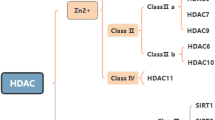
Abstract
Silent information regulator proteins (SIRT), or sirtuins, are evolutionarily conserved NAD+-dependent deacetylases and ADP-mono-ribosyltransferases. In mammalian, seven sirtuins have been identified, namely SIRT1–7, with different subcellular localization. Nuclear sirtuins, including SIRT1, SIRT6 and SIRT7, localize predominantly in the nucleus and are implicated in many vital biological processes, including stress response, transcription, genome maintenance, tumorigenesis and aging. Dysregulation of nuclear sirtuins is associated with the development of many diseases, including cancer and metabolic disorders. Therefore, the activities of nuclear sirtuins must be properly regulated. In this review, we summarize the current knowledge on the post-translational modifications of nuclear sirtuins and discuss how these modifications modulate their functions.

Similar content being viewed by others
References
Ahuja, N., Schwer, B., Carobbio, S., Waltregny, D., North, B. J., Castronovo, V., et al. (2007). Regulation of insulin secretion by SIRT4, a mitochondrial ADP-ribosyltransferase. The Journal of Biological Chemistry,282, 33583–33592.
Back, J. H., Rezvani, H. R., Zhu, Y., Guyonnet-Duperat, V., Athar, M., Ratner, D., et al. (2011). Cancer cell survival following DNA damage-mediated premature senescence is regulated by mammalian target of rapamycin (mTOR)-dependent Inhibition of sirtuin 1. The Journal of Biological Chemistry,286, 19100–19108.
Bai, B., Liang, Y., Xu, C., Lee, M. Y., Xu, A., Wu, D., et al. (2012). Cyclin-dependent kinase 5-mediated hyperphosphorylation of sirtuin-1 contributes to the development of endothelial senescence and atherosclerosis. Circulation,126, 729–740.
Barber, M. F., Michishita-Kioi, E., Xi, Y., Tasselli, L., Kioi, M., Moqtaderi, Z., et al. (2012). SIRT7 links H3K18 deacetylation to maintenance of oncogenic transformation. Nature,487, 114–118.
Bian, Y., Song, C., Cheng, K., Dong, M., Wang, F., Huang, J., et al. (2014). An enzyme assisted RP-RPLC approach for in-depth analysis of human liver phosphoproteome. Journal of Proteomics,96, 253–262.
Buler, M., Andersson, U., & Hakkola, J. (2016). Who watches the watchmen? Regulation of the expression and activity of sirtuins. FASEB Journal: Official Publication of the Federation of American Societies for Experimental Biology,30, 3942–3960.
Cai, J., Zuo, Y., Wang, T., Cao, Y., Cai, R., Chen, F. L., et al. (2016). A crucial role of SUMOylation in modulating Sirt6 deacetylation of H3 at lysine 56 and its tumor suppressive activity. Oncogene,35, 4949–4956.
Caito, S., Rajendrasozhan, S., Cook, S., Chung, S., Yao, H., Friedman, A. E., et al. (2010). SIRT1 is a redox-sensitive deacetylase that is post-translationally modified by oxidants and carbonyl stress. FASEB Journal: Official Publication of the Federation of American Societies for Experimental Biology,24, 3145–3159.
Chalkiadaki, A., & Guarente, L. (2015). The multifaceted functions of sirtuins in cancer. Nature Reviews Cancer,15, 608–624.
Chen, S., Blank, M. F., Iyer, A., Huang, B., Wang, L., Grummt, I., et al. (2016). SIRT7-dependent deacetylation of the U3–55k protein controls pre-rRNA processing. Nature Communications,7, 10734.
Chen, S., Seiler, J., Santiago-Reichelt, M., Felbel, K., Grummt, I., & Voit, R. (2013). Repression of RNA polymerase I upon stress is caused by inhibition of RNA-dependent deacetylation of PAF53 by SIRT7. Molecular Cell,52, 303–313.
