Abstract
Derivatives of Carya illinoinensis (pecan) are used as dietary supplements and their leaf and nuts-in-shuck extracts are used in the treatment of various pathologies due to their significant composition in phenolic compounds that act as natural antifungal and anticancer agents. This study aimed to evaluate the antifungal activity of acetone extracts (AEs) from leaves (L) and nuts-in-shucks (S) of cultivars (cv.) Wichita (Wic) and Ukulinga (Uku) of South African C. illinoinensis against A. alternata pathogen. The AEs of Wic-L and Uku-L showed higher antifungal efficacy against all tested A. alternata isolates, with inhibition zones ranging from 11 to 39 mm. Acetone crude extracts of cv. Wichita and cv. Ukulinga showed efficacy against the isolates and were significantly different (p < 0.001). Scanning electron microscopy (SEM) showed major morphological damages on the conidia from assayed cultures, which resulted in inhibition. We further evaluated the in vitro cytotoxic effects of Carya illinoinensis on human embryonic kidney 293 (HEK-293T) cell lines with methanolic extracts (MEs). HEK-293T cell lines (10 × 103 cells/well) were treated with each sample of the extracts at various concentration (1.56, 6.25, 12.5, 25, 50, and 100 µg/mL) and cell viability was quantitated using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay after 48 h. The HEK-293 cell viabilities when exposed to MEs of Wic-L, Wic-S, and Uku-L were 88.2%, 75.2%, and 86.3% respectively, confirming non-toxic properties in accordance with International Organisation for Standardization (ISO) norms. However, the viability of cell culture after the treatment with MEs of Uku-S was 37% and was as toxic. We further used High-performance liquid chromatography (HPLC) to investigate individual phenolic constituents and total phenolic content in the leaves and shucks of both cultivars. Twelve individual phenols were detected through HPLC analysis in the respective extracts. The total phenolic content was higher in the extracts of Wic-L (102.19 mg GAE/g) and Uku-L (110.13 mg GAE/g) relative to the Wic-S (62.03 mg GAE/g) and Uku-S (85.07 mg GAE/g) extracts. Finally, these findings highlighted the possibility of pecan leaf extracts as a potential natural bioactive antifungal agent against black spot disease on pecans in South Africa.
Highlights
-
The acetone extracts (AEs) of Wichita leaves (Wic-L) and Ukulinga leaves (Uku-L) of Carya illinoinensis had the height antifungal efficacy against all tested A. alternata isolates compared to the AEs of both nuts-in-shucks cultivars (Wic-S and Uku-S).
-
Scanning electron microscopy showed major morphological damages on the conidia from the antifungal activity assayed cultures.
-
The methanolic extracts (MEs) of Uku-S extract had toxic effects towards the HEK-293 cells relative to the non-toxic properties exhibited by Wic-L, Wic-S, and Uku-L.
-
Twelve individual phenols were detected in the respective tested extracts and Wic-L and Uku-L extracts had higher total phenolic content compared to the Wic-S and Uku-S extracts.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The upsurge modern phytomedicine and ethnopharmacology of medicinal plants and their extracts has attracted the attention of researchers over the years [1], since their high phytochemical content is used as antitumor, antioxidant [2] and antifungal agents in pathogenesis [3]. Herbal therapies are often considered less toxic and induce less side effects than synthetic ones [4], and the World Health Organization estimates that up to 80% of the world’s population uses conventional medicines [5, 6], which as commonly used to alleviate diseases. Carya illinoinensis, commonly known as pecan can be beneficial for its abundant sources of unsaturated fatty acids, proteins [7] and the diverse bioactivities of secondary metabolites was found to be responsible for its therapeutic use as cytotoxic agents [8, 9] and antifungal agents [10].
