (Tubbs and Nussenzweig 2017).
Nuclear genomic instability is a central feature of carcinogenesis (Jeggo et al. 2016; Negrini et al. 2010), but it is also strongly implicated in a range of other pathologies. The impact of genomic instability on tissue homeostasis is underlined by the more than 50 disorders currently known to be caused by mutations in genes that function in DNA repair (Petr et al. 2020). Because of its compelling link to cellular degeneration, genomic instability is widely recognized as one of the primary hallmarks of ageing (Vijg and Suh 2013). However, whereas the role of genomic instability in carcinogenesis is well-documented, how it can drive degenerative processes is far less understood. In this regard, an often underexposed side of genomic instability is its possible impact on the proteome.
To maintain a balanced proteome (i.e. protein homeostasis or proteostasis) inside the complex and crowded intracellular environment, cells rely on the constant surveillance of an elaborate, interlinked system of molecular chaperones, regulators and protein degradation pathways, referred to as the proteostasis- or protein quality control (PQC) network (Hipp et al. 2019). The PQC network ensures that proteins are synthesized at the right time and in the right quantity, that they are folded correctly, and that proteins that are misfolded, aggregated or no longer needed are degraded. Safeguarding protein homeostasis is crucial for any cell, as ‘proteome instability’ can result in protein aggregation and proteotoxic stress, which drive dysregulation of cellular pathways and functionality impairment, degeneration and cell death (Klaips et al. 2018; Labbadia and Morimoto 2015).
If and how genomic instability challenges protein homeostasis remains incompletely understood, but emerging data suggests that they may indeed be inherently connected. This is illustrated tellingly in cancer cells, which suffer from severe proteotoxic stress, resulting not only from their increased metabolism—elevating the protein folding demand—but also from a high burden of genomic alterations (Anon 2020; Dai et al. 2012; Deshaies 2014; Priestley et al. 2019; Vogelstein et al. 2013). Genomic instability has also been implicated in several (age-related) degenerative disorders believed to be primarily caused by a loss of protein homeostasis, including Alzheimer’s (Hou et al. 2017) and Parkinson’s (Sepe et al. 2016) disease. Vice versa, several recent studies have reported that proteotoxic stress plays a central role in disorders strongly associated with genomic instability (Hamczyk et al. 2019; Zhu et al. 2019). Although the DDR and the PQC network have long been approached as separate entities, over the last few years it has become clear that they are intricately interwoven with other core cellular signaling pathways, and importantly, with each other as well (Chatzidoukaki et al. 2020; Xie and Jarosz 2018). Together, these findings point at the possibility that genomic instability and proteome instability converge on a path to disease.
In this review we discuss the intricate relationship between genomic instability and protein homeostasis. We start by outlining the functionality of the PQC network in some more detail, and by explaining the concepts of protein aggregation and proteotoxicity. After this, we continue with an overview of the emerging interconnectivity between DDR and PQC network components. Next, we focus on the often complex proteomic impact of distinct genomic alterations—which frequently venture far beyond a simple ‘loss of function’—and discuss their molecular links with protein stability, misfolding and aggregation. In the last section, we explore the possibility of augmenting the capacity of the PQC network to mitigate the detrimental consequences of genomic instability.
Protein aggregation and proteotoxicity
Most proteins are thermodynamically only marginally stable in the physiological context of the cell (DePristo et al. 2005), and some are even found to be inherently unstable due to their specific sequence properties (Deller et al. 2016). Up to 30–50% of the human proteome appears to be made up of proteins that contain large regions of low complexity (intrinsically disordered regions, IDRs), most of which are only stabilized upon binding to specific partners (Gsponer et al. 2008; Uversky 2019). A large number of these proteins are expressed at concentrations close to their solubility limit in the cellular environment, forming a ‘metastable subproteome’ that is highly susceptible to misfolding (Tartaglia et al. 2007). These features likely overlap with the functional use of liquid–liquid phase-separation of biomolecules (proteins and nucleic acids) in cells, a process that needs to be closely regulated to avoid off-pathway phase transitions (Box 1). All of this underlines the extreme complexity of the human proteome, in which thousands of marginally stable protein species are coordinately expressed, the majority of which need to fold into a well-defined three-dimensional structure (i.e. their ‘native state’) and be maintained at precise abundances to perform their function. In addition, stresses like elevated temperatures (Ghosh and Dill 2010), heavy metals (Tamás et al. 2014) and oxidative stress (Lévy et al. 2019) pose an added burden on the proteome by damaging proteins and impairing protein production.

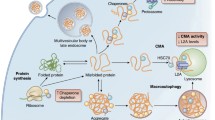
For most (globular) proteins the native state is a finite ensemble of closely related three-dimensional conformations (‘conformers’). Within the native state, a protein can (locally) adapt its structure, for example upon ligand binding and release (including nucleic acids and other proteins) (Janin et al. 2008), or during enzymatic activity (Chiti and Dobson 2017). In the complex environment of the cell, proteins rely on molecular chaperones of the PQC network to guide them into this conformation, prevent them from becoming misfolded, or even refold them when needed (Hartl et al. 2011). Several conserved families of molecular chaperones exist, two core machineries of which are particularly relevant for the topic of this review: HSP70 and HSP90 (Fig. 1).
A simplified overview of the chaperone machinery of the PQC network, responsible for proper protein (re)folding, maturation, and maintenance of conformational stability, all to avoid aggregation and enable protein function; through these functions the PQC network is also of crucial importance for regulating the function of clients in DDR pathways. Interaction of HSP70 with protein substrates is allosterically controlled by ATP and the substrate itself, and co-regulated by different JDPs, which function as ‘targeting factors’ that further increase substrate affinity. Through iterative cycles of substrate binding and release, where the substrate-binding domain of the HSP70 machinery alternatingly adopts an ‘open’ or ‘closed’ conformation, substrate folding is promoted. Substrates can also be handed over—mediated by co-chaperones (e.g. HOP)—to the HSP90 machinery when they are metastable and/or require HSP90 for full maturation. HSP90 exists as a homodimer that assumes an extended conformation when bound to ADP. Its folding activity depends on alternating between this ‘open’ state and a ‘closed’ state, which is favored by ATP-binding to the N-terminal domain that subsequently dimerizes. When proteins are terminally misfolded, they can be targeted for degradation, mediated by co-chaperones (e.g. the E3 ubiquitin ligase CHIP). BER base excision repair, NER nucleotide excision repair, MMR mismatch repair, PRR post-replication repair, NHEJ non-homologous end-joining, HR homologous recombination, JDP J-domain proteins, NEF nucleotide exchange factor
HSP70 has a strong affinity for hydrophobic stretches, which in the native state of a globular protein are hidden in the core, but become exposed when proteins unfold. HSP70 assists in the (re)folding of these substrates by binding and subsequently releasing them in iterative, ATP-dependent cycles, thus preventing aggregation and allowing their folding to take place (Rudiger et al. 1997; Kampinga and Craig 2010; Mayer 2018). The HSP70 cycle is closely regulated by J-domain proteins (JDPs, also called HSP40s) which facilitate client engagement (Kampinga and Craig 2010). HSP70 works in close concert with the chaperone machinery of HSP90, which is thought to take over partially folded clients directly and facilitate their complete (re)folding (Morán Luengo et al. 2019). Besides acting downstream of HSP70 in protein folding, HSP90 also fulfills many crucial roles in cell physiology by facilitating the maturation and conformational stability of client proteins, often assisted by various ‘co-chaperones’ (reviewed in Biebl and Buchner 2019). Many DNA repair proteins rely extensively on HSP70 often in combination with HSP90 to shape their conformational stability, and control their assembly into multiprotein DNA repair complexes (Knighton and Truman 2019; Sottile and Nadin 2018) (Fig. 1, bottom table).
When proteins fail to reach or hold their native state—i.e. when chaperones are unable to meet the folding demand—they can misfold and lose their function. Misfolded and superfluous proteins are sent for degradation (Fig. 1), or they can aggregate (see below). The two main intracellular proteolytic pathways are the ubiquitin–proteasome system (UPS) and the autophagy–lysosomal system. The UPS is a highly specific pathway that is responsible for most of the individual protein degradation. UPS substrates are recognized and posttranslationally tagged by ubiquitin in a three-step enzymatic cascade to target them for degradation (Amm et al. 2014). The autophagy–lysosomal system is an umbrella term that describes three major forms of proteolysis: chaperone-mediated autophagy, microautophagy and macroautophagy. Importantly, all three make use of the same general principle of lysosomal degradation, and only differ in how they deliver substrates to the lysosome (Hansen et al. 2018). Autophagy occurs primarily in response to cellular stress, to free up molecules like amino acids or lipids for reuse, or to degrade large unwanted substrates, including damaged organelles like mitochondria (Dikic and Elazar 2018). It starts by the engulfment of sequestered cytosolic cargo by a double-membrane structure known as an autophagosome. This autophagosome then translocates to the lysosome with which it fuses, after which the inner membrane together with the cargo are degraded by the hydrolytic enzymes inside the lysosomal lumen (Bento et al. 2016).
Protein aggregation, proteotoxicity and pathology
When the PQC network is unable to guide or hold proteins in their native state, they can misfold and convert into a nonfunctional, aggregated state, which is believed to frequently render a protein toxic to its environment (i.e. a ‘proteotoxic gain of function’) (Fig. 1). Protein aggregates exist in a range of different conformations, but overall, they can be divided into two main classes: disordered/amorphous aggregates, and amyloids. Whereas amorphous aggregates arise typically as a result of off-pathway, hydrophobic interactions (Hipp et al. 2019), amyloids are formed by the self-assembly of β-strand containing proteins into a ‘cross-β’ filament structure (Dobson 2017). How these protein aggregates can drive pathology through proteotoxicity lies beyond the scope of this review, but is thoroughly reviewed elsewhere in Kampinga and Bergink (2016), and in Klaips et al. (2018). Importantly, metastable or aggregation-prone proteins can also affect the stability of the global proteome, for example by increasing the aggregation propensity of other proteins. This is believed to be mainly the result of a competition for limited chaperone-mediated folding capacity and/or sequestration of chaperones in protein aggregates (Gidalevitz et al. 2010; Hipp et al. 2019). In addition, protein aggregates (in particular amyloids) can directly induce the ‘co-aggregation’ of other proteins, which likely occurs through various mechanisms (Bondarev et al. 2018). These and other findings indicate that an initial aggregation event can drive a cascade (or ‘snowballing’) of subsequent misfolding and aggregation events, which ultimately leads to a complete loss of protein homeostasis.
Protein homeostasis mechanisms are interlinked with genome maintenance
Protein aggregation poses a threat to the integrity of the genome
A growing body of experimental data points at protein aggregation as a possible cause of DNA damage. Aggregation of certain disease-associated proteins, including amyloid-β fragments and α-synuclein, has been associated with elevated levels of DNA strand breaks (Farmer et al. 2020; Illuzzi et al. 2009; Vasquez et al. 2017), indicating that DNA damage can be an ancillary consequence of protein aggregation. Two primary biological cascades have been proposed to underlie this damage. First, aggregated proteins can elicit genotoxic oxidative stress by engaging mitochondria and driving mitochondrial dysfunction (Lévy et al. 2019). One example comes from pathogenic α-synuclein aggregates, which can bind mitochondrial membranes and impair respiratory chain components, hampering oxidative phosphorylation (Ludtmann et al. 2018). This in turn can lead to the dissipation of the mitochondrial membrane potential and to the formation of harmful reactive oxygen species (ROS). Although cause and consequence can sometimes be difficult to disentangle, aggregation of, among others, mutant SOD1 (Vehvilainen et al. 2014), TDP-43 (Wang et al. 2019), Huntingtin(Htt) (Bossy-Wetzel et al. 2008) and amyloid-β (Moreira et al. 2010) fragments have been reported to lead to a similar impairment of mitochondrial function.
Second, aggregating protein species can sequester factors required for DNA repair, thus draining the functional pool of proteins involved in maintaining genome integrity. Although it is not always clear if the sequestration of DNA repair factors is able to completely explain the observed impairment of genome maintenance, this appears to be a general phenomenon in several neurodegenerative disorders associated with protein aggregation (Enokido et al. 2010; Gao et al. 2019; Nakamura et al. 2019; Suberbielle et al. 2015).
Related to this, the native, soluble isoforms of certain disease-associated proteins, including Tau, FUS, SOD1 and α-synuclein, have been directly linked to genome maintenance in vivo, and genomic instability caused by their mutant species has been attributed to their effective loss from the nucleus (Bordoni et al. 2019; Maina et al. 2016; Schaser et al. 2019; Wang et al. 2018). Importantly, it is not always understood if this is a direct consequence of their misfolding, or a result of their accelerated aggregation in the cytoplasm.
Although several studies have investigated the relationship between protein aggregates and reduced genome maintenance, it is still unclear to what extent this connection is limited to aggregation of specific disease-associated proteins. Recent experimental work suggests that it extends to protein aggregation in general, as artificial aggregation of firefly luciferase has also been found to impair genome maintenance in human cells (Ben Yehuda et al. 2017).
The PQC network is crucial to maintain genome integrity
The PQC network safeguards protein homeostasis by carefully regulating protein synthesis, folding, and degradation, and through these functions it also plays a role in coordinating genome maintenance pathways. Many DNA repair proteins rely extensively on PQC network chaperones to shape their conformational stability, and control their assembly into multiprotein DNA repair complexes (Knighton and Truman 2019; Sottile and Nadin 2018). A well-studied example is HSP90, which has emerged as a central player in many DNA repair processes (Dubrez et al. 2020). HSP90 accumulates in DNA damage sites (Oda et al. 2007; Quanz et al. 2012), and its inhibition sensitizes human cells to both UV (Sekimoto et al. 2010) and γ-irradiation (Dote et al. 2006). HSP90 chaperones multiple DNA repair factors in different pathways, including RAD51 (Ko et al. 2012), FANCA (both homologous recombination, HR) (Oda et al. 2007), DNA-PK (non-homologous end-joining, NHEJ) (Solier et al. 2012), Pol eta (translesion synthesis, TLS) (Sekimoto et al. 2010) and XRCC1 (base-excision repair, BER) (Fang et al. 2014). It also has a critical role in the recruitment of the DSB repair machinery by stabilizing the MRN complex and stimulating the activity of ATM (Cheng et al. 2017). HSP90’s function complements that of HSP70 in various genome maintenance pathways, including BER, mismatch repair (MMR) and HR (Dubrez et al. 2020). These findings appear to reflect a broad nuclear activity of the HSP90 chaperone machinery, which is further underlined by the conserved role of the HSP90 co-chaperone p23 in several DNA repair pathways (Echtenkamp et al. 2011).