Choi, S. E., Kwon, S., Seok, S., Xiao, Z., Lee, K.-W., Kang, Y., et al. (2017). Obesity-linked phosphorylation of SIRT1 by casein kinase 2 inhibits its nuclear localization and promotes fatty liver. Molecular and Cellular Biology,37, e00006–00017.
Choudhary, C., Kumar, C., Gnad, F., Nielsen, M. L., Rehman, M., Walther, T. C., et al. (2009). Lysine acetylation targets protein complexes and co-regulates major cellular functions. Science,325, 834–840.
Conrad, E., Polonio-Vallon, T., Meister, M., Matt, S., Bitomsky, N., Herbel, C., et al. (2016). HIPK2 restricts SIRT1 activity upon severe DNA damage by a phosphorylation-controlled mechanism. Cell Death and Differentiation,23, 110–122.
Dalle-Donne, I., Aldini, G., Carini, M., Colombo, R., Rossi, R., & Milzani, A. (2006). Protein carbonylation, cellular dysfunction, and disease progression. Journal of Cellular and Molecular Medicine,10, 389–406.
Dephoure, N., Zhou, C., Villen, J., Beausoleil, S. A., Bakalarski, C. E., Elledge, S. J., et al. (2008). A quantitative atlas of mitotic phosphorylation. Proceedings of the National Academy of Sciences of the United States of America,105, 10762–10767.
Dowling, D. P., Gantt, S. L., Gattis, S. G., Fierke, C. A., & Christianson, D. W. (2008). Structural studies of human histone deacetylase 8 and its site-specific variants complexed with substrate and inhibitors. Biochemistry,47, 13554–13563.
Du, J., Zhou, Y., Su, X., Yu, J. J., Khan, S., Jiang, H., et al. (2011). Sirt5 is a NAD-dependent protein lysine demalonylase and desuccinylase. Science,334, 806–809.
Fang, J., Ianni, A., Smolka, C., Vakhrusheva, O., Nolte, H., Krüger, M., et al. (2017). Sirt7 promotes adipogenesis in the mouse by inhibiting autocatalytic activation of Sirt1. Proceedings of the National Academy of Sciences,114, E8352–E8361.
Feldman, J. L., Baeza, J., & Denu, J. M. (2013). Activation of the protein deacetylase SIRT6 by long-chain fatty acids and widespread deacylation by mammalian sirtuins. The Journal of Biological Chemistry,288, 31350–31356.
Finnin, M. S., Donigian, J. R., Cohen, A., Richon, V. M., Rifkind, R. A., Marks, P. A., et al. (1999). Structures of a histone deacetylase homologue bound to the TSA and SAHA inhibitors. Nature,401, 188–193.
Flick, F., & Luscher, B. (2012). Regulation of sirtuin function by posttranslational modifications. Frontiers in Pharmacology,3, 29.
Ford, E., Voit, R., Liszt, G., Magin, C., Grummt, I., & Guarente, L. (2006). Mammalian Sir2 homolog SIRT7 is an activator of RNA polymerase I transcription. Genes & Development,20, 1075–1080.
Ford, J., Ahmed, S., Allison, S., Jiang, M., & Milner, J. (2008). JNK2-dependent regulation of SIRT1 protein stability. Cell Cycle,7, 3091–3097.
Gantt, S. L., Joseph, C. G., & Fierke, C. A. (2010). Activation and inhibition of histone deacetylase 8 by monovalent cations. The Journal of Biological Chemistry,285, 6036–6043.
Gerhart-Hines, Z., Dominy, J. E., Jr., Blattler, S. M., Jedrychowski, M. P., Banks, A. S., Lim, J. H., et al. (2011). The cAMP/PKA pathway rapidly activates SIRT1 to promote fatty acid oxidation independently of changes in NAD(+). Molecular Cell,44, 851–863.
Ghosh, S., & Zhou, Z. (2015). SIRTain regulators of premature senescence and accelerated aging. Protein & Cell,6, 322–333.