In South Africa, industrial processing of C. illinoinensis is on the rise [11], however there is little or no knowledge of its ethnopharmacological use in the prevention and treatment of diseases such as diabetes, obesity, hypertension, hypercholesterolemia, cancer, and inflammatory diseases. Not to mention that phytopathogenic fungi such as Alternaria alternata, evidently known to cause Alternaria black spot (ABS) disease on C. illinoinensis in South Africa [12, 13] which in reduces the quality and production yield. Fungicides are used to manage the spread of the disease caused by A. alternata on various crops [14] and on C. illinoinensis ABS pathogen [15]. However, the hazardous effects of fungicides can be detrimental to human health and the environment. For example, Bastos et al. [16] used mice cells to demonstrate that non-azole agrochemicals used to improve crop productivity can induce permanent cross-resistance with clinical antifungals. It is worth developing cheaper and more environmentally compatible biofungicides to attenuate plant diseases [17]. Therefore, the increasing occurrence of degenerative and pathogenic diseases on humans and plants worldwide has stimulated the search for new sources of bioactive compounds, and alternative treatments are strongly encouraged.
Phytomedicinal extracts are considered safe for use as a potential natural agent in place of fungicides [18] and C. illinoinensis tissues has been shown to have promising application for control of phytopathogens, including Alternaria [19]. In addition, has exhibited strong antioxidant and anti-hyperglycaemic effects against streptozotocin (STZ)-induced diabetes in Sprague–Dawley mice [20] and streptozotocin-induced diabetic rats [21]. This association between the high intake of phenolic compounds from herbal sources and protection against different types of human diseases and phytopathogenic disease has been established but little or no information is available on the antifungal effects of acetone C. illinoinensis leaf and shuck extracts against A. alternata ABS pathogen and the effects of the extracts as cytotoxic agents.
In this study, the potential in vitro antifungal activity of acetone extracts (AEs) of leaf (L) and shuck (S) of two South African C. illinoinensis cultivars (cv.) Wichita (Wic) and Ukulinga (Uku) against A. alternata was investigated. Scanning electron microscope (SEM) images were used to corroborate changes in the cell membrane of A. alternata, possibly induced by crude extracts with high inhibitory activity. We determined the relevant phenolic acid composition and the total phenolic content in the leaves and shucks of Wic- and Uku-cv. using High-Performance Liquid Chromatography (HPLC). Finally, we investigated the in vitro cytotoxic effects of C. illinoinensis methanolic extracts (MEs) on normal human embryonic kidney 293 (HEK-293T) cell lines using MTT 3-(4,5dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide) assay commonly used to demonstrate cell viability after exposure to toxic substances [22].
2 Material and methods
2.1 Carya illinoinensis samples and crude extracts preparation
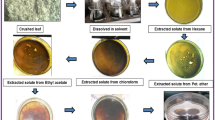
Fresh C. illinoinensis leaves and nuts-in-shucks of matured Wic-cv and Uku-cv trees were acquired from an orchard in Hartswater (S 27° 49′ 32.6 E 024° 50′ 11.7), Northern Cape province of South Africa in October 2022. The pecan samples were transported to the laboratory following due protocol [13]. Plant materials were surface-sterilised by washing with tap water, disinfected in 2% sodium hypochlorite (NaOCl) for 3–5 min, rinsed with sterilised distilled water (dH2O) for 2 min and then dried using laboratory tissue paper. Peeled shucks and leaves for each cultivar were dried and grounded into a fine powder by a blender (Fig. 1a–h). Plant crude extracts were performed by implementing a cold extraction method [23, 24], with solid/liquid separation techniques of acetone (99.5%) (Sigma-Aldrich, Johannesburg, South Africa) as extraction solvents. The homogenised plant materials (5 g) were soaked in their respective solvent (40 mL) and shaked for 72 h at room temperature in an orbital shaker (Heidolph-Instruments, Schwabach, Germany) (Fig. 1e). The plant extracts were filtered with a Buchner funnel and Whatman No. 1 filter paper (Fig. 1f). Filtrates were concentrated at 35 ± 2 °C using a shaker incubator (Labotec IncoShake, Gauteng, South Africa) at a speed of 25 rpm (Fig. 1g, h) and stored at − 20 °C until use.
South African Carya illinoinensis leaves and shuck of Wichita and Ukulinga cultivars used in preparing crude extracts. a Shuck samples. b Leaves samples. c Blending process. d Fine powder samples. e Orbital shaker process of the samples soaked in the respective solvents. e Filtration of the homogenised samples. g Shaker incubator to concentrate sample filtrates. h Residue crude extracts
2.2 Antifungal activity assay
Three A. alternata isolates (CGJM3006, CGJM3078, CGJM3142), shown to cause ABS disease in a previous study [13], were used. The cultures are maintained in the culture collection of Gert Johannes Marais (CGJM), Department of Plant Sciences, University of the Free State, South Africa. Cultures of each isolate were inoculated in 45 mL Potato dextrose broth (PDB) media and placed in a shake-incubator (Apex-Scientific, Gauteng, South Africa), at a speed of 25 rpm for 21 days. Spore concentrations were adjusted to (1 × 106 spores/mL) using a hemocytometer [25], corresponding to the standardised number of viable spore counts per grid required for filamentous fungi.