The two main proteolytic pathways of the PQC network, the autophagy–lysosomal system and the UPS, can also impact genome integrity. Not only do they mitigate oxidative DNA damage by controlling mitochondrial quality (Pickles, Vigié, and Youle 2018; Ravanelli et al. 2020), they also influence the dynamics of genome maintenance by controlling the turnover of many key DNA repair proteins (Brinkmann et al. 2015; Guo and Zhao 2020 ) and the DNA replication machinery (Roseaulin et al. 2013; Walter et al. 2016). Autophagy also appears to play a key role in maintaining nuclear homeostasis by selectively degrading other nuclear components (i.e. ‘nucleophagy’), including nucleolar factors and nuclear lamina proteins (Otto and Thumm 2020; Papandreou and Tavernarakis 2019). Although it is not always clear to what extent nucleophagy-substrates constitute damaged nuclear content, or whether it is a reflection of normal turnover, inhibiting autophagy has been shown to result in an aberrant nuclear morphology (Park et al. 2009), which may affect the integrity of the genome as well. Turnover of nuclear components is sometimes mediated by crosstalk between the two systems through the autophagy adaptor protein SQSTM1/p62 (Hewitt et al. 2016). For example, autophagy inhibition results in the nuclear accumulation of p62, which can indirectly alter HR by facilitating the proteasomal degradation of CHK1, FLNA and RAD51 (Hewitt and Korolchuk 2017). The UPS also plays a central role in genome maintenance by orchestrating a vast amount of ubiquitylation events, most of which are however not linked to client degradation (reviewed in Bergink and Jentsch 2009). Interestingly, although impairment of both autophagy and the UPS has been increasingly linked to genomic instability, several studies have also reported decreased DNA repair after inhibition of the proteasome (Arlow et al. 2013; Karpov et al. 2013; Sciascia et al. 2020). Together, this indicates that the autophagy–lysosomal system and the UPS have a complex—and still incompletely understood—role in the context of genome maintenance.
The strong dependency of DNA repair on the PQC network also poses a risk. During chronic proteotoxic stress, an excessive protein folding and degradation demand can overwhelm the capacity of the PQC network, depleting free chaperone pools (Hipp et al. 2019) and disrupting the function of both autophagy (Monaco and Fraldi 2020) and the UPS (Thibaudeau et al. 2018). This could potentially lower their net functional availability for other cellular processes, including genome maintenance. An interesting example of such a possible trade-off between protein homeostasis and genome integrity is proteotoxic stress-induced aneuploidy, which has been shown to result from a reduced availability of the HSP90 machinery for kinetochore assembly, leading to karyotype changes following cell division (Chen et al. 2012). While this mechanism may benefit the population in the long term by increasing genetic variation in the face of changing environments (Chen et al. 2012; Kaya et al. 2020; Rancati et al. 2008), it has substantial consequences for the individual cell. Another example is the widespread use in both contexts of ubiquitin and ubiquitin-like proteins (most notably NEDD8 and SUMO) as posttranslational modifications. These small polypeptides (8–11 kDa) are conjugated to target proteins and act as signaling molecules, often in concert with each other. They perform crucial regulatory roles in genome maintenance as modulators of protein–protein and protein-DNA interactions (reviewed elsewhere in Bergink and Jentsch 2009; Brown and Jackson 2015; Wang et al. 2017), but in the PQC network they function primarily as coordinators of the UPS and the autophagy-lysosomal pathway (Liebelt and Vertegaal 2016; Pohl and Dikic 2019), and as regulators of protein aggregation. The pervasive use of ubiquitin and ubiquitin-like protein modifications in both genome maintenance and protein homeostasis mechanisms has led to the idea that under proteotoxic stress, the PQC network competes for free ubiquitin with other ubiquitin-dependent processes, including genome maintenance and chromatin regulation pathways (Dantuma et al. 2006; Park and Ryu 2014). In line with this, proteasome dysfunction and aggregation of ubiquitin-positive substrates have been shown to specifically deplete the nuclear pool of unconjugated ubiquitin (Farrawell et al. 2018; Mimnaugh et al. 1997), and one recent study reported that DNA repair capacity was hampered as a consequence of this (ben Yehuda et al. 2017). However, mechanistic intervention studies are lacking so far, and although ubiquitin-, NEDD8- and SUMO-conjugated substrates all accumulate in protein aggregates upon proteotoxic stress (Bence 2001; Enchev et al. 2015; Liebelt and Vertegaal 2016), it is still unclear if competition for these posttranslational modifiers can explain increased genomic instability upon a loss of protein homeostasis.
Genome maintenance defects are causally linked to a loss of protein homeostasis
Overall, safeguarding protein homeostasis appears to be important to preserve genomic integrity. Importantly, this relationship between cellular protein homeostasis and genome integrity extends in the both directions. For example, protein misfolding and aggregation can affect genome maintenance, but genome maintenance defects are also causally linked to a loss of protein homeostasis.
A first indication of this is the notion that genome maintenance processes have been picked up in genetic screens designed to identify possible modulators of protein aggregation in various model organisms (van Ham et al.2009). More direct evidence for this connection is provided by heritable defects in several genome maintenance pathways that are causally linked to a loss of protein homeostasis. A well-studied example is ATM, a PI3K-like kinase that functions as a master switch in genome maintenance and cell cycle checkpoint regulation. The absence of functional ATM—which causes the severe neurodegenerative disorder ataxia–telangiectasia (A–T) (McKinnon 2012)—results in a hypersensitivity to double-strand breaks (DSBs) and to oxidative stress-inducing drugs, and leads to higher intracellular ROS levels (Barzilai et al. 2002). This increase in baseline ROS is associated with reduced cellular health, and in particular with a loss of protein homeostasis, including endoplasmic reticulum (ER) stress and activation of the unfolded protein response (UPR) (Barzilai et al. 2002; Liu et al. 2005; Yan et al. 2008). More recent work has revealed that ATM acts as a central regulator of cellular redox homeostasis, and that this function can, surprisingly, be genetically separated from ATM’s role in the response to DNA damage (Guo et al. 2010). In the same study, impaired activation of ATM by either DNA damage or oxidation both resulted in the accumulation of aggregated protein species. Additional oxidative stress further exacerbated protein aggregation only in the latter. This indicates that a loss of ATM can potently affect protein homeostasis via a dysregulated redox homeostasis, but also through impaired genome maintenance. In agreement with this, loss of kinase activity of the yeast ATM/ATR kinase Mec1—or its downstream signaling targets—also causes widespread protein aggregation and confers sensitivity to stresses challenging protein homeostasis (Corcoles-Saez et al. 2018). Considering the notion that in A–T, it is—arguably—the absence of ATM’s central role in the response to DNA damage which is responsible for the strong cerebellar degeneration observed (Shiloh 2020), this raises the question if genomic instability-induced loss of protein homeostasis could be an underlying pathogenic mechanism in this context.
Interestingly, a similar destabilization of the proteome has been found after impairments of other genome maintenance pathways, mechanistically largely unrelated to ATM. For example, Werner syndrome (WS) is a progeroid disorder cause by mutations in WRN, a DNA helicase involved NHEJ and HR (Croteau et al. 2014). Fibroblasts from WS patients accumulate protein aggregates and exhibit a dramatic upregulation of autophagy (Talaei et al. 2013). Cockayne syndrome (CS) is another severe progeroid disorder, caused by mutations in the transcription-coupled nucleotide excision repair (TC-NER) genes CSA or CSB (Vessoni et al. 2020). A recent study showed that CS patient-derived cells exhibit increased levels of misfolded proteins and ER stress, postulated to result from a reduced ribosomal translation fidelity (Alupei et al. 2018). Similarly, loss of the central NER protein XPA, which is associated with neurodegeneration (Kraemer 1987), has also been shown to lead to increased levels of polyubiquitylated proteins (Arczewska et al. 2013), impaired UPR function and accelerated protein aggregation (de Sousa Leal et al. 2020). For most of these examples, the molecular chain of events connecting a genome maintenance defect to a loss of protein homeostasis is still far from understood, and different pathological mechanisms have been hypothesized for each of them. However, the notion that impairments of mechanistically distinct genome maintenance pathways all lead to an eventual loss of protein homeostasis suggests that they may also share a common underlying cause: a destabilization of the proteome resulting from genomic instability.
Genomic instability intrinsically challenges protein homeostasis
How can genomic instability affect global protein homeostasis? Over the last two decades, studies focusing on age-related disorders, including Alzheimer’s and Parkinson’s diseases, have contributed enormously to our appreciation of the broad proteome-destabilizing impact of specific inherited and de novo mutations (Chiti and Dobson 2017; Vendruscolo et al. 2011). Accumulating evidence suggests that this connection between genomic instability and a loss of protein homeostasis may extend to somatically acquired alterations and persistent DNA damage as well. For example, recent advances in single-cell sequencing techniques that enable the profiling of cell-to-cell genomic variation (i.e. mosaicism) in high-throughput have revealed that—in parallel to declining protein homeostasis—genomic instability increases widespread in ageing tissues (Blokzijl et al. 2016; Brazhnik et al. 2020; Laurie et al. 2012; Lodato et al. 2018; Martincorena et al. 2015; Zhang et al. 2019). Moreover, we now appreciate that a large array of different types of genomic alterations, including persistent DNA damage, has the potential to destabilize the proteome, either directly or indirectly (Fig. 2). In the next sections, we will review the main mechanisms linking these genomic alterations to a loss of protein homeostasis.
Single nucleotide alterations: conformational instability and synthesis of aberrant mRNA
The potential of genetic alterations to affect protein homeostasis is first highlighted by the numerous base substitution mutations linked to protein conformational diseases, for example in Parkinson’s disease (Chiti and Dobson 2017). Many of these mutations alter the conformation of a single protein, which is believed to drive a cascade of misfolding and aggregation events that ultimately destabilizes the proteome, leading to pathology (Vendruscolo et al. 2011). From a molecular perspective, an intrinsic connection between base-substitutions and protein conformational instability is evident. The marginal thermodynamic stability of proteins leaves the protein folding process highly vulnerable to mutations that result in a change in the amino acid sequence, so-called missense mutations, as most of these are destabilizing (Redler et al. 2016). In certain cases, depending on the stability of the native protein and its folding intermediates, and on the location (e.g. hydrophobic core residues are generally less tolerant than hydrophilic surface residues (Matsui et al. 2017)), even a single missense mutation can completely destabilize a protein, causing it to misfold and/or increase its propensity to aggregate. Examples of this include certain mutations in α-synuclein (Dettmer et al. 2015), PFN1 (Boopathy et al. 2015), p53 (Wilcken et al. 2012), lysozyme (Booth et al. 1997) and transthyretin (Lim et al. 2017), and this list is far from exhaustive. In general, disease-associated mutations appear to occur more frequently at loci vulnerable to substitution-induced protein destabilization and aggregation (de Baets et al. 2015), adding further support to the notion that protein aggregation has a pervasive impact on human disease.
Other mechanisms by which missense mutations can lead to protein aggregation have been reported as well—amino acid substitutions that are not directly destabilizing may still drive a protein into an aggregation-prone conformation. For example, most of the disease-linked mutations in tau reduce its binding affinity for cytoskeletal microtubules, resulting in the accumulation of unbound tau which is highly aggregation-prone (Spillantini and Goedert 2013). A related mechanism has been uncovered for gelsolin, where mutations can impair its ability to bind calcium, leading to the gradual destabilization of the protein. However, unlike tau, the conformational change does not lead to the aggregation of gelsolin itself, but instead exposes a previously buried cleavage site, resulting in the production of small, highly amyloidogenic gelsolin fragments (Solomon et al. 2012). High levels of aggregating amyloid-β and apolipoprotein A-I fragments are the result of a similar mutation-induced dysregulated proteolysis events (Chen et al. 2017; Raimondi et al. 2011).
The incorporation of a different amino acid is not the only mechanism through which point mutations can challenge protein homeostasis. The removal or introduction of a premature stop codon (i.e. ‘nonsense’ mutation) can prevent a protein from ever being properly synthesized in the first place, as illustrated in the case of Apolipoprotein A-II and PrP, respectively (Benson et al. 2001; Bernardi and Bruni 2019). In both examples, translation is halted at the wrong place of the transcript, leading to the production of (partially) unfolded, aggregation-prone polypeptide fragments. Mutations can also affect protein production by altering splicing patterns, which can result in unstable and/or aggregation-prone polypeptides. In this regard, accumulating evidence suggests that also synonymous (long referred to as ‘silent’) mutations can profoundly affect both protein expression and conformation. For instance, next to many missense mutations, synonymous mutations in the MAPT gene (encoding for tau) can cause altered splicing of the MAPT transcript, resulting in increased synthesis of the disease-associated 4R tau isoform (Niblock and Gallo 2012). Synonymous mutations can even act more subtle, by altering mRNA stability, or by affecting translation rates leading to disrupted co-translational folding (Sauna and Kimchi-Sarfaty 2011). A recent study in E. coli showed that synonymous mutations can impair cellular fitness by driving misfolding of the native protein (Walsh et al. 2020), supporting the idea that these mutations can lead to proteotoxicity as well. Although far less studied, mutations located outside of the coding sequence of a gene, including promoter and enhancer regions, introns, and 3′ and 5′ UTRs may all affect protein homeostasis through similar mechanisms (Sauna and Kimchi-Sarfaty 2011).
Of special interest are insertion and deletion mutations (‘indels’). Indels spanning a number of nucleotides divisible by three will lead to the incorporation or deletion of one or more amino acids from the polypeptide, which may challenge folding stability. However, indels of any other size, including single-nucleotide alterations, can dramatically affect protein biogenesis because they change the reading frame of the genetic sequence. For example, frameshift mutations in the transcription factor p63 have been shown to lead to extensions of its C-terminus, resulting in the production of aggregating peptide fragments that display a toxic gain-of-function (Russo et al. 2018). Frameshift mutations in the tumor suppressor protein PTEN were also found to increase aggregation propensity, far stronger than both missense mutations and non-frameshifting indels (Palumbo et al. 2020). The extent to which frameshift mutations, especially those occurring in somatic cells, contribute to a loss of protein homeostasis is still largely unknown—they are difficult to detect in conventional short read sequencing data (Shigemizu et al. 2013) and likely much less frequent than substitutions (Brazhnik et al. 2020). Moreover, their pathological impact has been investigated mainly in the context of carcinogenesis. Nevertheless, their potentially profound impact on the proteome supports the idea that they can play a strong role in disrupting protein homeostasis.
Structural variants and ploidy changes: supersaturation and stoichiometric imbalances
A large, but relatively poorly understood group of genomic alterations is formed by structural variants (SVs), here defined as inversions, translocations, duplications and large indels. SVs typically comprise DNA segments spanning more than 50 basepairs (Baker 2012), leading to either chromosomal rearrangements or changes in absolute DNA content. Although the existence of SVs was initially met with skepticism, a growing body of evidence has shown that SVs are pervasive (Abel et al. 2020), and that they accumulate with age (Forsberg et al. 2012). As a group, SVs are thought to account for most of the interindividual variation among human genomes in terms of total nucleotides involved (Weischenfeldt et al. 2013). Their relationship to pathology and degeneration has been studied mainly in the context of carcinogenesis (Yi and Ju 2018), and although SVs can potentially have a strong proteomic impact—through gene disruption or fusion, or by altering gene expression (Weischenfeldt et al. 2013)—their global effect on protein homeostasis is still largely unexplored.
The proteomic impact of SVs is better characterized in the case of copy number variants (CNVs), resulting from either large duplications or deletions. CNVs are associated with a range of diseases and phenotypic outcomes, including ageing and neurodegeneration (Potter et al. 2019; Shepherd, Yang, and Halliday 2018). Of particular interest here are the CNVs of SNCA and APP which have been directly linked to an accelerated loss of protein homeostasis and disease progression in Parkinson’s (Perez-Rodriguez et al. 2019) and Alzheimer’s diseases (Rovelet-Lecrux et al. 2006), respectively. These extra-copy CNVs are thought to increase the expression of aggregation-prone α-synuclein and amyloid-β. Interestingly, Down’s syndrome patients, carrying an extra APP gene due to trisomy 21, are highly prone to Alzheimer’s disease as well (Lott and Head 2019). These findings may reflect the phenomenon of protein supersaturation, where an increased abundance of marginally stable proteins causes them to supersede their in vivo solubility, catalyzing aggregation (Tartaglia et al. 2007). This is supported by findings showing that in yeast, aneuploidy causes widespread proteotoxicity, irrespective of the chromosome involved (Oromendia et al. 2012). Moreover, the proteotoxicity resulting from a single extra chromosome leads to a decrease in yeast replicative lifespan, the extent of which is proportionate to the size of the chromosome (Sunshine et al. 2016). Recent work has uncovered an additional mechanism through which aneuploidy may lead to proteotoxic stress: loss of protein complex stoichiometry. Eukaryotes rely on coordinated protein expression to maintain the proper stoichiometry required for multiprotein complex assembly. The significant expression changes caused by aneuploidy result in a net surplus of protein complex subunits, which have to be dealt with by the PQC network—they are either degraded, or they aggregate (Brennan et al. 2019; Stingele et al. 2012).