Grob, A., Roussel, P., Wright, J. E., McStay, B., Hernandez-Verdun, D., & Sirri, V. (2009). Involvement of SIRT7 in resumption of rDNA transcription at the exit from mitosis. Journal of Cell Science,122, 489–498.
Gu, W., & Roeder, R. G. (1997). Activation of p53 sequence-specific DNA binding by acetylation of the p53 C-terminal domain. Cell,90, 595–606.
Guo, X., Williams, J. G., Schug, T. T., & Li, X. (2010). DYRK1A and DYRK3 promote cell survival through phosphorylation and activation of SIRT1. The Journal of Biological Chemistry,285, 13223–13232.
Hu, S., Liu, H., Ha, Y., Luo, X., Motamedi, M., Gupta, M. P., et al. (2015). Posttranslational modification of Sirt6 activity by peroxynitrite. Free Radical Biology & Medicine,79, 176–185.
Imai, S., Armstrong, C. M., Kaeberlein, M., & Guarente, L. (2000). Transcriptional silencing and longevity protein Sir2 is an NAD-dependent histone deacetylase. Nature,403, 795–800.
Jackson, M. D., & Denu, J. M. (2002). Structural identification of 2'- and 3'-O-acetyl-ADP-ribose as novel metabolites derived from the Sir2 family of beta -NAD+-dependent histone/protein deacetylases. The Journal of Biological Chemistry,277, 18535–18544.
Jiang, H., Khan, S., Wang, Y., Charron, G., He, B., Sebastian, C., et al. (2013). SIRT6 regulates TNF-alpha secretion through hydrolysis of long-chain fatty acyl lysine. Nature,496, 110–113.
Jiang, L., Xiong, J., Zhan, J., Yuan, F., Tang, M., Zhang, C., et al. (2017). Ubiquitin-specific peptidase 7 (USP7)-mediated deubiquitination of the histone deacetylase SIRT7 regulates gluconeogenesis. The Journal of Biological Chemistry,292, 13296–13311.
Kaidi, A., Weinert, B. T., Choudhary, C., & Jackson, S. P. (2010). Human SIRT6 promotes DNA end resection through CtIP deacetylation. Science,329, 1348–1353.
Kanfi, Y., Naiman, S., Amir, G., Peshti, V., Zinman, G., Nahum, L., et al. (2012). The sirtuin SIRT6 regulates lifespan in male mice. Nature,483, 218–221.
Kang, H., Jung, J. W., Kim, M. K., & Chung, J. H. (2009). CK2 is the regulator of SIRT1 substrate-binding affinity, deacetylase activity and cellular response to DNA-damage. PLoS One,4, e6611.
Kang, H., Suh, J. Y., Jung, Y. S., Jung, J. W., Kim, M. K., & Chung, J. H. (2011). Peptide switch is essential for Sirt1 deacetylase activity. Molecular Cell,44, 203–213.
Kim, E. J., Kho, J. H., Kang, M. R., & Um, S. J. (2007). Active regulator of SIRT1 cooperates with SIRT1 and facilitates suppression of p53 activity. Molecular Cell,28, 277–290.
Kim, J. E., Chen, J., & Lou, Z. (2008). DBC1 is a negative regulator of SIRT1. Nature,451, 583–586.
Kim, S. C., Sprung, R., Chen, Y., Xu, Y., Ball, H., Pei, J., et al. (2006). Substrate and functional diversity of lysine acetylation revealed by a proteomics survey. Molecular Cell,23, 607–618.
Kornberg, M. D., Sen, N., Hara, M. R., Juluri, K. R., Nguyen, J. V., Snowman, A. M., et al. (2010). GAPDH mediates nitrosylation of nuclear proteins. Nature Cell Biology,12, 1094–1100.
Lau, A. W., Liu, P., Inuzuka, H., & Gao, D. (2014). SIRT1 phosphorylation by AMP-activated protein kinase regulates p53 acetylation. American Journal of Cancer Research,4, 245–255.