The in vitro antifungal activity screening for the crude extracts was investigated using a well-diffusion method [26]. The PDB media (5 mL) with conidial suspension of A. alternata were mixed with potato dextrose agar (PDA) medium (50 °C) and poured in separate 90 mm Petri plates (Lasec, Bloemfontein, South Africa). Plates were left under a laminar air flow system to solidify. The PDA plates were bored (5 mm) using a sterile plug borer and inoculated with 60 μL of each crude extract at different concentrations (60, 70, 80 and 90 mg/mL in sterile dH2O). Sodium hypochlorite (5% NaOCl) was used as positive control, while sterile dH2O was used as the negative control for each plate. The treatment was performed in triplicate. Test plates were incubated in a Labcon LTGC-M40 incubator (Labcon, Gauteng, South Africa) for 7 days at 25 ± 1 °C under 12 h alternating cycles of Near Ultra-Violet (NUV) light and darkness.
The diameter (mm) of the zone of inhibitions of the crude extracts and the positive controls on fungal growth were measured using ImageJ v. 1.53 software [27]. Two orthogonal diameter measurements of the plate (200 mm) were calibrated as the scale standard of the images. The inhibition zone diameters were determined at 0° and 90° measurement, and the mean diameter values were used consequently. The values from the respective wells were subtracted from the zone of inhibition of the crude extracts to obtain standardised sizes of the inhibition zones.
2.3 Scanning electron microscopy
A representative culture of A. alternata isolate (CGJM3006) was treated with 90 mg/mL of AEs derived from leaves and shucks of Wic-cv and Uku-cv showing the largest zone of inhibition were examined by scanning electron microscopy. Small blocks (5 mm) of agar containing the edge of zones of inhibition and the untreated (negative control) mycelial growth zones were aseptically cut out from the treated Petri plates. The samples were fixed in 3% glutaraldehyde (Sigma Aldrich, St. Louis, Missouri, United States), rinsed in 0.1 M (pH 7.0) sodium phosphate buffer solution for 3 h, and post-fixed in 1% osmium tetroxide (OsO4) for 1 h. The samples were placed on 0.2 µm polycarbonate membrane filters and dehydrated in a graded ethanol series (50%, 70%, and 95%) for 20 min in each phase, followed by two changes in 100% for 1 h in each phase. Samples were dried using a critical point dryer (Tousimis, Maryland, USA), mounted on stubs (Cambridge pin type, 10 mm) using double sided carbon tape and gold coated (± 60 nm) with a Bio-Rad sputter coater (Bio-Rad, Oxfordshire, United Kingdom). Specimens were examined and analysed with a JSM-7800F Extreme-resolution Analytical Field Emission Scanning Electron Microscope (JEOL Ltd, Tokyo, Japan) at the Centre for Microscopy, University of the Free State, South Africa.
2.4 Phenolic acid composition and total phenolic content
HPLC analyses for MEs of leaves and shucks of Wic-cv and Uku-cv were carried out using an Agilent 1260 Infinity HPLC series (Agilent Technologies, Santa Clara, CA, USA), equipped and separated with a quaternary pump and a Zorbax Eclipse plus Eclipse C18 column (4.6 mm × 250 mm i.d., 5 μm). The column temperature was maintained at 40 °C. The mobile phase consisted of HPLC grade water (solution A) and 0.05% trifluoroacetic acid in acetonitrile (solution B) at a flow rate 1 mL/min. The mobile phase was programmed consecutively in a linear gradient as follows: 0 min (82% A); 0–5 min (80% A); 5–8 min (60% A); 8–12 min (60% A); 12–15 min (82% A); 15–16 min (82% A), and 16–20 min (82% A). The injection volume was 5 μL for each of the sample solutions. The multiwavelength detector was monitored at 280 nm. The 17 standard phenolic compounds used were gallic acid, chlorogenic acid, catechin, methyl gallate, coffeic acid, syringic acid, pyrocatechol, rutin, ellagic acid, coumaric acid, vanillin, ferulic acid, naringenin, quercetin, cinnamic acid, kaempferol, and hesperetin (Sigma-Aldrich, Cairo, Egypt).