Like other SVs, CNVs and aneuploidy can pose a significant threat to the stability of the proteome (Oromendia and Amon 2014), but their contribution to for example the age-related decline in protein homeostasis has not been fully elucidated. One of the reasons for this is that most studies investigating the proteomic consequences of CNVs and aneuploidy have approached it mostly from a germline perspective. Nonetheless, despite at times conflicting data (Bos et al. 2017), many studies have reported that both CNVs, including large megabase variants, and aneuploidy accumulate with age (Revay et al. 2017), also in humans (Forsberg et al. 2012; Villela et al. 2018). Their impact on protein homeostasis may very well depend on the proteins involved, and future studies will therefore have to establish if they have a degenerative role in the general population.
A related class of genomic alterations that can disrupt protein homeostasis is formed by expansions of repetitive DNA sequences. Although such repeat expansions (or ‘tandem repeats’) can also be considered SVs, underneath we discuss these alterations separately as they can have profoundly distinct proteomic consequences (Spielmann et al. 2018).
Tandem repeats: aggregation-proneness, RAN translation and somatic expansion
Currently, 13 different types of tandem repeats (tri-, tetra-, penta- or hexanucleotide) have been identified, together causing over 40 distinct hereditary disorders (Paulson 2018). In many of these diseases, the expanded tandem repeat leads to the production of a highly aggregation-prone protein that gradually destabilizes the proteome, ultimately leading to a loss of protein homeostasis (Gidalevitz 2006), as is the case for certain polyalanine expansions (Pirone et al. 2019; Polling et al. 2015). One of the most prevalent expansions is the CAG expansion, which occurs in several different proteins. The resulting polyglutamine stretch (i.e. polyQ) causes diseases like Huntington’s disease (HD) and most spinocerebellar ataxias (SCAs) (Adegbuyiro et al. 2017). In all known polyglutamine diseases, the size of the expanded CAG tract is inversely correlated to the age of disease onset (Kuiper et al. 2017). This is attributed mainly to the length-dependent ability of polyQ stretches to form stable β-hairpins, resulting in a highly amyloidogenic conformation, although other factors have been shown to affect polyQ aggregation as well (Kuiper et al. 2017). Recently, CAG expansions in huntingtin were also shown to drive its aggregation by altering phase separation dynamics (Box 1).
Although close to half of the repeat expansion disorders are thought be primarily driven by RNA-dependent gain-of-function mechanisms (Ellerby 2019), most of these have been associated with a loss of protein homeostasis as well. One important reason for this is that repeat expansion transcripts can produce proteins in multiple reading frames without the need for a canonical AUG start codon (i.e. repeat-associated non-AUG or RAN translation) (Banez-Coronel and Ranum 2019). Hence, even when an expansion lies outside a protein-coding region, both sense and antisense transcripts can produce different aggregation-prone repetitive polypeptides (Cleary, Pattamatta, and Ranum 2018). This is illustrated by the CTG expansion in junctophilin-3 (JPH3) which causes an HD-like syndrome (HDL2). Here, RAN translation of the antisense CAG transcript results in the production of polyglutamine stretches that aggregate, which is thought to be a main driver of HDL2 pathology (Swinnen et al. 2020). A similar mechanism may also play a role in myotonic dystrophy type 1 (DM1), which is caused by a CTG expansion in the 3′ UTR of DMPK (Gudde et al. 2017; Zu et al. 2013). RAN translation is also responsible for the production of proteotoxic dipeptide-repeats from the G4C2 repeat expansion located in the first intron of C9orf72, which is strongly linked to ALS and frontotemporal dementia (FTD) (Balendra and Isaacs 2018; Zu et al. 2013). Interestingly, RAN translation of both G4C2 and CGG (associated with fragile X-associated tremor/ataxia syndrome or FXTAS) repeats has been shown to be activated in a PERK- and eIF2a-dependent manner by the integrated stress response (ISR). This points at the existence of a pathological feed-forward loop, where a gradual destabilization of the proteome favors additional RAN translation of toxic proteins, accelerating the protein homeostasis decline (Green et al. 2017).
Recently, advanced genome profiling techniques like long-read sequencing have unveiled previously unknown neurodegeneration-associated repeat expansions linked to protein aggregation (Cortese et al. 2019; Ishiura et al. 2019), suggesting that pathological tandem repeats may be more common than generally thought. In addition, known tandem repeats may also contribute more to the age-related decline of protein homeostasis than currently believed. Repeat expansions are often highly unstable, expanding further from one generation to the next, a phenomenon referred to as anticipation (Paulson 2018). However, for several tandem repeats, including CAG, CTG, and C9orf72, ongoing expansion has also been observed in somatic cells (Castel et al. 2010; Nordin et al. 2015), in some (but not all, see (Cancel et al. 1998) cases specifically in those tissues most prominently involved in pathology (Kennedy 2003), and correlating with disease progression (Ciosi et al. 2019; Morales et al. 2012; Swami et al. 2009). This supports the idea that in certain situations, somatic expansion can influence disease progression and perhaps even pathogenesis. In line with this, recent work has found that expansion of the only naturally occurring mouse polymorphic CAG repeat (located in the Tbp gene) takes place in aged WT mice (Sanchez-Contreras and Cardozo-Pelaez 2017). Although studies investigating ongoing somatic expansion of tandem repeats have so far been largely correlative in nature, it is tempting to speculate about their possible impact on the stability of the proteome. Additional studies combining for example long-read single-cell sequencing with proteomics are therefore needed to address the global effects of expansions on protein homeostasis in the context of both disease and normal ageing.
Persistent DNA damage: transcription blockage and transcriptional mutagenesis
Wrongly repaired DNA damage can lead to mutations and other stable genetic alterations, but importantly, even unrepaired damage can impact protein homeostasis. Although accurately measuring the steady-state levels of such persistent DNA lesions in high-throughput is still difficult, they do appear to accumulate with age, and this has been proposed to be one of the main drivers of the ageing process itself (Lans et al. 2019; Ou and Schumacher 2018; Petr et al. 2020). DNA lesions can affect transcription by impairing or even completely blocking the progression of RNA polymerase II (Pol II), resulting in the reduced production of mRNA which can hamper cellular function. In addition, complete transcription blockage has been linked to the formation of vulnerable (i.e. unpaired) DNA R-loops that are lesion-prone, which may in turn lead to a vicious cycle of genotoxic events (Lans et al. 2019). Although such a molecular cascade has been associated with increased apoptosis and cellular senescence (Petr et al. 2020), it may also influence the stability of the proteome, for example by altering the stoichiometry of protein engaged in multiprotein complexes. Alternatively, many DNA lesions can also be bypassed by Pol II, but this can severely reduce transcriptional fidelity and lead to transcriptional mutagenesis (Brégeon and Doetsch 2011). In these cases, transcription-coupled repair is not triggered, which can result in a rapid build-up of faulty transcripts (Brégeon et al. 2003), a process that has been hypothesized to contribute to the protein aggregation observed in neurodegenerative diseases (Basu et al. 2015; Brégeon and Doetsch 2011). Although both transcriptional blockage and transcriptional mutagenesis have the potential to drive a destabilization of the proteome, their (relative) contributions on a genome-wide level in vivo remain incompletely understood. Interestingly, persistent DNA damage has recently been found to drive the activation of the integrated stress response (ISR), a signaling network important for maintaining protein homeostasis (Clementi et al. 2020). In this study, activation of the ISR was shown to promote cell survival through increased translation of ATF4, a transcription factor controlling various stress response genes. Although the transcriptional response initiated by ATF4 in this context has yet to be unveiled, notable downstream targets of the ISR and of ATF4 in particular include key PQC network components (Fusakio et al. 2016). Future research should investigate the relative proteomic impact of transcriptional blockage and transcriptional mutagenesis, and determine whether ATF4 dependent stress signaling indeed plays a role in maintaining protein homeostasis upon persistent DNA damage.
Additional factors: transposons, 3D genome organization, and natural variation
Factors that increase the instability of the genome largely independent of conventional DNA damage have in recent years also been brought under attention. Although these processes are likely very important for cellular function and disease, there is still much unknown about them and how they interface with pathology. Importantly, their potential impact on protein homeostasis would likely occur through similar processes as discussed above. For these reasons, we will only touch upon two examples briefly.
The first is dysregulated retrotransposon activity. A substantial fraction of the human genome is made up of transposable elements (TEs), among which retrotransposons, a class of TEs that includes for example the long interspersed nuclear elements (LINEs) (Cordaux and Batzer 2009). Retrotransposons are mobile genetic elements that can be copied and randomly re-inserted (i.e. transposition) into the genome. Not only can this process be highly mutagenic and change coding or regulatory sequences (Muotri et al. 2005; Upton et al. 2015), but transcription of retrotransposons may also result in cytotoxic polypeptides (Li et al. 2015). In healthy cells most of them are thought to be silenced, but a loss of their silencing has been associated with age-related degenerative disorders, most notably Alzheimer’s disease and ALS (Jönsson et al. 2020).
A second example is aberrant three-dimensional (3D) genome organization. Instead of floating around freely or being randomly folded inside the nucleus, dynamic genome organization is tightly controlled. Each chromosome occupies a defined territory, and within each chromosome, several layers of organization appear to determine the position of specific chromosomal regions relative to each other, and to the nuclear lamina (reviewed in Yu and Ren 2017). This organization is crucial in DNA replication and gene regulation, underlying the formation of closed and open chromatin and the function of distal cis-regulatory elements, but also larger processes like X-chromosome inactivation in humans. Aberrant 3D genome organization has been linked to increased genomic instability and disease, for example in Hutchinson–Gilford progeria syndrome (HGPS) (Evans et al. 2019). Interestingly, a recent study found that proteotoxic stress plays is a crucial driver of atherosclerosis in HGPS, arguably the most debilitating symptom of this disease (Hamczyk et al. 2019).
Both examples reflect a growing awareness that our genomes are far from static, but that they are instead shaped by numerous internal and external factors, many of which are active throughout life. Together with the more established sources of genomic instability discussed above, they illustrate the vastly complex relation between genome and proteome. Although it is still unclear whether specifically dysregulated TE activity and aberrant 3D genome organization can challenge protein homeostasis, each has the potential to profoundly shape this relation.
Finally, the broad potential of genomic alterations to impact protein homeostasis also raises the question to what extent ‘naturally’ occurring genetic variation (e.g. single-nucleotide polymorphisms, SNPs) may contribute to a loss of protein homeostasis. Importantly, such variation is generally not considered as the outcome of genomic instability, but as a result of the inherent stochasticity of genome maintenance processes coupled to neutral and adaptive genetic processes, the main driving forces of evolution in a population (Prohaska et al. 2019). The impact of this variation on disease and lifespan is still far from understood, but it is thought to contribute substantially to the variation observed in protein homeostasis decline with age as well (Gidalevitz et al. 2013; Gidalevitz, Prahlad, and Morimoto 2011).
Proteome instability as a targetable pathological mechanism of genomic instability
Although genome alterations—in particular single nucleotide alterations—can lead to loss of protein function, it is clear that many (if not all) types of DNA changes and lesions also have the potential to disrupt proteome stability and drive protein aggregation through a proteotoxic gain of function in many different ways (Fig. 2). Importantly, this is not restricted to specific disease-associated proteins, but extends to a large fraction of the proteome, and as a consequence, it does not necessarily take specific or large genomic changes to challenge protein homeostasis (Gidalevitz 2006; Gidalevitz et al. 2009). From the inherent metastability of the proteome (Gidalevitz et al. 2011), the pervasive aggregation-prone characteristics (Ciryam et al. 2016; Goldschmidt et al. 2010), and possible feed-forward loops in place (Green et al. 2017), it can thus be inferred that even a seemingly small amount of random genetic alterations—mutations or SVs; either inherited, arisen in the germline, or acquired somatically—may at some point in time (when the PQC network is unable or no longer able to deal with it) set off a cascade of aggregation events (Vendruscolo et al. 2011), driving a loss of protein homeostasis.
How pervasive the link between genomic instability and proteome instability really is, and what role it plays in disease, is a crucial question that has so far been largely left unanswered. As a loss of protein homeostasis can profoundly impact cellular function, and potently drive pathology, could a loss of protein homeostasis then also contribute to the degeneration resulting from genomic instability? Intriguingly, emerging data suggest that, at least in certain situations, a loss of protein homeostasis is not only a consequence of genomic instability, but could even be one of the primary mechanisms underlying downstream pathology, as we will discuss below. First, we need to briefly outline how the PQC network can be used to control the consequences of genomic alterations.
The PQC network can modulate the degenerative consequences of genomic instability
For almost two decades it is known that the PQC network plays an important role in shaping the consequences of genomic alterations. Early work from the field of evolutionary biology found that bacterial strains engineered to accumulate a large amount of mutations survive by upregulating the expression of the chaperonin GroEL and the molecular chaperone DnaK, the bacterial HSP70 (Fares et al. 2002; Maisnier-Patin et al. 2005). Normally, these heat shock proteins collaborate in the folding and assembly of proteins, and prevent them from becoming misfolded (Gragerov et al. 1992). During increased genomic instability, they buffer the effects of mutations by similarly engaging mutated client proteins and kinetically stabilizing them so that they remain functionally active (Jarosz, Taipale, and Lindquist 2010; Tokuriki and Tawfik 2009; Zhao et al. 2019).
This role of the PQC network appears to be highly conserved. To sustain their inherent proteotoxic stress and even thrive under it, cancer cells hijack the PQC network, including the UPS and chaperone systems (Calderwood and Gong 2016; Deshaies 2014). The abundant heat shock protein HSP90 (Borkovich et al. 1989) has emerged as a particularly interesting chaperone in eukaryotes in this regard. Like GroEL and DnaK in bacteria, HSP90 can bind and stabilize genetically altered proteins, allowing them to explore new functions, thus potentiating genetic variation (Jarosz et al. 2010). Cancer cells make extensive use of HSP90’s ability as a potentiator as a means of stabilizing oncogenic proteins (Whitesell and Lindquist 2005), including mutant p53 (Nagata et al. 1999) (often in concert with HSP70 (Boysen et al. 2019)).
Importantly, this modulatory role of the PQC network is not just limited to cancer. In a recent study by Karras and colleagues, HSP90 was shown to act as a pervasive buffer against mutations, mitigating their detrimental effects on protein function (Karras et al. 2017). The buffering of HSP90 comes at the price of rendering the manifestation of detrimental genetic variation vulnerable to cellular stresses—e.g. upon heat stress, Hps90’s ability to potentiate genetic alterations is compromised (Karras et al. 2017)—which could be highly relevant in situations of chronic stress like ageing.