Lee, C. W., Wong, L. L., Tse, E. Y., Liu, H. F., Leong, V. Y., Lee, J. M., et al. (2012). AMPK promotes p53 acetylation via phosphorylation and inactivation of SIRT1 in liver cancer cells. Cancer Research,72, 4394–4404.
Li, L., Shi, L., Yang, S., Yan, R., Zhang, D., Yang, J., et al. (2016). SIRT7 is a histone desuccinylase that functionally links to chromatin compaction and genome stability. Nature Communications,7, 12235.
Lin, Z., Yang, H., Kong, Q., Li, J., Lee, S. M., Gao, B., et al. (2012). USP22 antagonizes p53 transcriptional activation by deubiquitinating Sirt1 to suppress cell apoptosis and is required for mouse embryonic development. Molecular Cell,46, 484–494.
Lin, Z., Yang, H., Tan, C., Li, J., Liu, Z., Quan, Q., et al. (2013). USP10 antagonizes c-Myc transcriptional activation through SIRT6 stabilization to suppress tumor formation. Cell Reports,5, 1639–1649.
Liszt, G., Ford, E., Kurtev, M., & Guarente, L. (2005). Mouse Sir2 homolog SIRT6 is a nuclear ADP-ribosyltransferase. The Journal of Biological Chemistry,280, 21313–21320.
Liu, X., Wang, D., Zhao, Y., Tu, B., Zheng, Z., Wang, L., et al. (2011). Methyltransferase Set7/9 regulates p53 activity by interacting with Sirtuin 1 (SIRT1). Proceedings of the National Academy of Sciences of the United States of America,108, 1925–1930.
Mao, Z., Hine, C., Tian, X., Van Meter, M., Au, M., Vaidya, A., et al. (2011). SIRT6 promotes DNA repair under stress by activating PARP1. Science,332, 1443–1446.
McCord, R. A., Michishita, E., Hong, T., Berber, E., Boxer, L. D., Kusumoto, R., et al. (2009). SIRT6 stabilizes DNA-dependent Protein Kinase at chromatin for DNA double-strand break repair. Aging,1, 109–121.
Michishita, E., McCord, R. A., Berber, E., Kioi, M., Padilla-Nash, H., Damian, M., et al. (2008). SIRT6 is a histone H3 lysine 9 deacetylase that modulates telomeric chromatin. Nature,452, 492–496.
Michishita, E., Park, J. Y., Burneskis, J. M., Barrett, J. C., & Horikawa, I. (2005). Evolutionarily conserved and nonconserved cellular localizations and functions of human SIRT proteins. Molecular Biology of the Cell,16, 4623–4635.
Min, J., Landry, J., Sternglanz, R., & Xu, R. M. (2001). Crystal structure of a SIR2 homolog-NAD complex. Cell,105, 269–279.
Miteva, Y. V., & Cristea, I. M. (2014). A proteomic perspective of Sirtuin 6 (SIRT6) phosphorylation and interactions and their dependence on its catalytic activity. Molecular & Cellular Proteomics,13, 168–183.
Mostoslavsky, R., Chua, K. F., Lombard, D. B., Pang, W. W., Fischer, M. R., Gellon, L., et al. (2006). Genomic instability and aging-like phenotype in the absence of mammalian SIRT6. Cell,124, 315–329.
Nakamura, T., & Lipton, S. A. (2013). Emerging role of protein-protein transnitrosylation in cell signaling pathways. Antioxidants & Redox Signaling,18, 239–249.
Nasrin, N., Kaushik, V. K., Fortier, E., Wall, D., Pearson, K. J., de Cabo, R., et al. (2009). JNK1 phosphorylates SIRT1 and promotes its enzymatic activity. PLoS One,4, e8414.