Total phenolic content (TPC) in the four extracts was determined by a FluoStar Omega Microplate Reader (BMG Labtech, Kaliobyea, Egypt), according to the minor modification of Microplate method [24]. Briefly, a methanol extract (3 mg/mL) of the samples were prepared. Separately, gallic acid standard stock solution of 1 mg/mL was prepared in methanol and 9 serial dilutions were subsequently prepared in the concentrations of (12.5, 25, 50, 100, 200, 400, 500, 800 and 1000 μg/mL). The TPC was determined by comparing absorbance of the samples against a standard curve of gallic acid (µg/mL) equivalents per gram (mg GAE/g) of dry extract, thus, the average absorbance was recorded in three replicates.
2.5 Cytotoxicity assay
Cell viability for cytotoxicity against normal human embryonic kidney cells (HEK-293T) was assessed by the mitochondrial-dependent reduction of yellow MTT to purple formazan by following standard protocol for MTT assay [22]. The HEK-293T cells were obtained from American Type Culture Collection (ATCC, Manassas, VA, USA) were suspended in DMEM-F12 medium, 1% antibiotic–antimycotic mixture (10,000 U/mL Potassium-penicillin, 10,000 µg/mL Streptomycin sulphate and 25 µg/mL Amphotericin B), and 1% l-glutamine at 37 °C in a 5% CO2 incubator.
Cells were batch grown for 10 days, then seeded in 96-well plates at a density of 10 × 103 cells/well, in complete growth medium, and incubated at 37 °C for 24 h in a 5% CO2 incubator. Medium was aspirated off and serum deprived fresh medium was added to negative control (treated with DMSO) or treated with individual samples of Pecan cultivars (WS, WL, US, UL) to a final concentration range of (1.56, 6.25, 12.5, 25, 50, and 100 µg/mL). After 48 h of incubation, medium was aspirated, 40 µL MTT salt (2.5 μg/mL) was added to each well. Plates were incubated four hours at 37 °C supported with 5% CO2. The 200 μL of 10% sodium dodecyl sulphate (SDS) in deionized water was added to each well to stop the reaction, then the plates were incubated overnight at 37 °C. Doxycycline (100 µg/mL) was used as a positive control to obtain 100% lethality under the same conditions [28]. Absorbance at 595 nm and reference absorbance at 620 nm was measured using a microplate reader (Bio-Rad Laboratories Inc., model 3350, Hercules, California, USA). The mean percentage of change in viability was calculated according to the formula:
2.6 Statistical analyses
Data for mycelial growth inhibition (mm) and total phenolic content were presented as means ± standard deviations (triplicate values). Data were subjected to three-way ANOVA to show the interaction effects of Alternaria alternata isolate, cultivar (Wichita and Ukulinga tissues) and the concentration of extracts using R version 4.1.0 [29] within R-Studio v. 1.3.959 [30], and Fisher’s LSD test (p = 0.05) function from the ‘agricolae’ [31] and ‘doebioresearch’ (Analysis of Factorial Randomised Block Design for 3 factors) [32] packages were used to determine the significant level of the data.
The data of cell viability for cytotoxicity were subjected to probability analysis to determine LC50 and LC90 using IBM SPSS program, version 11.0, while the independent t-test was employed to analyse the significant differences between negative control and samples.