A loss of protein homeostasis may be a major link between genomic instability and pathology
As HSP90 can mitigate the loss of function of individual proteins, this raises the question whether the PQC network can also dampen the phenotypic consequences of increased genomic instability. Recent studies indicate that this may be the case. Overexpression of the transcription factor HSF1 (the major transcriptional regulator of the PQC network) is able to counteract not only the global proteotoxicity caused by aneuploidy in human cells, but also rescue the associated growth defects (Donnelly et al. 2014). More recently, a loss of protein homeostasis was found to play a crucial role in the etiology of Down syndrome (DS) and Hutchinson–Gilford progeria syndrome (HGPS), two disorders for which, respectively, a large genomic alteration (trisomy) or global genomic instability has been well-established as a primary underlying cause (Antonarakis et al. 2020; Gonzalo and Kreienkamp 2015; Musich and Zou 2009). In cell culture and mouse models of both syndromes, increased ER stress and UPR activation have been observed (Aivazidis et al. 2017; Hamczyk et al. 2019; Lanzillotta et al. 2018), along with an elevated sensitivity to either induction of ER stress or heat shock (Paradisi et al. 2005). Importantly, the use of chemical chaperones was shown to reduce protein aggregation, and in addition prevent cell degeneration and death in DS (Hirata et al. 2020; Nawa et al. 2019). A similar strategy in an HGPS mouse model was able to diminish vascular pathology, and extend lifespan (Hamczyk et al. 2019).
In a particularly insightful study, Zhu et al. inferred that the proteome instability in DS may even be responsible for a substantial part of the DS cognitive phenotype (Zhu et al. 2019). They discovered that the DS-associated defects in long-term memory and synaptic plasticity are driven by a maladapted downregulation of protein synthesis by the ISR, repressing transcriptional programs that are crucial for memory formation. Suppression of the ISR reversed these transcriptional changes, and restored synaptic plasticity and cognitive function (Zhu et al. 2019). This poses the interesting hypothesis that proteome instability may also affect cellular function by triggering a transcriptional rewiring at the expense of normal cellular functioning. Future studies should determine how intervening in this rewiring affects long-term pathological outcomes, as restoring transcription would be expected to also increase the protein folding burden, potentially further destabilizing the proteome.
Together, these findings show that the PQC network plays an important role in shaping the downstream consequences of genomic instability in these disorders, suggesting that a loss of proteome instability is—either directly or indirectly—a key intermediate event leading to disease. Whether a loss of protein homeostasis is a general pathological consequence of genomic instability remains unknown, but it represents a very promising avenue for future research, as it could help explain the substantial overlap between pathologies associated with a loss of protein homeostasis and genomic instability, including cancer and neurodegeneration, and may provide an answer as to why many genome maintenance disorders exhibit strong (neuro) degenerative symptoms (Jeppesen et al. 2011).
Targeting proteome instability to break a vicious cycle of degeneration?
The data discussed in this review indicate that genomic instability and proteome instability are closely interconnected phenomena, similar to what has been proposed by others (Xie and Jarosz 2018). Importantly, the fact that defects in one can result in impairments of the other points at the possibility of a vicious cycle of events (Fig. 3), which could be important for disease. Genomic alterations also increase widespread in somatic cells over time (Blokzijl et al. 2016; Brazhnik et al. 2020; Martincorena et al. 2015; Martincorena and Campbell 2015; Zhang et al. 2019), and the cumulative impact of these changes on the proteome is still largely unknown. Considering that this increase in genomic instability frequently correlates with the progression of degenerative pathologies associated with a loss of protein homeostasis (Ciosi et al. 2019; Lodato et al. 2018; Park et al. 2019; Vijg and Dong 2020), this indicates that this cycle may even play a fundamental role in ageing.
Genomic instability and proteome instability locked in a vicious cycle. Proteome instability is closely associated with (neuro) degenerative phenotypes, and genomic instability is strongly linked to cancer. However, significant overlap between pathologies associated with each of them exists as well, which may be explained by a vicious cycle of events. Proteome instability can result in genomic instability through the formation of protein aggregates which either drive oxidative stress or sequester genome maintenance components. In addition, it can cause a reduction in the availability of PQC network components involved in genome maintenance. Vice versa, genomic instability may further increase proteome instability through the accumulation of genomic alterations that, either directly or indirectly, challenge protein homeostasis. Auxiliary stresses like ROS or heavy metals, as well as declining capacities of genome maintenance systems and the PQC network can add additional momentum to this cycle
Dissipating momentum from this cycle could be a very interesting opportunity to mitigate pathologies associated with both a loss of protein homeostasis and genomic instability. It seems unlikely that this can be achieved by targeting genomic instability, as several crucial issues would need to be overcome. Although over the last few years several studies have reported that DNA repair can be selectively improved (Georgiadis et al. 2016; Gioia et al. 2019; Mason et al. 2014), this can be a dangerous endeavor, as it is often dysregulated DNA repair (Curtin 2012)—instead of absent repair—that leads to genomic instability, and hyperactive DNA repair has been linked to carcinogenesis as well (Bryant et al. 2019; Herrero et al. 2015; Sy et al. 2020). Moreover, DNA damage occurs largely stochastic, both in its nature and in genomic location, and through a range of different processes, and so the spectrum of lesions will be different from cell to cell. Even though protein aggregates can sequester DNA repair factors, it would therefore be impossible to precisely restore DNA repair capacity without the risk of adverse effects. Complicating this problem even further is the fact that, for this strategy to be effective (i.e. to repair lesions and prevent them from becoming ‘locked-in’ alterations), DNA repair would not only need to be boosted precisely, but also continuously, from an early age onwards. Enhancing DNA repair capacity sufficiently to prevent or even alleviate global genomic instability may therefore not be possible.
In contrast, attenuating proteome instability by enhancing the capacity of the PQC network is likely a far more feasible strategy (Balch et al. 2008; Labbadia and Morimoto 2015). Nature frequently relies on activation and broad transcriptional upregulation of the PQC network to maintain protein homeostasis upon stress situations, for example during elevated temperatures or starvation (Åkerfelt et al. 2010). In addition, the PQC network has been shown to be able to dampen the detrimental consequences of genomic alterations (this review). It would be highly insightful to investigate whether PQC network components can be used to mitigate degenerative phenotypes associated with genomic instability, for example in DNA repair syndromes. There are several particularly interesting targets in this regard, including the core PQC network machinery of HSP90, but other chaperones, specifically those with broad substrate ranges like small heat shock proteins, may be interesting too, as these have been shown to form a first line of defense against protein aggregation under a range of cellular stresses (Haslbeck and Vierling 2015). Stimulation of the two proteolytic systems, autophagy and the UPS, via drug-mediated manipulation of specific components in these pathways could also be an attractive strategy to safeguard protein homeostasis upon genomic instability (Njomen and Tepe 2019; Rubinsztein et al. 2012). Importantly, much more work is needed to investigate the value of each of these approaches in this context. As many PQC network components are intricately involved genome maintenance, altering their capacity might not always be beneficial, and may in some cases even lead to increased genomic instability (Poletto et al. 2017). Future studies should determine how and when modulation of the PQC network could abate the pathological consequences of genomic instability.
Concluding remarks
Genomic integrity and proteome stability rely on intricately connected regulatory pathways. As a result, the relationship between these two is highly complex, with disruptions in either one often affecting the other. Together with the notion that the PQC network can be wielded to mitigate the degenerative consequences of genomic alterations, this suggests that a loss of protein homeostasis could be an important consequence of genomic instability. This may be highly relevant for disorders such as DNA repair syndromes, and could help explain the overall overlap in pathology associated with genomic instability and proteotoxicity.
For now, uncovering whether the relationship between genomic instability and a loss of protein homeostasis plays an important role in vivo, and if so, which of these phenomena contributes the most to the associated disease phenotypes still stands as a major future goalpost. Nevertheless, the data reviewed here already indicates that targeting proteome instability may be a promising therapeutic strategy to mitigate the degenerative consequences of genomic instability.
Data accessibility
This article does not contain any additional data.
References
Abel, H. J., Larson, D. E., Regier, A. A., Chiang, C., Das, I., Kanchi, K. L., et al. (2020). Mapping and characterization of structural variation in 17,795 human genomes. Nature, 583(7814), 83–89. https://doi.org/10.1038/s41586-020-2371-0
Adegbuyiro, A., Sedighi, F., Pilkington, A. W., Groover, S., & Legleiter, J. (2017). Proteins containing expanded polyglutamine tracts and neurodegenerative disease. Biochemistry, 56(9), 1199–1217. https://doi.org/10.1021/acs.biochem.6b00936
Aguilera, A., & Gómez-González, B. (2008). Genome instability: A mechanistic view of its causes and consequences. Nature Reviews Genetics, 9(3), 204–217. https://doi.org/10.1038/nrg2268
Aivazidis, S., Coughlan, C. M., Rauniyar, A. K., Hua Jiang, L., Liggett, A., Maclean, K. N., & Roede, J. R. (2017). The burden of trisomy 21 disrupts the proteostasis network in down syndrome. PLoS ONE, 12(4), e0176307. https://doi.org/10.1371/journal.pone.0176307
Åkerfelt, M., Morimoto, R. I., & Sistonen, L. (2010). Heat shock factors: Integrators of cell stress, development and lifespan. Nature Reviews Molecular Cell Biology, 11(8), 545–555. https://doi.org/10.1038/nrm2938
Alupei, M. C., Maity, P., Esser, P. R., Krikki, I., Tuorto, F., Parlato, R., et al. (2018). Loss of proteostasis is a pathomechanism in cockayne syndrome. Cell Reports, 23(6), 1612–1619. https://doi.org/10.1016/j.celrep.2018.04.041
Amm, I., Sommer, T., & Wolf, D. H. (2014). Protein quality control and elimination of protein waste: The role of the ubiquitin–proteasome system. Biochimica et Biophysica Acta (BBA) - Molecular Cell Research, 1843(1), 182–196. https://doi.org/10.1016/j.bbamcr.2013.06.031
Anon. (2020). Pan-cancer analysis of whole genomes. Nature, 578(7793), 82–93. https://doi.org/10.1038/s41586-020-1969-6
Antonarakis, S. E., Skotko, B. G., Rafii, M. S., Strydom, A., Pape, S. E., Bianchi, D. W., et al. (2020). Down syndrome. Nature Reviews Disease Primers, 6(1), 9. https://doi.org/10.1038/s41572-019-0143-7
Arczewska, K. D., Tomazella, G. G., Lindvall, J. M., Kassahun, H., Maglioni, S., Torgovnick, A., et al. (2013). Active transcriptomic and proteomic reprogramming in the C. Elegans nucleotide excision repair mutant xpa-1. Nucleic Acids Research, 41(10), 5368–5381. https://doi.org/10.1093/nar/gkt225
Arlow, T., Scott, K., Wagenseller, A., & Gammie, A. (2013). Proteasome inhibition rescues clinically significant unstable variants of the mismatch repair protein Msh2. Proceedings of the National Academy of Sciences, 110(1), 246–251. https://doi.org/10.1073/pnas.1215510110
Baker, M. (2012). Structural variation: The genome’s hidden architecture. Nature Methods, 9(2), 133–137. https://doi.org/10.1038/nmeth.1858
Balch, W. E., Morimoto, R. I., Dillin, A., & Kelly, J. W. (2008). Adapting proteostasis for disease intervention. Science, 319(5865), 916–919. https://doi.org/10.1126/science.1141448
Balendra, R., & Isaacs, A. M. (2018). C9orf72-mediated ALS and FTD: Multiple pathways to disease. Nature Reviews Neurology, 14(9), 544–558. https://doi.org/10.1038/s41582-018-0047-2
Banez-Coronel, M., & Ranum, L. P. W. (2019). Repeat-associated non-AUG (RAN) translation: Insights from pathology. Laboratory Investigation, 99(7), 929–942. https://doi.org/10.1038/s41374-019-0241-x
Barzilai, A., Rotman, G., & Shilow, Y. (2002). ATM deficiency and oxidative stress: A new dimension of defective response to DNA damage. DNA Repair, 1(1), 3–25. https://doi.org/10.1016/S1568-7864(01)00007-6
Basu, S., Je, G., & Kim, Y.-S. (2015). Transcriptional mutagenesis by 8-OxodG in α-synuclein aggregation and the pathogenesis of Parkinson’s disease. Experimental & Molecular Medicine, 47(8), e179–e179. https://doi.org/10.1038/emm.2015.54