Olsen, J.V., Vermeulen, M., Santamaria, A., Kumar, C., Miller, M.L., Jensen, L.J., Gnad, F., Cox, J., Jensen, T.S., Nigg, E.A., et al. (2010). Quantitative phosphoproteomics reveals widespread full phosphorylation site occupancy during mitosis. Science signaling3, ra3.
Peng, C., Lu, Z., Xie, Z., Cheng, Z., Chen, Y., Tan, M., et al. (2011). The first identification of lysine malonylation substrates and its regulatory enzyme. Molecular & Cellular Proteomics,10(M111), 012658.
Peng, L., Yuan, Z., Li, Y., Ling, H., Izumi, V., Fang, B., et al. (2015). Ubiquitinated sirtuin 1 (SIRT1) function is modulated during DNA damage-induced cell death and survival. The Journal of Biological Chemistry,290, 8904–8912.
Rahman, S., & Islam, R. (2011). Mammalian Sirt1: insights on its biological functions. Cell Communication and Signaling,9, 11.
Revollo, J. R., & Li, X. (2013). The ways and means that fine tune Sirt1 activity. Trends in Biochemical Sciences,38, 160–167.
Ronnebaum, S. M., Wu, Y., McDonough, H., & Patterson, C. (2013). The ubiquitin ligase CHIP prevents SirT6 degradation through noncanonical ubiquitination. Molecular and Cellular Biology,33, 4461–4472.
Sasaki, T., Maier, B., Koclega, K. D., Chruszcz, M., Gluba, W., Stukenberg, P. T., et al. (2008). Phosphorylation regulates SIRT1 function. PLoS One,3, e4020.
Sauve, A. A., Celic, I., Avalos, J., Deng, H., Boeke, J. D., & Schramm, V. L. (2001). Chemistry of gene silencing: the mechanism of NAD+-dependent deacetylation reactions. Biochemistry,40, 15456–15463.
Sebastian, C., Zwaans, B. M., Silberman, D. M., Gymrek, M., Goren, A., Zhong, L., et al. (2012). The histone deacetylase SIRT6 is a tumor suppressor that controls cancer metabolism. Cell,151, 1185–1199.
Smith, B. C., & Denu, J. M. (2006). Sir2 protein deacetylases: evidence for chemical intermediates and functions of a conserved histidine. Biochemistry,45, 272–282.
Somoza, J.R., Skene, R.J., Katz, B.A., Mol, C., Ho, J.D., Jennings, A.J., Luong, C., Arvai, A., Buggy, J.J., Chi, E., et al. (2004). Structural snapshots of human HDAC8 provide insights into the Class I histone deacetylases. Structure 12, 1325–1334.
Sun, L., Fan, G., Shan, P., Qiu, X., Dong, S., Liao, L., et al. (2016). Regulation of energy homeostasis by the ubiquitin-independent REGgamma proteasome. Nature Communications,7, 12497.
Tan, M., Peng, C., Anderson, K. A., Chhoy, P., Xie, Z., Dai, L., et al. (2014). Lysine glutarylation is a protein posttranslational modification regulated by SIRT5. Cell Metabolism,19, 605–617.
Tasselli, L., Xi, Y., Zheng, W., Tennen, R. I., Odrowaz, Z., Simeoni, F., et al. (2016). SIRT6 deacetylates H3K18ac at pericentric chromatin to prevent mitotic errors and cellular senescence. Nature Structural & Molecular Biology,23, 434.
Thirumurthi, U., Shen, J., Xia, W., LaBaff, A.M., Wei, Y., Li, C.W., Chang, W.C., Chen, C.H., Lin, H.K., Yu, D., et al. (2014). MDM2-mediated degradation of SIRT6 phosphorylated by AKT1 promotes tumorigenesis and trastuzumab resistance in breast cancer. Science signaling7, ra71.
Toiber, D., Erdel, F., Bouazoune, K., Silberman, D. M., Zhong, L., Mulligan, P., et al. (2013). SIRT6 recruits SNF2H to DNA break sites, preventing genomic instability through chromatin remodeling. Molecular Cell,51, 454–468.