3 Results
3.1 Antifungal activity effects
Analyses of variance for the antifungal activity of AEs of leaf and shuck showed a significant interaction between the A. alternata isolate, cultivar (Wichita and Ukulinga tissues), and concentration of extracts (p < 0.001) (Tables 1, 2, Fig. 2, and Supplementary Table S1). Negative controls (dH2O) had no antifungal effect with 0% inhibition of mycelial growth and differed significantly (p < 0.001) from positive controls (5% NaOCl) and the AEs treatment. The extracts showed significant antifungal effects against the treated fungal isolates at different concentrations, where zones of inhibition increased consistently with increased concentrations (60–90 mg/mL) (Table 1). Leaf extracts for both cultivars differed significantly (p < 0.001) compared to the inhibition by shuck extracts, indicating better antifungal efficacy. Alternaria alternata isolate CGJM3006 was the most susceptible to AEs concentrations from 60 to 90 mg/mL (inhibition zones ranging from 11 to 39 mm), and significantly different (p < 0.001) from A. alternata CGJM3142 having zones of 8–36 mm (p < 0.001), and A. alternata CGJM3078 with slightly different zones of 5–17 mm (p < 0.001). The AEs of Wic-cv had zones of inhibition ranging from 8 to 39 mm, and were significantly different (p < 0.001) from those of Uku-cv, with inhibition zones from 5 to 29 mm.
Plates representing inhibition zones of acetone leaf and shuck extracts of Carya illinoinensis leaves and shucks (90 mg/mL) against Alternaria alternata isolate (CGJM3006). Black arrow heads indicate antifungal activities caused by the crude extracts, (+ve) indicates the bleach (NaOCl) positive control, and (−ve) the sterile distilled water that served as negative control
3.2 Scanning electron microscopy
The conducted SEM analysis showed structural damage to A. alternata conidia after treatment with pecan leaves and shucks AEs of both cultivars (Fig. 3a–f). Treated conidia showed plasmolysis and distorted, squashed, collapsed or rough conidial surfaces. The untreated A. alternata conidia were well-developed with a smooth conidial surface. Evidently, the results established a significant morphological alteration and damage between the untreated (control) and treated conidia.
Scanning electron microscopy (SEM) micrographs of Alternaria alternata conidia (CGJM3006) treated with acetone leaf and shuck extracts (90 mg/mL). a, b Untreated (control) fungal conidia. c Conidia treated with Wichita shuck extracts. d Conidia treated with Wichita leaf extracts. e Conidia treated with Ukulinga shuck extracts. f Conidia treated with Ukulinga leaf extracts. The arrow depicts collapsed or rough conidial surface. Scale bar = 1 μm
3.3 Phenolic acid composition and total phenolic content
Seventeen phenolic acids were detected (Fig. 4a–e) in the pecan leaf and shuck extracts of the South African C. illinoinensis cultivars. The most abundant phenolic compounds detected in an ascending order (Supplementary Table S2) in the leaf extracts of Wic-cv ranged from pyrocatechol (112.24 µg/17.5 mg) to the lowest in naringenin (14.09 µg/17.5 mg), and pyrocatechol (21.61 µg/17.9 mg) to chlorogenic acid (12.25 µg/17.9 mg) in the shuck extracts of Wic-cv. In the leaf extracts of Uku-cv, pyrocatechol (71.38 µg/17.4 mg) was the most abundant and coumaric acid (11.41 µg/17.4 mg) was the lowest detected. Whereas in the shuck extracts of Uku-cv, pyro catechol (41.14.38 µg/21.8 mg) was the most abundant phenolic compound and catechin (29.11 µg/21.8 mg) was detected the lowest.
HPLC chromatogram profiles of phenolic standards detected in leaf and shuck extracts of Carya illinoinensis cultivars. a Wichita leaf extracts. b Wichita shuck extracts. c Ukulinga leaf extracts. d Ukulinga shuck extracts. e Phenolic standards. f Gallic acid standards absorbance verses calibration curve for total phenolic content (TPC). HPLC profiles of the phenols were simultaneously detected at 280 nm of absorbance (mAU) versus 20 min retention time (minutes). The 17 phenolic compound standards are gallic acid (G-a), chlorogenic acid (CG-a), catechin (CAT), methyl gallate (meGAL), coffeic acid (COFF-a), syringic acid S-a), pyro catechol (PC), rutin (RUT), ellagic acid (E-a), coumaric acid (COU-a), vanillin (VA), ferulic acid (F-a), naringenin (NA), quercetin (QUA), cinnamic acid (C-a), kaempferol (KEM), and hesperetin (HES)
For the total phenolic content, the analytic curve showed linearity in the concentration range used as standard for gallic acid (Regression equation: y = 0.0036x − 0.1174 and R2 = 0.9917), where y absorbance at 630 nm and x total phenol in the extracts (Fig. 4f). Total phenolic content of the four extracts (Table 3) were expressed as gallic acid equivalent (GAE) and found higher in the leaf extracts of Wic-cv (102.19 mg GAE/g) and Uku-cv (110.13 mg GAE/g), with p = 2.6e−13. The shuck extracts of Wic-cv and Uku-cv were significant (p = 0.018) and showed relatively lower amounts (62.03 and 85.07 mg GAE/g), respectively.