Ben Yehuda, A., Risheq, M., Novoplansky, O., Bersuker, K., Kopito, R. R., Goldberg, M., & Brandeis, M. (2017). Ubiquitin accumulation on disease associated protein aggregates is correlated with nuclear ubiquitin depletion, histone de-ubiquitination and impaired DNA damage response. PLoS ONE, 12(1), e0169054. https://doi.org/10.1371/journal.pone.0169054
Bence, N. F. (2001). Impairment of the ubiquitin-proteasome system by protein aggregation. Science, 292(5521), 1552–1555. https://doi.org/10.1126/science.292.5521.1552
Benson, M. D., Liepnieks, J. J., Yazaki, M., Yamashita, T., Asl, K. H., Guenther, B., & Kluve-Beckerman, B. (2001). A New human hereditary amyloidosis: The result of a stop-codon mutation in the apolipoprotein aii gene. Genomics, 72(3), 272–277. https://doi.org/10.1006/geno.2000.6499
Bento, C. F., Renna, M., Ghislat, G., Puri, C., Ashkenazi, A., Vicinanza, M., et al. (2016). Mammalian autophagy: How does it work? Annual Review of Biochemistry, 85(1), 685–713. https://doi.org/10.1146/annurev-biochem-060815-014556
Bergink, S., & Jentsch, S. (2009). Principles of ubiquitin and SUMO modifications in DNA repair. Nature, 458(7237), 461–467. https://doi.org/10.1038/nature07963
Bernardi, L., & Bruni, A. C. (2019). Mutations in prion protein gene: Pathogenic mechanisms in C-terminal vs. N-terminal domain, a review. International Journal of Molecular Sciences, 20(14), 3606. https://doi.org/10.3390/ijms20143606
Biebl, M. M., & Buchner, J. (2019). Structure, function, and regulation of the Hsp90 machinery. Cold Spring Harbor Perspectives in Biology, 11(9), a034017. https://doi.org/10.1101/cshperspect.a034017
Blokzijl, F., De Ligt, J., Jager, M., Sasselli, V., Roerink, S., Sasaki, N., et al. (2016). Tissue-specific mutation accumulation in human adult stem cells during life. Nature, 538(7624), 260–264. https://doi.org/10.1038/nature19768
Bondarev, S., Antonets, K., Kajava, A., Nizhnikov, A., & Zhouravleva, G. (2018). Protein co-aggregation related to amyloids: Methods of investigation, diversity, and classification. International Journal of Molecular Sciences, 19(8), 2292. https://doi.org/10.3390/ijms19082292
Boopathy, S., Silvas, T. V., Tischbein, M., Jansen, S., Shandilya, S. M., Zitzewitz, J. A., et al. (2015). Structural basis for mutation-induced destabilization of profilin 1 in ALS. Proceedings of the National Academy of Sciences, 112(26), 7984–7989. https://doi.org/10.1073/pnas.1424108112
Booth, D. R., Sunde, M., Bellotti, V., Robinson, C. V., Hutchinson, W. L., Fraser, P. E., et al. (1997). Instability, unfolding and aggregation of human lysozyme variants underlying amyloid fibrillogenesis. Nature, 385(6619), 787–793. https://doi.org/10.1038/385787a0
Bordoni, M., Pansarasa, O., Dell’Orco, M., Crippa, V., Gagliardi, S., Sproviero, D., et al. (2019). Nuclear phospho-SOD1 protects DNA from oxidative stress damage in amyotrophic lateral sclerosis. Journal of Clinical Medicine, 8(5), 729. https://doi.org/10.3390/jcm8050729
Borkovich, K. A., Farrelly, F. W., Finkelstein, D. B., Taulien, J., & Lindquist, S. (1989). Hsp82 is an essential protein that is required in higher concentrations for growth of cells at higher temperatures. Molecular and Cellular Biology, 9(9), 3919–3930. https://doi.org/10.1128/MCB.9.9.3919
Bossy-Wetzel, E., Petrilli, A., & Knott, A. B. (2008). Mutant huntingtin and mitochondrial dysfunction. Trends in Neurosciences, 31(12), 609–616. https://doi.org/10.1016/j.tins.2008.09.004
Boysen, M., Kityk, R., & Mayer, M. P. (2019). Hsp70- and Hsp90-mediated regulation of the conformation of P53 DNA binding domain and P53 cancer variants. Molecular Cell, 74(4), 831-843.e4. https://doi.org/10.1016/j.molcel.2019.03.032
Brazhnik, K., Sun, S., Alani, O., Kinkhabwala, M., Wolkoff, A. W., Maslov, A. Y., et al. (2020). Single-cell analysis reveals different age-related somatic mutation profiles between stem and differentiated cells in human liver. Science Advances, 6(5), 2659. https://doi.org/10.1126/sciadv.aax2659
Brégeon, D., Doddridge, Z. A., You, H. J., Weiss, B., & Doetsch, P. W. (2003). Transcriptional mutagenesis induced by uracil and 8-oxoguanine in Escherichia Coli. Molecular Cell, 12(4), 959–970. https://doi.org/10.1016/S1097-2765(03)00360-5
Brégeon, D., & Doetsch, P. W. (2011). Transcriptional mutagenesis: Causes and involvement in tumour development. Nature Reviews Cancer, 11(3), 218–227. https://doi.org/10.1038/nrc3006
Brennan, C. M., Vaites, L. P., Wells, J. N., Santaguida, S., Paulo, J. A., Zuzana Storchova, J., et al. (2019). Protein aggregation mediates stoichiometry of protein complexes in aneuploid cells. Genes & Development, 33(15–16), 1031–1047. https://doi.org/10.1101/gad.327494.119
Brinkmann, K., Schell, M., Hoppe, T., & Kashkar, H. (2015). Regulation of the DNA damage response by ubiquitin conjugation. Frontiers in Genetics. https://doi.org/10.3389/fgene.2015.00098
Brown, J. S., & Jackson, S. P. (2015). Ubiquitylation, neddylation and the DNA damage response. Open Biology, 5(4), 150018. https://doi.org/10.1098/rsob.150018
Bryant, E. E., Šunjevarić, I., Berchowitz, L., Rothstein, R., & Reid, R. J. D. (2019). Rad5 dysregulation drives hyperactive recombination at replication forks resulting in cisplatin sensitivity and genome instability. Nucleic Acids Research, 47(17), 9144–9159. https://doi.org/10.1093/nar/gkz631
Calderwood, S. K., & Gong, J. (2016). Heat shock proteins promote cancer: It’s a protection racket. Trends in Biochemical Sciences, 41(4), 311–323. https://doi.org/10.1016/j.tibs.2016.01.003
Cancel, G., Gourfinkel-An, I., Stevanin, G., Didierjean, O., Abbas, N., Hirsch, E., et al. (1998). Somatic mosaicism of the CAG repeat expansion in spinocerebellar ataxia type 3/machado-joseph disease. Human Mutation, 11(1), 23–27. https://doi.org/10.1002/(SICI)1098-1004(1998)11:1%3c23::AID-HUMU4%3e3.0.CO;2-M
Carvalho, C. M. B., & Lupski, J. R. (2016). Mechanisms underlying structural variant formation in genomic disorders. Nature Reviews Genetics, 17(4), 224–238. https://doi.org/10.1038/nrg.2015.25
Castel, A. L., Cleary, J. D., & Pearson, C. E. (2010). Repeat instability as the basis for human diseases and as a potential target for therapy. Nature Reviews Molecular Cell Biology, 11(3), 165–170. https://doi.org/10.1038/nrm2854
Chatzidoukaki, O., Goulielmaki, E., Schumacher, B., & Garinis, G. A. (2020). DNA damage response and metabolic reprogramming in health and disease. Trends in Genetics. https://doi.org/10.1016/j.tig.2020.06.018
Chen, G., Bradford, W. D., Seidel, C. W., & Li, R. (2012). Hsp90 stress potentiates rapid cellular adaptation through induction of aneuploidy. Nature, 482(7384), 246–250. https://doi.org/10.1038/nature10795
Chen, G.-F., Ting-hai, Xu., Yan, Y., Zhou, Y.-R., Jiang, Yi., Melcher, K., & Eric Xu, H. (2017). Amyloid beta: Structure, biology and structure-based therapeutic development. Acta Pharmacologica Sinica, 38(9), 1205–1235. https://doi.org/10.1038/aps.2017.28
Cheng, A. N., Fan, C.-C., Lo, Y.-K., Kuo, C.-L., Hui-Chun Wang, I., Lien, H., et al. (2017). Cdc7-Dbf4-mediated phosphorylation of HSP90-S164 stabilizes HSP90-HCLK2-MRN complex to enhance ATR/ATM signaling that overcomes replication stress in cancer. Scientific Reports, 7(1), 17024. https://doi.org/10.1038/s41598-017-17126-2
Chiti, F., & Dobson, C. M. (2017). Protein misfolding, amyloid formation, and human disease: A summary of progress over the last decade. Annual Review of Biochemistry, 86(1), 27–68. https://doi.org/10.1146/annurev-biochem-061516-045115
Ciosi, M., Maxwell, A., Cumming, S. A., Hensman, D. J., Moss, A. M., Alshammari, M. D., et al. (2019). A genetic association study of glutamine-encoding DNA sequence structures, somatic CAG expansion, and DNA repair gene variants, with huntington disease clinical outcomes. EBioMedicine, 48, 568–580. https://doi.org/10.1016/j.ebiom.2019.09.020
Ciryam, P., Kundra, R., Freer, R., Morimoto, R. I., Dobson, C. M., & Vendruscolo, M. (2016). A transcriptional signature of Alzheimer’s disease is associated with a metastable subproteome at risk for aggregation. Proceedings of the National Academy of Sciences, 113(17), 4753–4758. https://doi.org/10.1073/pnas.1516604113
Cleary, J. D., Pattamatta, A., & Ranum, L. P. W. (2018). Repeat-associated non-ATG (RAN) translation. Journal of Biological Chemistry, 293(42), 16127–16141. https://doi.org/10.1074/jbc.R118.003237
Clementi, E., Inglin, L., Beebe, E., Gsell, C., Garajova, Z., & Markkanen, E. (2020). Persistent DNA damage triggers activation of the integrated stress response to promote cell survival under nutrient restriction. BMC Biology, 18(1), 36. https://doi.org/10.1186/s12915-020-00771-x
Corcoles-Saez, I., Dong, K., Johnson, A. L., Waskiewicz, E., Costanzo, M., Boone, C., & Cha, R. S. (2018). Essential function of Mec1, the budding yeast ATM/ATR checkpoint-response kinase, in protein homeostasis. Developmental Cell, 46(4), 495-503.e2. https://doi.org/10.1016/j.devcel.2018.07.011
Cordaux, R., & Batzer, M. A. (2009). The impact of retrotransposons on human genome evolution. Nature Reviews Genetics, 10(10), 691–703. https://doi.org/10.1038/nrg2640
Cortese, A., Simone, R., Sullivan, R., Vandrovcova, J., Tariq, H., Yau, W. Y., et al. (2019). Biallelic expansion of an intronic repeat in RFC1 Is a common cause of late-onset Ataxia. Nature Genetics, 51(4), 649–658. https://doi.org/10.1038/s41588-019-0372-4
Croteau, D. L., Popuri, V., Opresko, P. L., & Bohr, V. A. (2014). Human RecQ helicases in DNA repair, recombination, and replication. Annual Review of Biochemistry , 83(1), 519–552. https://doi.org/10.1146/annurev-biochem-060713-035428
Curtin, N. J. (2012). DNA repair dysregulation from cancer driver to therapeutic target. Nature Reviews Cancer, 12(12), 801–817. https://doi.org/10.1038/nrc3399
Dai, C., Dai, S., & Cao, J. (2012). Proteotoxic stress of cancer: Implication of the heat-shock response in oncogenesis. Journal of Cellular Physiology, 227(8), 2982–2987. https://doi.org/10.1002/jcp.24017
Dantuma, N. P., Groothuis, T. A. M., Salomons, F. A., & Neefjes, J. (2006). A dynamic ubiquitin equilibrium couples proteasomal activity to chromatin remodeling. Journal of Cell Biology, 173(1), 19–26. https://doi.org/10.1083/jcb.200510071
de Baets, G., van Doorn, L., Rousseau, F., & Schymkowitz, J. (2015). Increased aggregation is more frequently associated to human disease-associated mutations than to neutral polymorphisms. PLOS Computational Biology, 11(9), e1004374. https://doi.org/10.1371/journal.pcbi.1004374
de Sousa Leal, A. M., de Azevedo Medeiros, L. B., Muñoz-Cadavid, C. O., de Paula, O. R., de Souza Timóteo, A. R., de Oliveira, A. H., et al. (2020). XPA deficiency affects the ubiquitin-proteasome system function. DNA Repair, 94, 102937. https://doi.org/10.1016/j.dnarep.2020.102937
Deller, M. C., Kong, L., & Rupp, B. (2016). Protein stability: A crystallographer’s perspective. Acta Crystallographica Section F Structural Biology Communications, 72(2), 72–95. https://doi.org/10.1107/S2053230X15024619
DePristo, M. A., Weinreich, D. M., & Hartl, D. L. (2005). Missense meanderings in sequence space: A biophysical view of protein evolution. Nature Reviews Genetics, 6(9), 678–687. https://doi.org/10.1038/nrg1672
Deshaies, R. J. (2014). Proteotoxic crisis, the ubiquitin-proteasome system, and cancer therapy. BMC Biology, 12(1), 94. https://doi.org/10.1186/s12915-014-0094-0
Dettmer, U., Newman, A. J., Soldner, F., Luth, E. S., Kim, N. C., von Saucken, V. E., et al. (2015). Parkinson-causing α-synuclein missense mutations shift native tetramers to monomers as a mechanism for disease initiation. Nature Communications, 6(1), 7314. https://doi.org/10.1038/ncomms8314
Dikic, I., & Elazar, Z. (2018). Mechanism and medical implications of mammalian autophagy. Nature Reviews Molecular Cell Biology, 19(6), 349–364.
Dobson, C. M. (2017). The amyloid phenomenon and its links with human disease. Cold Spring Harbor Perspectives in Biology, 9(6), a023648. https://doi.org/10.1101/cshperspect.a023648
Donnelly, N., Passerini, V., Dürrbaum, M., Stingele, S., & Storchová, Z. (2014). HSF1 deficiency and impaired HSP90-dependent protein folding are hallmarks of aneuploid human cells. The EMBO Journal, 33(20), 2374–2387. https://doi.org/10.15252/embj.201488648
Dote, H., Burgan, W. E., Camphausen, K., & Tofilon, P. J. (2006). Inhibition of Hsp90 compromises the DNA damage response to radiation. Cancer Research, 66(18), 9211–9220. https://doi.org/10.1158/0008-5472.CAN-06-2181
Dubrez, L., Causse, S., Bonan, N. B., Dumétier, B., & Garrido, C. (2020). Heat-shock proteins: Chaperoning DNA repair. Oncogene, 39(3), 516–529. https://doi.org/10.1038/s41388-019-1016-y
Echtenkamp, F. J., Zelin, E., Oxelmark, E., Woo, J. I., Andrews, B. J., Garabedian, M., & Freeman, B. C. (2011). Global functional map of the P23 molecular chaperone reveals an extensive cellular network. Molecular Cell, 43(2), 229–241. https://doi.org/10.1016/j.molcel.2011.05.029
Ellerby, L. M. (2019). Repeat expansion disorders: Mechanisms and therapeutics. Neurotherapeutics, 16(4), 924–927. https://doi.org/10.1007/s13311-019-00823-3
Enchev, R. I., Schulman, B. A., & Peter, M. (2015). Protein neddylation: Beyond Cullin–RING ligases. Nature Reviews Molecular Cell Biology, 16(1), 30–44. https://doi.org/10.1038/nrm3919
Enokido, Y., Tamura, T., Ito, H., Arumughan, A., Komuro, A., Shiwaku, H., et al. (2010). Mutant huntingtin impairs Ku70-mediated DNA repair. Journal of Cell Biology, 189(3), 425–443. https://doi.org/10.1083/jcb.200905138
Evans, S. A., Horrell, J., & Neretti, N. (2019). The three-dimensional organization of the genome in cellular senescence and age-associated diseases. Seminars in Cell & Developmental Biology, 90, 154–160. https://doi.org/10.1016/j.semcdb.2018.07.022
Fang, Q., Inanc, B., Schamus, S., Wang, X.-H., Wei, L., Brown, A. R., et al. (2014). HSP90 regulates DNA repair via the interaction between XRCC1 and DNA polymerase β. Nature Communications, 5(1), 5513. https://doi.org/10.1038/ncomms6513