Van Meter, M., Simon, M., Tombline, G., May, A., Morello, T. D., Hubbard, B. P., et al. (2016). JNK Phosphorylates SIRT6 to Stimulate DNA Double-Strand Break Repair in Response to Oxidative Stress by Recruiting PARP1 to DNA Breaks. Cell Reports,16, 2641–2650.
Vannini, A., Volpari, C., Filocamo, G., Casavola, E. C., Brunetti, M., Renzoni, D., et al. (2004). Crystal structure of a eukaryotic zinc-dependent histone deacetylase, human HDAC8, complexed with a hydroxamic acid inhibitor. Proceedings of the National Academy of Sciences of the United States of America,101, 15064–15069.
Vazquez, B. N., Thackray, J. K., Simonet, N. G., Kane-Goldsmith, N., Martinez-Redondo, P., Nguyen, T., et al. (2016). SIRT7 promotes genome integrity and modulates non-homologous end joining DNA repair. The EMBO Journal,35, 1488–1503.
Vidali, G., Gershey, E. L., & Allfrey, V. G. (1968). Chemical studies of histone acetylation. The distribution of epsilon-N-acetyllysine in calf thymus histones. The Journal of Biological Chemistry,243, 6361–6366.
Wang, H., Liu, S., Liu, S., Wei, W., Zhou, X., Lin, F., et al. (2017). Enhanced expression and phosphorylation of Sirt7 activates smad2 and ERK signaling and promotes the cardiac fibrosis differentiation upon angiotensin-II stimulation. PLoS One,12, e0178530.
Wen, L., Chen, Z., Zhang, F., Cui, X., Sun, W., Geary, G. G., et al. (2013). Ca2+/calmodulin-dependent protein kinase kinase beta phosphorylation of Sirtuin 1 in endothelium is atheroprotective. Proceedings of the National Academy of Sciences of the United States of America,110, E2420–2427.
Yamakuchi, M. (2012). MicroRNA Regulation of SIRT1. Frontiers in Physiology,3, 68.
Yan, W.W., Liang, Y.L., Zhang, Q.X., Wang, D., Lei, M.Z., Qu, J., He, X.H., Lei, Q.Y., Wang, Y.P. (2018). Arginine methylation of SIRT7 couples glucose sensing with mitochondria biogenesis. EMBO Reports, e46377.
Yang, B., Zwaans, B. M., Eckersdorff, M., & Lombard, D. B. (2009). The sirtuin SIRT6 deacetylates H3 K56Ac in vivo to promote genomic stability. Cell Cycle (Georgetown, Tex),8, 2662–2663.
Yang, Y., Fu, W., Chen, J., Olashaw, N., Zhang, X., Nicosia, S. V., et al. (2007). SIRT1 sumoylation regulates its deacetylase activity and cellular response to genotoxic stress. Nature Cell Biology,9, 1253–1262.
Yu, J., & Auwerx, J. (2009). The role of sirtuins in the control of metabolic homeostasis. Annals of the New York Academy of Sciences,1173(Suppl 1), E10–19.
Yuan, J., Luo, K., Liu, T., & Lou, Z. (2012). Regulation of SIRT1 activity by genotoxic stress. Genes & development,26, 791–796.
Zhao, W., Kruse, J. P., Tang, Y., Jung, S. Y., Qin, J., & Gu, W. (2008). Negative regulation of the deacetylase SIRT1 by DBC1. Nature,451, 587–590.
Zschoernig, B., & Mahlknecht, U. (2009). Carboxy-terminal phosphorylation of SIRT1 by protein kinase CK2. Biochemical and biophysical research communications,381, 372–377.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhao, K., Zhou, Z. Post-translational modifications of nuclear sirtuins. GENOME INSTAB. DIS. 1, 34–45 (2020). https://doi.org/10.1007/s42764-019-00001-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42764-019-00001-x