3.4 Cytotoxicity effects of C. illinoinensis extracts on HEK-293T cells
The cytotoxicity effects of C. illinoinensis Wic-cv and Uku-cv MEs on HEK-293Tcell lines based on the MTT cell viability assay after 48 h are presented in Table 4 and Fig. 5. Cell viability decreased with increasing MEs concentrations (1.56–100 µg/mL) of leaves and shucks of Wic-cv, leaves of Uku-cv on the cells increased after 48 h incubation (Fig. 5). Accordingly, three MEs were toxic to the cells compared to doxycycline (positive control) and DMSO (negative control) (Table 4), with leaf extract of Wic-cv (LC50 = 56.0 µg/mL, LC90 = 93.4 µg/mL, and 88.2% at 100 pmm), shuck extract of Wic-cv LC50 = 63.2 µg/mL, LC90 = 110.0 µg/mL, and 72.2% at 100 pmm), leaf extract of Uku-cv (LC50 = 49.1 µg/mL, LC90 = 86.8 µg/mL, and 86.3% at 100 pmm) and thus, no toxic effects for shuck extract of Uku-cv (37.8% at 100 pmm) less than the LC50 and LC90 values.
Probability transformed response effects of methanolic extracts (MEs) of Carya illinoinensis cultivars on HEK-293T cells viability analysed by mitochondrial metabolic activity (MTT) assay. Cells were treated for 48 h with increased concentration of MEs of Wichita leafs (a), Wichita shucks (b), Ukulinga leafs (c), and no toxic effects for Ukulinga shucks. Data are represented as means ± standard deviations (n = 3)
4 Discussion
No data as yet have been published to support the efficacy of South African C. illinoinensis tissue extracts against A. alternata, besides data on other plant pathogens. In this study, the antifungal activity of AEs leaf and shuck extracts of C. illinoinensis Wic-cv and Uku-cv were evaluated against A. alternata isolates, causing ABS on C. illinoinensis in South Africa. The AEs demonstrated antifungal efficacy on the tested pathogen by inhibiting growth, resulting in plasmolysis and conidial distortion. In addition, the leaf and shuck extracts of both cultivars showed activity but leaves of Wic-cv were more effective. Phytochemical analysis of C. illinoinensis tissues of both cultivars showed the presence of phenols. The cytotoxicity of the cell viability effects of leaf and shuck MEs for both cultivars showed strong toxic cytotoxic effects on the HEK-293T cells, except for the Uku-cv shuck extracts with non-toxic effects.
In vitro antifungal activity showed that the different concentrations and crude extracts for the pecan cultivars exhibited varying levels of antifungal activity against the A. alternata isolates. Acetone crude extracts of Wic-cv and Uku-cv were efficient, demonstrating larger inhibition zones on A. alternata growth. A previous study, for instance, showed that the volatile oil extracts of pecan leaves from Wic-cv showed antifungal activity against Aspergillus flavus with inhibition zones of 10 mm [20]. The current study showed that acetone extracts inhibited the growth of the pathogen A. alternata. In addition, changes in the conidia suggest that the extracts may potentially be utilised as antifungal agents.
The efficacy of antifungal activity demonstrated by AEs could be attributed to the solubility properties of the solvent [33,34,35]. Altogether, we suggest that the antifungal activity of acetone plant extracts could depend on the polar constituents that contributed to the inhibitory effects on A. alternata [36]. Inhibitory effects of AEs against A. alternata have been previously reported. For example, Wianowska et al. [24] demonstrated that acetone extracts of Juglans regia strongly inhibited the mycelial growth of A. alternata. Similarly, acetone extracts of Allium, Datura and Zingiber [37] and Withania somnifera leaves showed strong antifungal activity against A. alternata [38].