Fares, M. A., Ruiz-González, M. X., Moya, A., Elena, S. F., & Barrio, E. (2002). GroEL buffers against deleterious mutations. Nature, 417(6887), 398–398. https://doi.org/10.1038/417398a
Farmer, K. M., Ghag, G., Puangmalai, N., Montalbano, M., Bhatt, N., & Kayed, R. (2020). P53 aggregation, interactions with tau, and impaired DNA damage response in Alzheimer’s disease. Acta Neuropathologica Communications, 8(1), 132. https://doi.org/10.1186/s40478-020-01012-6
Farrawell, N. E., Lambert-Smith, I., Mitchell, K., McKenna, J., McAlary, L., Ciryam, P., et al. (2018). SOD1-A4V aggregation alters ubiquitin homeostasis in a cell model of ALS. Journal of Cell Science, 131(11), 209122. https://doi.org/10.1242/jcs.209122
Forsberg, L. A., Rasi, C., Razzaghian, H. R., Pakalapati, G., Waite, L., Thilbeault, K. S., et al. (2012). Age-related somatic structural changes in the nuclear genome of human blood cells. The American Journal of Human Genetics, 90(2), 217–228. https://doi.org/10.1016/j.ajhg.2011.12.009
Fusakio, M. E., Willy, J. A., Wang, Y., Mirek, E. T., Al Baghdadi, R. J., Adams, C. M., et al. (2016). Transcription factor ATF4 directs basal and stress-induced gene expression in the unfolded protein response and cholesterol metabolism in the liver. Molecular Biology of the Cell, 27(9), 1536–1551. https://doi.org/10.1091/mbc.E16-01-0039
Gao, R., Chakraborty, A., Geater, C., Pradhan, S., Gordon, K. L., Snowden, J., et al. (2019). Mutant huntingtin impairs PNKP and ATXN3, disrupting DNA repair and transcription. ELife. https://doi.org/10.7554/eLife.42988
Georgiadis, M. M., Chen, Q., Meng, J., Guo, C., Wireman, R., Reed, A., et al. (2016). Small molecule activation of apurinic/apyrimidinic endonuclease 1 reduces DNA damage induced by cisplatin in cultured sensory neurons. DNA Repair, 41, 32–41. https://doi.org/10.1016/j.dnarep.2016.03.009
Ghosh, K., & Dill, K. (2010). Cellular proteomes have broad distributions of protein stability. Biophysical Journal, 99(12), 3996–4002. https://doi.org/10.1016/j.bpj.2010.10.036
Gidalevitz, T. (2006). Progressive disruption of cellular protein folding in models of polyglutamine diseases. Science, 311(5766), 1471–1474. https://doi.org/10.1126/science.1124514
Gidalevitz, T., Kikis, E. A., & Morimoto, R. I. (2010). A cellular perspective on conformational disease: The role of genetic background and proteostasis networks. Current Opinion in Structural Biology, 20(1), 23–32. https://doi.org/10.1016/j.sbi.2009.11.001
Gidalevitz, T., Krupinski, T., Garcia, S., & Morimoto, R. I. (2009). Destabilizing protein polymorphisms in the genetic background direct phenotypic expression of mutant SOD1 toxicity. PLoS Genetics, 5(3), e1000399. https://doi.org/10.1371/journal.pgen.1000399
Gidalevitz, T., Prahlad, V., & Morimoto, R. I. (2011). The stress of protein misfolding: From single cells to multicellular organisms. Cold Spring Harbor Perspectives in Biology, 3(6), a009704–a009704. https://doi.org/10.1101/cshperspect.a009704
Gidalevitz, T., Wang, N., Deravaj, T., Alexander-Floyd, J., & Morimoto, R. I. (2013). Natural genetic variation determines susceptibility to aggregation or toxicity in a C. elegansmodel for polyglutamine disease. BMC Biology, 11(1), 100. https://doi.org/10.1186/1741-7007-11-100
Giglia-Mari, G., Zotter, A., & Vermeulen, W. (2011). DNA damage response. Cold Spring Harbor Perspectives in Biology, 3(1), a000745–a000745. https://doi.org/10.1101/cshperspect.a000745
Gioia, U., Francia, S., Cabrini, M., Brambillasca, S., Michelini, F., Jones-Weinert, C. W., & di Fagagna, F. D. (2019). Pharmacological boost of DNA damage response and repair by enhanced biogenesis of DNA damage response RNAs. Scientific Reports, 9(1), 6460. https://doi.org/10.1038/s41598-019-42892-6
Goldschmidt, L., Teng, P. K., Riek, R., & Eisenberg, D. (2010). Identifying the amylome, proteins capable of forming amyloid-like fibrils. Proceedings of the National Academy of Sciences, 107(8), 3487–3492. https://doi.org/10.1073/pnas.0915166107
Gonzalo, S., & Kreienkamp, R. (2015). DNA repair defects and genome instability in Hutchinson-Gilford progeria syndrome. Current Opinion in Cell Biology, 34, 75–83. https://doi.org/10.1016/j.ceb.2015.05.007
Gragerov, A., Nudler, E., Komissarova, N., Gaitanaris, G. A., Gottesman, M. E., & Nikiforov, V. (1992). Cooperation of GroEL/GroES and DnaK/DnaJ heat shock proteins in preventing protein misfolding in Escherichia Coli. Proceedings of the National Academy of Sciences, 89(21), 10341–10344. https://doi.org/10.1073/pnas.89.21.10341
Green, K. M., Rebecca Glineburg, M., Kearse, M. G., Flores, B. N., Linsalata, A. E., Fedak, S. J., et al. (2017). RAN translation at C9orf72-associated repeat expansions is selectively enhanced by the integrated stress response. Nature Communications, 8(1), 2005. https://doi.org/10.1038/s41467-017-02200-0
Gsponer, J., Futschik, M. E., Teichmann, S. A., & Babu, M. M. (2008). Tight regulation of unstructured proteins: From transcript synthesis to protein degradation. Science, 322(5906), 1365–1368. https://doi.org/10.1126/science.1163581
Guo, C., & Zhao, Y. (2020). Autophagy and DNA damage repair. Genome Instability & Disease. https://doi.org/10.1007/s42764-020-00016-9
Guo, Z., Kozlov, S., Lavin, M. F., Person, M. D., & Paull, T. T. (2010). ATM activation by oxidative stress. Science, 330(6003), 517–521. https://doi.org/10.1126/science.1192912
Hamczyk, M. R., Villa‐Bellosta. R., Quesada, V., Gonzalo, P., Vidak, S., Nevado, R. M., Andrés‐Manzano, M. J., Misteli, T., López‐Otín, C., Andrés, V. (2019) Progerin accelerates atherosclerosis by inducing endoplasmic reticulum stress in vascular smooth muscle cells. EMBO Molecular Medicine 11 (4)
Hansen, M., Rubinsztein, D. C., & Walker, D. W. (2018). Autophagy as a promoter of longevity: Insights from model organisms. Nature Reviews Molecular Cell Biology, 19(9), 579–593. https://doi.org/10.1038/s41580-018-0033-y
Hartl, F. U., Bracher, A., & Hayer-Hartl, M. (2011). Molecular chaperones in protein folding and proteostasis. Nature, 475(7356), 324–332. https://doi.org/10.1038/nature10317
Haslbeck, M., & Vierling, E. (2015). A first line of stress defense: Small heat shock proteins and their function in protein homeostasis. Journal of Molecular Biology, 427(7), 1537–1548. https://doi.org/10.1016/j.jmb.2015.02.002
Herrero, A. B., Miguel, J. S., & Gutierrez, N. C. (2015). Deregulation of DNA double-strand break repair in multiple myeloma: Implications for genome stability. PLoS ONE, 10(3), e0121581. https://doi.org/10.1371/journal.pone.0121581
Hewitt, G., Carroll, B., Sarallah, R., Correia-Melo, C., Ogrodnik, M., Nelson, G., et al. (2016). SQSTM1/P62 mediates crosstalk between autophagy and the UPS in DNA repair. Autophagy, 12(10), 1917–1930. https://doi.org/10.1080/15548627.2016.1210368
Hewitt, G., & Korolchuk, V. I. (2017). Repair, reuse, recycle: The expanding role of autophagy in genome maintenance. Trends in Cell Biology, 27(5), 340–351. https://doi.org/10.1016/j.tcb.2016.11.011
Hipp, M. S., Kasturi, P., & Ulrich Hartl, F. (2019). The proteostasis network and its decline in ageing. Nature Reviews Molecular Cell Biology, 20(7), 421–435. https://doi.org/10.1038/s41580-019-0101-y
Hirata, K., Nambara, T., Kawatani, K., Nawa, N., Yoshimatsu, H., Kusakabe, H., et al. (2020). 4-Phenylbutyrate ameliorates apoptotic neural cell death in down syndrome by reducing protein aggregates. Scientific Reports, 10(1), 14047. https://doi.org/10.1038/s41598-020-70362-x
Hou, Y., Song, H., Croteau, D. L., Akbari, M., & Bohr, V. A. (2017). Genome instability in Alzheimer disease. Mechanisms of Ageing and Development, 161, 83–94. https://doi.org/10.1016/j.mad.2016.04.005
Illuzzi, J., Yerkes, S., Parekh-Olmedo, H., & Kmiec, E. B. (2009). DNA breakage and induction of DNA damage response proteins precede the appearance of visible mutant huntingtin aggregates. Journal of Neuroscience Research, 87(3), 733–747. https://doi.org/10.1002/jnr.21881
Ishiura, H., Shibata, S., Yoshimura, J., Suzuki, Y., Wei, Qu., Koichiro Doi, M., et al. (2019). Noncoding CGG repeat expansions in neuronal intranuclear inclusion disease, oculopharyngodistal myopathy and an overlapping disease. Nature Genetics, 51(8), 1222–1232. https://doi.org/10.1038/s41588-019-0458-z
Janin, J., Bahadur, R. P., & Chakrabarti, P. (2008). Protein–protein interaction and quaternary structure. Quarterly Reviews of Biophysics, 41(2), 133–180. https://doi.org/10.1017/S0033583508004708
Jarosz, D. F., Taipale, M., & Lindquist, S. (2010). Protein homeostasis and the phenotypic manifestation of genetic diversity: Principles and mechanisms. Annual Review of Genetics, 44(1), 189–216. https://doi.org/10.1146/annurev.genet.40.110405.090412
Jeggo, P. A., Pearl, L. H., & Carr, A. M. (2016). DNA repair, genome stability and cancer: A historical perspective. Nature Reviews Cancer, 16(1), 35–42. https://doi.org/10.1038/nrc.2015.4
Jeppesen, D. K., Bohr, V. A., & Stevnsner, T. (2011). DNA repair deficiency in neurodegeneration. Progress in Neurobiology, 94(2), 166–200. https://doi.org/10.1016/j.pneurobio.2011.04.013
Jönsson, M. E., Garza, R., Johansson, P. A., & Jakobsson, J. (2020). Transposable elements: A common feature of neurodevelopmental and neurodegenerative disorders. Trends in Genetics, 36(8), 610–623. https://doi.org/10.1016/j.tig.2020.05.004
Kampinga, H. H., & Bergink, S. (2016). Heat shock proteins as potential targets for protective strategies in neurodegeneration. The Lancet Neurology, 15(7), 748–759. https://doi.org/10.1016/S1474-4422(16)00099-5
Kampinga, H. H., & Craig, E. A. (2010). The HSP70 chaperone machinery: J proteins as drivers of functional specificity. Nature Reviews Molecular Cell Biology, 11(8), 579–592. https://doi.org/10.1038/nrm2941
Karpov, D. S., Spasskaya, D. S., Tutyaeva, V. V., Mironov, A. S., & Karpov, V. L. (2013). Proteasome inhibition enhances resistance to DNA damage via upregulation of Rpn4-dependent DNA repair genes. FEBS Letter, 587(18), 3108–3114. https://doi.org/10.1016/j.febslet.2013.08.007
Karras, G. I., Yi, S., Sahni, N., Fischer, M., Xie, J., Vidal, M., et al. (2017). HSP90 shapes the consequences of human genetic variation. Cell, 168(5), 856-866.e12. https://doi.org/10.1016/j.cell.2017.01.023
Kaya, A., Mariotti, M., Tyshkovskiy, A., Zhou, X., Hulke, M. L., Ma, S., et al. (2020). Molecular signatures of aneuploidy-driven adaptive evolution. Nature Communications, 11(1), 588. https://doi.org/10.1038/s41467-019-13669-2
Kennedy, L. (2003). Dramatic tissue-specific mutation length increases are an early molecular event in huntington disease pathogenesis. Human Molecular Genetics, 12(24), 3359–3367. https://doi.org/10.1093/hmg/ddg352
Klaips, C. L., Jayaraj, G. G., & Ulrich Hartl, F. (2018). Pathways of cellular proteostasis in aging and disease. Journal of Cell Biology, 217(1), 51–63. https://doi.org/10.1083/jcb.201709072
Knighton, L. E., & Truman, A. W. (2019). Role of the molecular chaperones Hsp70 and Hsp90 in the DNA damage response (pp. 345–358). Cham: Springer.
Ko, J.-C., Chen, H.-J., Huang, Y.-C., Tseng, S.-C., Weng, S.-H., Wo, T.-Y., et al. (2012). HSP90 inhibition induces cytotoxicity via down-regulation of Rad51 expression and DNA repair capacity in non-small cell lung cancer cells. Regulatory Toxicology and Pharmacology, 64(3), 415–424. https://doi.org/10.1016/j.yrtph.2012.10.003
Kraemer, K. H. (1987). Xeroderma pigmentosum. Cutaneous, ocular, and neurologic abnormalities in 830 published cases. Archives of Dermatology, 123(2), 241–250. https://doi.org/10.1001/archderm.123.2.241
Kuiper, E. F. E., de Mattos, E. P., Jardim, L. B., Kampinga, H. H., & Bergink, S. (2017). Chaperones in polyglutamine aggregation: Beyond the Q-stretch. Frontiers in Neuroscience. https://doi.org/10.3389/fnins.2017.00145
Labbadia, J., & Morimoto, R. I. (2015). The biology of proteostasis in aging and disease. Annual Review of Biochemistry, 84(1), 435–464. https://doi.org/10.1146/annurev-biochem-060614-033955
Lans, H., Hoeijmakers, J. H. J., Vermeulen, W., & Marteijn, J. A. (2019). The DNA damage response to transcription stress. Nature Reviews Molecular Cell Biology, 20(12), 766–784. https://doi.org/10.1038/s41580-019-0169-4
Lanzillotta, C., Tramutola, A., Meier, S., Schmitt, F., Barone, E., Perluigi, M., et al. (2018). Early and selective activation and subsequent alterations to the unfolded protein response in down syndrome mouse models. Journal of Alzheimer’s Disease, 62(1), 347–359. https://doi.org/10.3233/JAD-170617
Laurie, C. C., Laurie, C. A., Rice, K., Doheny, K. F., Zelnick, L. R., McHugh, C. P., et al. (2012). Detectable clonal mosaicism from birth to old age and its relationship to cancer. Nature Genetics, 44(6), 642–650. https://doi.org/10.1038/ng.2271
Lévy, E., el Banna, N., Baïlle, D., Heneman-Masurel, A., Truchet, S., Rezaei, H., et al. (2019). Causative links between protein aggregation and oxidative stress: A review. International Journal of Molecular Sciences, 20(16), 3896. https://doi.org/10.3390/ijms20163896
Li, W., Lee, M. H., Henderson, L., Tyagi, R., Bachani, M., Steiner, J., et al. (2015). Human endogenous retrovirus-K contributes to motor neuron disease. Science Translational Medicine, 7(307), 307153. https://doi.org/10.1126/scitranslmed.aac8201
Liebelt, F., & Vertegaal, A. C. O. (2016). Ubiquitin-dependent and independent roles of SUMO in proteostasis. American Journal of Physiology-Cell Physiology, 311(2), C284–C296. https://doi.org/10.1152/ajpcell.00091.2016
Lim, K. H., Dasari, A. K. R., Ma, R., Hung, I., Gan, Z., Kelly, J. W., & Fitzgerald, M. C. (2017). Pathogenic mutations induce partial structural changes in the native β-sheet structure of transthyretin and accelerate aggregation. Biochemistry, 56(36), 4808–4818. https://doi.org/10.1021/acs.biochem.7b00658
Liu, Na., Stoica, G., Yan, M., Scofield, V. L., Qiang, W., Lynn, W. S., & Wong, P. K. Y. (2005). ATM deficiency induces oxidative stress and endoplasmic reticulum stress in astrocytes. Laboratory Investigation, 85(12), 1471–1480. https://doi.org/10.1038/labinvest.3700354
Lodato, M. A., Rodin, R. E., Bohrson, C. L., Coulter, M. E., Barton, A. R., Kwon, M., et al. (2018). Aging and neurodegeneration are associated with increased mutations in single human neurons. Science, 359(6375), 555–559. https://doi.org/10.1126/science.aao4426
Lott, I. T., & Head, E. (2019). Dementia in down syndrome: Unique insights for Alzheimer disease research. Nature Reviews Neurology, 15(3), 135–147. https://doi.org/10.1038/s41582-018-0132-6
Ludtmann, M. H., Angelova, P. R., Horrocks, M. H., Choi, M. L., Rodrigues, M., Baev, A. Y., et al. (2018). α-synuclein oligomers interact with ATP synthase and open the permeability transition pore in parkinson’s disease. Nature Communications, 9(1), 2293. https://doi.org/10.1038/s41467-018-04422-2
Maina, B., Mahmoud, Y.-H., & Serpell, L. (2016). Nuclear tau and its potential role in Alzheimer’s disease. Biomolecules, 6(1), 9. https://doi.org/10.3390/biom6010009
Maisnier-Patin, S., Roth, J. R., Fredriksson, Å., Nyström, T., Berg, O. G., & Andersson, D. I. (2005). Genomic buffering mitigates the effects of deleterious mutations in bacteria. Nature Genetics, 37(12), 1376–1379. https://doi.org/10.1038/ng1676
Martincorena, I., & Campbell, P. J. (2015). Somatic mutation in cancer and normal cells. Science, 349(6255), 1483–1489. https://doi.org/10.1126/science.aab4082
Martincorena, I., Roshan, A., Gerstung, M., Ellis, P., van Loo, P., McLaren, S., et al. (2015). High burden and pervasive positive selection of somatic mutations in normal human skin. Science, 348(6237), 880–886. https://doi.org/10.1126/science.aaa6806
Mason, J. M., Logan, H. L., Budke, B., Wu, M., Pawlowski, M., Weichselbaum, R. R., et al. (2014). The RAD51-stimulatory compound RS-1 can exploit the RAD51 overexpression that exists in cancer cells and tumors. Cancer Research, 74(13), 3546–3555. https://doi.org/10.1158/0008-5472.CAN-13-3220
Matsui, D., Nakano, S., Dadashipour, M., & Asano, Y. (2017). Rational identification of aggregation hotspots based on secondary structure and amino acid hydrophobicity. Scientific Reports, 7(1), 9558. https://doi.org/10.1038/s41598-017-09749-2
Mayer, M. P. (2018). Intra-molecular pathways of allosteric control in Hsp70s. Philosophical Transactions of the Royal Society B: Biological Sciences, 373(1749), 20170183. https://doi.org/10.1098/rstb.2017.0183
McKinnon, P. J. (2012). ATM and the molecular pathogenesis of ataxia telangiectasia. Annual Review of Pathology: Mechanisms of Disease, 7(1), 303–321. https://doi.org/10.1146/annurev-pathol-011811-132509
Mimnaugh, E. G., Chen, H. Y., Davie, J. R., Celis, J. E., & Neckers, L. (1997). Rapid deubiquitination of nucleosomal histones in human tumor cells caused by proteasome inhibitors and stress response inducers: Effects on replication, transcription, translation, and the cellular stress response †. Biochemistry, 36(47), 14418–14429. https://doi.org/10.1021/bi970998j
Monaco, A., & Fraldi, A. (2020). Protein aggregation and dysfunction of autophagy-lysosomal pathway: A vicious cycle in lysosomal storage diseases. Frontiers in Molecular Neuroscience. https://doi.org/10.3389/fnmol.2020.00037
Morales, F., Couto, J. M., Higham, C. F., Hogg, G., Cuenca, P., Braida, C., et al. (2012). Somatic instability of the expanded CTG triplet repeat in myotonic dystrophy type 1 is a heritable quantitative trait and modifier of disease severity. Human Molecular Genetics, 21(16), 3558–3567. https://doi.org/10.1093/hmg/dds185
Morán Luengo, T., Matthias, P. M., & Stefan, G. D. R. (2019). The Hsp70–Hsp90 chaperone cascade in protein folding. Trends in Cell Biology, 29(2), 164–177. https://doi.org/10.1016/j.tcb.2018.10.004
Moreira, P. I., Carvalho, C., Zhu, X., Smith, M. A., & Perry, G. (2010). Mitochondrial dysfunction is a trigger of Alzheimer’s disease pathophysiology. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease, 1802(1), 2–10. https://doi.org/10.1016/j.bbadis.2009.10.006
Muotri, A. R., Chu, V. T., Marchetto, M. C., Deng, W., Moran, J. V., & Gage, F. H. (2005). Somatic mosaicism in neuronal precursor cells mediated by L1 retrotransposition. Nature, 435(7044), 903–910. https://doi.org/10.1038/nature03663
Musich, P. R., & Zou, Y. (2009). Genomic instability and DNA damage responses in progeria arising from defective maturation of prelamin A. Aging, 1(1), 28–37. https://doi.org/10.18632/aging.100012
Nagata, Y., Anan, T., Yoshida, T., Mizukami, T., Taya, Y., Fujiwara, T., et al. (1999). The stabilization mechanism of mutant-type P53 by impaired ubiquitination: The loss of wild-type P53 function and the Hsp90 association. Oncogene, 18(44), 6037–6049. https://doi.org/10.1038/sj.onc.1202978
Nakamura, M., Kaneko, S., Dickson, D. W., & Kusaka, H. (2019). Aberrant accumulation of BRCA1 in Alzheimer disease and other tauopathies. Journal of Neuropathology & Experimental Neurology. https://doi.org/10.1093/jnen/nlz107
Nawa, N., Hirata, K., Kawatani, K., Nambara, T., Omori, S., Banno, K., et al. (2019). Elimination of protein aggregates prevents premature senescence in human trisomy 21 fibroblasts. PLoS ONE, 14(7), e0219592. https://doi.org/10.1371/journal.pone.0219592
Negrini, S., Gorgoulis, V. G., & Halazonetis, T. D. (2010). Genomic instability—an evolving Hallmark of cancer. Nature Reviews Molecular Cell Biology, 11(3), 220–228. https://doi.org/10.1038/nrm2858
Niblock, M., & Gallo, J.-M. (2012). Tau alternative splicing in familial and sporadic tauopathies. Biochemical Society Transactions, 40(4), 677–680. https://doi.org/10.1042/BST20120091
Niedernhofer, L. J., Gurkar, A. U., Wang, Y., Vijg, J., Hoeijmakers, J. H. J., & Robbins, P. D. (2018). Nuclear genomic instability and aging. Annual Review of Biochemistry, 87(1), 295–322. https://doi.org/10.1146/annurev-biochem-062917-012239
Njomen, E., & Tepe, J. J. (2019). Proteasome activation as a new therapeutic approach to target proteotoxic disorders. Journal of Medicinal Chemistry, 62(14), 6469–6481. https://doi.org/10.1021/acs.jmedchem.9b00101
Nordin, A., Akimoto, C., Wuolikainen, A., Alstermark, H., Jonsson, P., Birve, A., et al. (2015). Extensive size variability of the GGGGCC expansion in C9orf72 in both neuronal and non-neuronal tissues in 18 patients with ALS or FTD. Human Molecular Genetics, 24(11), 3133–3142. https://doi.org/10.1093/hmg/ddv064
Oda, T., Hayano, T., Miyaso, H., Takahashi, N., & Yamashita, T. (2007). Hsp90 regulates the fanconi anemia DNA damage response pathway. Blood, 109(11), 5016–5026. https://doi.org/10.1182/blood-2006-08-038638
Oromendia, A. B., & Amon, A. (2014). Aneuploidy: Implications for protein homeostasis and disease. Disease Models & Mechanisms, 7(1), 15–20. https://doi.org/10.1242/dmm.013391
Oromendia, A. B., Dodgson, S. E., & Amon, A. (2012). Aneuploidy causes proteotoxic stress in yeast. Genes & Development, 26(24), 2696–2708. https://doi.org/10.1101/gad.207407.112
Otto, F. B., & Thumm, M. (2020). Nucleophagy—implications for microautophagy and health. International Journal of Molecular Sciences, 21(12), 4506. https://doi.org/10.3390/ijms21124506
Ou, H.-L., & Schumacher, B. (2018). DNA damage responses and P53 in the aging process. Blood, 131(5), 488–495. https://doi.org/10.1182/blood-2017-07-746396
Palumbo, E., Zhao, Bi., Xue, B., Uversky, V. N., & Davé, V. (2020). Analyzing aggregation propensities of clinically relevant PTEN mutants: A new culprit in pathogenesis of cancer and other PTENopathies. Journal of Biomolecular Structure and Dynamics, 38(8), 2253–2266. https://doi.org/10.1080/07391102.2019.1630005
Papandreou, M.-E., & Tavernarakis, N. (2019). Nucleophagy: From homeostasis to disease. Cell Death & Differentiation, 26(4), 630–639. https://doi.org/10.1038/s41418-018-0266-5
Paradisi, M., McClintock, D., Boguslavsky, R. L., Pedicelli, C., Worman, H. J., & Djabali, K. (2005). Dermal fibroblasts in Hutchinson-Gilford progeria syndrome with the lamin A G608G mutation have dysmorphic nuclei and are hypersensitive to heat stress. BMC Cell Biology, 6(1), 27. https://doi.org/10.1186/1471-2121-6-27
Park, C.-W., & Ryu, K.-Y. (2014). Cellular ubiquitin pool dynamics and homeostasis. BMB Reports, 47(9), 475–482. https://doi.org/10.5483/BMBRep.2014.47.9.128
Park, J. S., Lee, J., Jung, E. S., Kim, M. H., Kim, I. B., Son, H., et al. (2019). Brain somatic mutations observed in Alzheimer’s disease associated with aging and dysregulation of tau phosphorylation. Nature Communications, 10(1), 3090. https://doi.org/10.1038/s41467-019-11000-7
Park, Y.-E., Hayashi, Y. K., Bonne, G., Arimura, T., Noguchi, S., Nonaka, I., & Nishino, I. (2009). Autophagic degradation of nuclear components in mammalian cells. Autophagy, 5(6), 795–804. https://doi.org/10.4161/auto.8901
Paulson, H. (2018). Repeat expansion diseases. Handb Clin Neurol., 2018(147), 105–123. https://doi.org/10.1016/B978-0-444-63233-3.00009-9.
Perez-Rodriguez, D., Kalyva, M., Leija-Salazar, M., Lashley, T., Tarabichi, M., Chelban, V., et al. (2019). Investigation of somatic CNVs in brains of synucleinopathy cases using targeted SNCA analysis and single cell sequencing. Acta Neuropathologica Communications, 7(1), 219. https://doi.org/10.1186/s40478-019-0873-5
Petr, M. A., Tulika, T., Carmona-Marin, L. M., & Scheibye-Knudsen, M. (2020). Protecting the aging genome. Trends in Cell Biology, 30(2), 117–132. https://doi.org/10.1016/j.tcb.2019.12.001
Pickles, S., Vigié, P., & Youle, R. J. (2018). Mitophagy and quality control mechanisms in mitochondrial maintenance. Current Biology, 28(4), R170–R185. https://doi.org/10.1016/j.cub.2018.01.004
Pirone, L., Caldinelli, L., di Lascio, S., di Girolamo, R., di Gaetano, S., Fornasari, D., et al. (2019). Molecular insights into the role of the polyalanine region in mediating <scp>PHOX</Scp> 2B aggregation. The FEBS Journal, 286(13), 2505–2521. https://doi.org/10.1111/febs.14841
Pohl, C., & Dikic, I. (2019). Cellular quality control by the ubiquitin-proteasome system and autophagy. Science, 366(6467), 818–822. https://doi.org/10.1126/science.aax3769
Poletto, M., Yang, Di., Fletcher, S. C., Vendrell, I., Fischer, R., Legrand, A. J., & Dianov, G. L. (2017). Modulation of proteostasis counteracts oxidative stress and affects DNA base excision repair capacity in ATM-deficient cells. Nucleic Acids Research, 45(17), 10042–10055. https://doi.org/10.1093/nar/gkx635
Polling, S., Ormsby, A. R., Wood, R. J., Lee, K., Shoubridge, C., Hughes, J. N., et al. (2015). Polyalanine expansions drive a shift into α-helical clusters without amyloid-fibril formation. Nature Structural & Molecular Biology, 22(12), 1008–1015. https://doi.org/10.1038/nsmb.3127
Potter, H., Chial, H. J., Caneus, J., Elos, M., Elder, N., Borysov, S., & Granic, A. (2019). Chromosome instability and mosaic aneuploidy in neurodegenerative and neurodevelopmental disorders. Frontiers in Genetics. https://doi.org/10.3389/fgene.2019.01092
Priestley, P., Baber, J., Lolkema, M. P., Steeghs, N., de Bruijn, E., Shale, C., et al. (2019). Pan-cancer whole-genome analyses of metastatic solid tumours. Nature, 575(7781), 210–216. https://doi.org/10.1038/s41586-019-1689-y
Prohaska, A., Racimo, F., Schork, A. J., Sikora, M., Stern, A. J., Ilardo, M., et al. (2019). Human disease variation in the light of population genomics. Cell, 177(1), 115–131. https://doi.org/10.1016/j.cell.2019.01.052
Quanz, M., Herbette, A., Sayarath, M., de Koning, L., Dubois, T., Sun, J.-S., & Dutreix, M. (2012). Heat shock protein 90α (Hsp90α) is phosphorylated in response to DNA damage and accumulates in repair foci. Journal of Biological Chemistry, 287(12), 8803–8815. https://doi.org/10.1074/jbc.M111.320887
Raimondi, S., Guglielmi, F., Giorgetti, S., di Gaetano, S., Arciello, A., Monti, D. M., et al. (2011). Effects of the known pathogenic mutations on the aggregation pathway of the amyloidogenic peptide of apolipoprotein A-I. Journal of Molecular Biology, 407(3), 465–476. https://doi.org/10.1016/j.jmb.2011.01.044
Rancati, G., Pavelka, N., Fleharty, B., Noll, A., Trimble, R., Walton, K., et al. (2008). Aneuploidy underlies rapid adaptive evolution of yeast cells deprived of a conserved cytokinesis motor. Cell, 135(5), 879–893. https://doi.org/10.1016/j.cell.2008.09.039
Ravanelli, S., den Brave, F., & Hoppe, T. (2020). Mitochondrial quality control governed by ubiquitin. Frontiers in Cell and Developmental Biology. https://doi.org/10.3389/fcell.2020.00270
Redler, R. L., Das, J., Diaz, J. R., & Dokholyan, N. V. (2016). Protein destabilization as a common factor in diverse inherited disorders. Journal of Molecular Evolution, 82(1), 11–16. https://doi.org/10.1007/s00239-015-9717-5
Revay, T., Oluwole, O., Kroetsch, T., & Allan King, W. (2017). In vivo and in vitro ageing results in accumulation of de Novo copy number variations in bulls. Scientific Reports, 7(1), 1631. https://doi.org/10.1038/s41598-017-01793-2
Roseaulin, L. C., Noguchi, C., & Noguchi, E. (2013). Proteasome-dependent degradation of replisome components regulates faithful DNA replication. Cell Cycle, 12(16), 2564–2569. https://doi.org/10.4161/cc.25692
Rovelet-Lecrux, A., Hannequin, D., Raux, G., le Meur, N., Laquerrière, A., Vital, A., et al. (2006). APP locus duplication causes autosomal dominant early-onset Alzheimer disease with cerebral amyloid angiopathy. Nature Genetics, 38(1), 24–26. https://doi.org/10.1038/ng1718
Rubinsztein, D. C., Codogno, P., & Levine, B. (2012). Autophagy modulation as a potential therapeutic target for diverse diseases. Nature Reviews Drug Discovery, 11(9), 709–730. https://doi.org/10.1038/nrd3802
Rudiger, S., Germeroth, L., Schneider-Mergener, J., & Bukau, B. (1997). Substrate specificity of the dnak chaperone determined by screening cellulose-bound peptide libraries. The EMBO Journal, 16(7), 1501–1507.