Crude acetone extract showed structural damage to the A. alternata conidia, with shrunken, distorted, and perforated cell walls. The morphological aberrations of A. alternata conidia observed in this study and inhibition of spore formation could be due to the abundance of bioactive compounds in the leaf and shuck extracts of C. illinoinensis, which led to the anomalies observed in the SEM micrographs. These morphological alterations may be linked to disruptions in the morphogenesis and growth of A. alternata, as previously reported [39, 40]. Plant extracts enriched with phenolic compounds are lipophilic in nature and can easily diffuse through cell membranes, interfering with cellular stability [41]. The action mechanism allows these phenolic compounds to interfere with the ABC transporter system (ATP binding cassette) [39]. This may inhibit the synthesis of ergosterol, ATP and aminoacyl tRNA synthetase, leading to cell dysfunction and altered sterol metabolism, ultimately results in fungal cell leakage, plasmolysis, and cell lysis. Such suggested mechanisms need to be investigated in the future.
HPLC profile of phenolic acid composition showed that leaves of Wic-cv and Uku-cv had abundant phenolic contents contrary to shucks. This was based on retention time, standards and the peaks depicted in the HPLC chromatogram profiles. The most phenols detected in leaves of Wic-cv and Uku-cv, respectively, include pyrocatechol (112.24 µg/17.5 mg and 71.38 µg/17.4 mg), chlorogenic acid (74.01 µg/17.5 mg and 58.65 µg/17.4 mg), gallic acid (27.26 µg/17.5 mg and 29.67 µg/17.4 mg), catechin (20.21 µg/17.5 mg and 44.47 µg/17.4 mg), naringenin (14.09 µg/17.5 mg and 25.35 µg/17.4 mg), and coumaric acid (17.03 µg/17.5 mg and 11.41 µg/17.4 mg). Pyrocatechol or catechol (1,2-dihydroxybenzene), along with other phenolics such as chlorogenic, gallic, catechin, naringenin, and coumaric, can be found in different edible and medicinal plants, for instance, acai fruit oil [42], green tea [43], mushrooms [44], and roselle [45]. These phenolic compounds exhibit a wide range of biological and pharmacological activities in pecans such as, antiviral, antibacterial, anti-inflammatory, anticancer and antioxidant [46,47,48]. Further studies of these enzyme activities can be evaluated against the pathogen A. alternata and potentially biosynthesised as biofungicides [49]. Other phenols were detected at lower levels, and kaempferol and hesperetin were not present. Similar peaks of these phenols from pecan tissues have been previously reported from cultivars such as Cheyenne, Choctaw, Pawnee, Stuart, Summer, Ukulinga, Western, and Wichita [20, 50].
It is worth mentioning that the concentration of the total phenolic content (TPC) assay in leaves of Wic-cv (102.19 mg GAE/g) and Uku-cv (110.13 mg GAE/g) was higher than the shucks (62.03 mg GAE/g and 85.07 mg GAE/g) respectively. The variation in individual phenols and TPC concentrations for the samples could result from the abundance of secondary metabolites at different concentrations in plant parts, like leaves [10, 51, 52]. This may account for the difference or absence of phenols between the studied pecan leaf and shuck organs. These variations have been attributed to different extraction procedures and solvent polarities responsible for dissolving endogenous compounds of plants [35]. Phenolics are more soluble in polar organic solvents [53], hence methanol and acetone were selected as the extracting solvent for TPC evaluation [20, 54].
Phenolics are usually more lipophilic and are set to penetrate biological membranes or alter membrane functionality because of the lipophilicity [55, 56], whereas hydroxyl groups of phenolics can influence the cell membrane by decoupling oxidative phosphorylation. In addition, lipophilic phenolic compounds can build up in the phospholipid bilayer and alter plasma membrane permeability for cations such as K+ and H+, causing disruption of ion homeostasis and pH homeostasis. In light of this, the action of gallic acid (TPC) might also target and damage the cell membranes of the tested A. alternata pathogen in our study since the phenolic compound is a hydrophobic and lipophilic phenolic acid [57]. There is therefore no doubt, that these phenolic compounds are likely responsible for the antifungal activity against A. alternata in this study.