Russo, C., Osterburg, C., Sirico, A., Antonini, D., Ambrosio, R., Würz, J. M., et al. (2018). Protein aggregation of the P63 transcription factor underlies severe skin fragility in AEC syndrome. Proceedings of the National Academy of Sciences, 115(5), E906–E915. https://doi.org/10.1073/pnas.1713773115
Sanchez-Contreras, M., & Cardozo-Pelaez, F. (2017). Age-related length variability of polymorphic CAG repeats. DNA Repair, 49, 26–32. https://doi.org/10.1016/j.dnarep.2016.10.003
Sauna, Z. E., & Kimchi-Sarfaty, C. (2011). Understanding the contribution of synonymous mutations to human disease. Nature Reviews Genetics, 12(10), 683–691. https://doi.org/10.1038/nrg3051
Schaser, A. J., Osterberg, V. R., Dent, S. E., Stackhouse, T. L., Wakeham, C. M., Boutros, S. W., et al. (2019). Alpha-synuclein Is a DNA binding protein that modulates DNA repair with implications for lewy body disorders. Scientific Reports, 9(1), 10919. https://doi.org/10.1038/s41598-019-47227-z
Sciascia, N., Wei, Wu., Zong, D., Sun, Y., Wong, N., John, S., et al. (2020). Suppressing proteasome mediated processing of topoisomerase II DNA-protein complexes preserves genome integrity. ELife. https://doi.org/10.7554/eLife.53447
Sekimoto, T., Oda, T., Pozo, F. M., Murakumo, Y., Masutani, C., Hanaoka, F., & Yamashita, T. (2010). The molecular chaperone Hsp90 regulates accumulation of DNA polymerase η at replication stalling sites in UV-irradiated cells. Molecular Cell, 37(1), 79–89. https://doi.org/10.1016/j.molcel.2009.12.015
Sepe, S., Milanese, C., Gabriels, S., Derks, K. W., Payan-Gomez, C., van Ijcken, W. F., et al. (2016). Inefficient DNA repair is an aging-related modifier of Parkinson’s disease. Cell Reports, 15(9), 1866–1875. https://doi.org/10.1016/j.celrep.2016.04.071
Shendure, J., & Akey, J. M. (2015). The origins, determinants, and consequences of human mutations. Science, 349(6255), 1478–1483. https://doi.org/10.1126/science.aaa9119
Shepherd, C. E., Yang, Y., & Halliday, G. M. (2018). Region- and cell-specific aneuploidy in brain aging and neurodegeneration. Neuroscience, 374, 326–334. https://doi.org/10.1016/j.neuroscience.2018.01.050
Shigemizu, D., Fujimoto, A., Akiyama, S., Abe, T., Nakano, K., Boroevich, K. A., et al. (2013). A practical method to detect SNVs and indels from whole genome and exome sequencing data. Scientific Reports, 3(1), 2161. https://doi.org/10.1038/srep02161
Shiloh, Y. (2020). The cerebellar degeneration in ataxia-telangiectasia: A case for genome instability. DNA Repair, 95, 102950. https://doi.org/10.1016/j.dnarep.2020.102950
Solier, S., Kohn, K. W., Scroggins, B., Xu, W., Trepel, J., Neckers, L., & Pommier, Y. (2012). Heat shock protein 90 (HSP90), a substrate and chaperone of DNA-PK necessary for the apoptotic response. Proceedings of the National Academy of Sciences, 109(32), 12866–12872. https://doi.org/10.1073/pnas.1203617109
Solomon, J. P., Page, L. J., Balch, W. E., & Kelly, J. W. (2012). Gelsolin amyloidosis: Genetics, biochemistry, pathology and possible strategies for therapeutic intervention. Critical Reviews in Biochemistry and Molecular Biology, 47(3), 282–296. https://doi.org/10.3109/10409238.2012.661401
Sottile, M. L., & Nadin, S. B. (2018). Heat shock proteins and DNA repair mechanisms: An updated overview. Cell Stress and Chaperones, 23(3), 303–315. https://doi.org/10.1007/s12192-017-0843-4
Spielmann, M., Lupiáñez, D. G., & Mundlos, S. (2018). Structural variation in the 3D genome. Nature Reviews Genetics, 19(7), 453–467. https://doi.org/10.1038/s41576-018-0007-0
Spillantini, M. G., & Goedert, M. (2013). Tau pathology and neurodegeneration. The Lancet Neurology, 12(6), 609–622. https://doi.org/10.1016/S1474-4422(13)70090-5
Stingele, S., Stoehr, G., Peplowska, K., Cox, J., Mann, M., & Storchova, Z. (2012). Global analysis of genome, transcriptome and proteome reveals the response to aneuploidy in human cells. Molecular Systems Biology, 8(1), 608. https://doi.org/10.1038/msb.2012.40
Suberbielle, E., Djukic, B., Evans, M., Kim, D. H., Taneja, P., Wang, X., et al. (2015). DNA repair factor BRCA1 depletion occurs in Alzheimer brains and impairs cognitive function in mice. Nature Communications, 6(1), 8897. https://doi.org/10.1038/ncomms9897
Sunshine, A. B., Ong, G. T., Nickerson, D. P., Carr, D., Murakami, C. J., Wasko, B. M., et al. (2016). Aneuploidy shortens replicative lifespan in Saccharomyces Cerevisiae. Aging Cell, 15(2), 317–324. https://doi.org/10.1111/acel.12443
Swami, M., Hendricks, A. E., Gillis, T., Massood, T., Mysore, J., Myers, R. H., & Wheeler, V. C. (2009). Somatic expansion of the Huntington’s disease CAG repeat in the brain is associated with an earlier age of disease onset. Human Molecular Genetics, 18(16), 3039–3047. https://doi.org/10.1093/hmg/ddp242
Sy, S.-H., Guo, Y., Lan, Y., Ng, H., & Huen, M.-Y. (2020). Preemptive homology-directed DNA repair fosters complex genomic rearrangements in hepatocellular carcinoma. Translational Oncology, 13(9), 100796. https://doi.org/10.1016/j.tranon.2020.100796
Talaei, F., van Praag, V. M., & Henning, R. H. (2013). Hydrogen sulfide restores a normal morphological phenotype in werner syndrome fibroblasts, attenuates oxidative damage and modulates MTOR pathway. Pharmacological Research, 74, 34–44. https://doi.org/10.1016/j.phrs.2013.04.011
Tamás, M., Sharma, S., Ibstedt, S., Jacobson, T., & Christen, P. (2014). Heavy metals and metalloids as a cause for protein misfolding and aggregation. Biomolecules, 4(1), 252–267. https://doi.org/10.3390/biom4010252
Tartaglia, G. G., Pechmann, S., Dobson, C. M., & Vendruscolo, M. (2007). Life on the edge: A link between gene expression levels and aggregation rates of human proteins. Trends in Biochemical Sciences, 32(5), 204–206. https://doi.org/10.1016/j.tibs.2007.03.005
Thibaudeau, T. A., Anderson, R. T., & Smith, D. M. (2018). A common mechanism of proteasome impairment by neurodegenerative disease-associated oligomers. Nature Communications, 9(1), 1097. https://doi.org/10.1038/s41467-018-03509-0
Tokuriki, N., & Tawfik, D. S. (2009). Stability effects of mutations and protein evolvability. Current Opinion in Structural Biology, 19(5), 596–604. https://doi.org/10.1016/j.sbi.2009.08.003
Tubbs, A., & Nussenzweig, A. (2017). Endogenous DNA damage as a source of genomic instability in cancer. Cell, 168(4), 644–656. https://doi.org/10.1016/j.cell.2017.01.002
Upton, K. R., Gerhardt, D. J., Samuel Jesuadian, J., Richardson, S. R., Sánchez-Luque, F. J., Bodea, G. O., et al. (2015). Ubiquitous L1 mosaicism in hippocampal neurons. Cell, 161(2), 228–239. https://doi.org/10.1016/j.cell.2015.03.026
Uversky, V. N. (2019). Intrinsically disordered proteins and their ‘mysterious’ (meta)physics. Frontiers in Physics. https://doi.org/10.3389/fphy.2019.00010
van den Bos, H., Spierings, D. C. J., Foijer, F., & Lansdorp, P. M. (2017). Does aneuploidy in the brain play a role in neurodegenerative disease? Chromosomal abnormalities—a hallmark manifestation of genomic instability. London: InTech.
van Ham TJ, Breitling R, Swertz MA, Nollen EA. (2009) Neurodegenerative diseases: Lessons from genome-wide screens in small model organisms. EMBO Molecular Medicine 1(8, 9):360–370. https://doi.org/10.1002/emmm.200900051
Vasquez, V., Mitra, J., Hegde, P. M., Pandey, A., Sengupta, S., Mitra, S., et al. (2017). Chromatin-bound oxidized α-synuclein causes strand breaks in neuronal genomes in in vitro models of Parkinson’s disease. Journal of Alzheimer’s Disease, 60(s1), S133–S150. https://doi.org/10.3233/JAD-170342
Vehvilainen, P., Koistinaho, J., & Gundars, G. (2014). Mechanisms of mutant SOD1 induced mitochondrial toxicity in amyotrophic lateral sclerosis. Frontiers in Cellular Neuroscience. https://doi.org/10.3389/fncel.2014.00126
Vendruscolo, M., Knowles, T. P. J., & Dobson, C. M. (2011). Protein solubility and protein homeostasis: A generic view of protein misfolding disorders. Cold Spring Harbor Perspectives in Biology, 3(12), a010454–a010454. https://doi.org/10.1101/cshperspect.a010454
Vessoni, A. T., Guerra, C. C., Kajitani, G. S., Nascimento, L. L., & Garcia, C. C. (2020). Cockayne syndrome: The many challenges and approaches to understand a multifaceted disease. Genetics and Molecular Biology. https://doi.org/10.1590/1678-4685-gmb-2019-0085
Vijg, J., & Dong, X. (2020). Pathogenic mechanisms of somatic mutation and genome mosaicism in aging. Cell, 182(1), 12–23. https://doi.org/10.1016/j.cell.2020.06.024
Vijg, J., & Suh, Y. (2013). Genome instability and aging. Annual Review of Physiology, 75(1), 645–668. https://doi.org/10.1146/annurev-physiol-030212-183715
Villela, D., Suemoto, C. K., Leite, R., Pasqualucci, C. A., Grinberg, L. T., Pearson, P., & Rosenberg, C. (2018). Increased DNA copy number variation mosaicism in elderly human brain. Neural Plasticity, 2018, 1–9. https://doi.org/10.1155/2018/2406170
Vogelstein, B., Papadopoulos, N., Velculescu, V. E., Zhou, S., Diaz, L. A., & Kinzler, K. W. (2013). Cancer genome landscapes. Science, 339(6127), 1546–1558. https://doi.org/10.1126/science.1235122
Walsh, I. M., Bowman, M. A., Soto, I. F., Santarriaga, A. R., & Clark, P. L. (2020). Synonymous codon substitutions perturb cotranslational protein folding in vivo and impair cell fitness. Proceedings of the National Academy of Sciences, 117(7), 3528–3534. https://doi.org/10.1073/pnas.1907126117
Walter, D., Hoffmann, S., Komseli, E.-S., Rappsilber, J., Gorgoulis, V., & Sørensen, C. S. (2016). SCFCyclin F-dependent degradation of CDC6 suppresses DNA re-replication. Nature Communications, 7(1), 10530. https://doi.org/10.1038/ncomms10530
Wang, H., Guo, W., Mitra, J., Hegde, P. M., Vandoorne, T., Eckelmann, B. J., et al. (2018). Mutant FUS causes DNA ligation defects to inhibit oxidative damage repair in amyotrophic lateral sclerosis. Nature Communications, 9(1), 3683. https://doi.org/10.1038/s41467-018-06111-6
Wang, P., Deng, J., Dong, J., Liu, J., Bigio, E. H., Mesulam, M., et al. (2019). TDP-43 induces mitochondrial damage and activates the mitochondrial unfolded protein response. PLOS Genetics, 15(5), e1007947. https://doi.org/10.1371/journal.pgen.1007947
Wang, Z., Zhu, W.-G., & Xingzhi, Xu. (2017). Ubiquitin-like Modifications in the DNA damage response. Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis, 803–805, 56–75. https://doi.org/10.1016/j.mrfmmm.2017.07.001
Weischenfeldt, J., Symmons, O., Spitz, F., & Korbel, J. O. (2013). Phenotypic impact of genomic structural variation: Insights from and for human disease. Nature Reviews Genetics, 14(2), 125–138. https://doi.org/10.1038/nrg3373
Whitesell, L., & Lindquist, S. L. (2005). HSP90 and the chaperoning of cancer. Nature Reviews Cancer, 5(10), 761–772. https://doi.org/10.1038/nrc1716
Wilcken, R., Wang, G., Boeckler, F. M., & Fersht, A. R. (2012). Kinetic mechanism of P53 oncogenic mutant aggregation and its inhibition. Proceedings of the National Academy of Sciences, 109(34), 13584–13589. https://doi.org/10.1073/pnas.1211550109
Xie, J. L., & Jarosz, D. F. (2018). Mutations, protein homeostasis, and epigenetic control of genome integrity. DNA Repair, 71, 23–32. https://doi.org/10.1016/j.dnarep.2018.08.004
Yan, M., Shen, J., Person, M. D., Kuang, X., Lynn, W. S., Atlas, D., & Wong, P. K. Y. (2008). Endoplasmic reticulum stress and unfolded protein response in atm-deficient thymocytes and thymic lymphoma cells are attributable to oxidative stress. Neoplasia, 10(2), 160–167. https://doi.org/10.1593/neo.07935
Yi, K., & Young Seok, Ju. (2018). Patterns and mechanisms of structural variations in human cancer. Experimental & Molecular Medicine, 50(8), 98. https://doi.org/10.1038/s12276-018-0112-3
Yu, M., & Ren, B. (2017). The three-dimensional organization of mammalian genomes. Annual Review of Cell and Developmental Biology, 33(1), 265–289. https://doi.org/10.1146/annurev-cellbio-100616-060531
Zhang, L., Dong, X., Lee, M., Maslov, A. Y., Wang, T., & Vijg, J. (2019). Single-cell whole-genome sequencing reveals the functional landscape of somatic mutations in B lymphocytes across the human lifespan. Proceedings of the National Academy of Sciences, 116(18), 9014–9019. https://doi.org/10.1073/pnas.1902510116
Zhao, L., Vecchi, G., Vendruscolo, M., Körner, R., Hayer-Hartl, M., & Ulrich Hartl, F. (2019). The Hsp70 chaperone system stabilizes a thermo-sensitive subproteome in E. Coli. Cell Reports, 28(5), 1335-1345.e6. https://doi.org/10.1016/j.celrep.2019.06.081
Zhu, P. J., Khatiwada, S., Cui, Ya., Reineke, L. C., Dooling, S. W., Kim, J. J., et al. (2019). Activation of the ISR mediates the behavioral and neurophysiological abnormalities in down syndrome. Science, 366(6467), 843–849. https://doi.org/10.1126/science.aaw5185
Zu, T., Liu, Y., Banez-Coronel, M., Reid, T., Pletnikova, O., Lewis, J., et al. (2013). RAN proteins and RNA foci from antisense transcripts in C9ORF72 ALS and frontotemporal dementia. Proceedings of the National Academy of Sciences, 110(51), E4968–E4977. https://doi.org/10.1073/pnas.1315438110
Funding
This work was supported by an Nederlandse organisatie voor Wetenschappelijk Onderzoek (NWO) grant to SB [ALW 824.15.004].
Author information
Authors and Affiliations
Contributions
WH and SB conceived the idea for the article; WH wrote the manuscript and made the artwork; WH and SB read and edited the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest.
Artwork
Artwork is fully original and was created using Adobe Illustrator 2020.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Huiting, W., Bergink, S. Locked in a vicious cycle: the connection between genomic instability and a loss of protein homeostasis. GENOME INSTAB. DIS. 2, 1–23 (2021). https://doi.org/10.1007/s42764-020-00027-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42764-020-00027-6