Several methods have been used to evaluate polyphenols in pecans [7]. Typically, high performance liquid chromatography (HPLC) analysis, specifically the Folin-Ciocalteu method is the most common, less costly, decrease runtime, and suitable for the determination of samples including phenolic compounds in plants [58]. Other than the types of phenolic compounds, the relative composition of these compounds are also given by HPLC analysis in the form of peak areas [59, 60]. Furthermore, the HLPC-microplate method has been described specifically for the determination of total phenolic compounds [61]. Moreover, this assay gives a short reaction time of 3 min and either gallic acid or tannic acid are used as a phenolic standard. Hence, the HLPC analysis can successfully determine the phenolic contents in pecan leaves and shucks, which are waste products that could be a high value raw material for the preparation of extracts or phenolic compounds.
Cytotoxicity studies performed with MTT using MEs leaf and shuck of Wic-cv, leaf extracts of Uku-cv showed stronger cytotoxic effects and was toxic to the HEK-293T cells, while the shuck extracts of Uku-cv lower cytotoxic effects and no toxic effects to the cells. This cytotoxic effects on HEK-293T cells may be attributed to the polyphenolic compositions of the C. illinoinensis methanolic extracts, and this agrees with previously reported studies, showing effects of MEs of peels from C. illinoinensis rich in polyphenols. In addition, cell prohibit mortality can be due to high concentrations of toxic bioactive compounds, including ellagic acid and gallic acid [62,63,64], as well as inorganic elements, whose safe dosage should be taken into consideration.
5 Conclusion
The need to find alternative applications to combat A. alternata pathogen infections on C. illinoinensis in South Africa has necessitated the current study. The observed efficacy of particularly leaves and shucks acetone extracts of Wic-cv and Uku-cv, indicates the possibilities of controlling the A. alternata pathogenesis. For instance, gallic acid and its derivatives (pyrogallic acid and syringic acid) inhibited the mycelial growth of A. solani and revealed dose-dependent fungistatic activities in vitro and suppressed the development of tomato early blight in vivo without any phytotoxic symptoms on treated tomato plants. Phenolic compounds are non-toxic to plants, but usually have toxic or lytic effect on pathogens, which reduces or inhibits infection. The application is inexpensive and could possibly eliminate the issues of synthetic molecules that are unsafe to the environment. Field trials to test the use in the field would be of great interest for future studies. Cytotoxicity study shed lights on the impact of extracts from C. illinoinensis leaves and shucks against normal HEK-293T cells. Interestingly, shucks extract of Uku-cv was non-cytotoxic, which would encourage its use as a natural bioactive product for therapeutic purpose to prevent cytotoxic events. However, further in vivo studies are needed to validate the use of Uku-cv shucks extract and conduct stringent screening for the effective compounds.
Data availability
The datasets used during the current study available from the corresponding author on reasonable request.
References
Pirintsos S, Panagiotopoulos A, Bariotakis M, Daskalakis V, Lionis C, Sourvinos G, Karakasiliotis I, Kampa M, Castanas E. From traditional ethnopharmacology to modern natural drug discovery: a methodology discussion and specific examples. Molecules. 2022;27:4060. https://doi.org/10.3390/MOLECULES27134060.
Qanash H, Yahya R, Bakri MM, Bazaid AS, Qanash S, Shater AF, Abdelghany TM. Anticancer, antioxidant, antiviral and antimicrobial activities of Kei apple (Dovyalis caffra) fruit. Sci Rep. 2022;12:1–15. https://doi.org/10.1038/s41598-022-09993-1.
Belmimoun A, Meddah B, Meddah ATT, Gabaldon J, Sonnet P. Antifungal activity of Myrtus communis and Zygophyllum album extracts against human pathogenic fungi. Eur J Biol Res. 2020;10:45–56.
Karimi A, Majlesi M, Rafieian-Kopaei M. Herbal versus synthetic drugs; beliefs and facts. J Nephropharmacol. 2015;4:30.
Reid AM, Oosthuizen CB, Fibrich BD, Twilley D, Lambrechts IA, Canha MN, Rademan S, Lall N. Traditional medicine: the ancient roots of modern practice. In: Medicinal plants for holistic health and well-being. London: Academic Press; 2018. p. 1–11. ISBN 978-0-12-812475-8.
WHO. WHO fungal priority pathogens list to guide research development and public health. Geneva: WHO; 2022. p. 1–48.
Atanasov AG, Sabharanjak SM, Zengin G, Mollica A, Szostak A, Simirgiotis M, Huminiecki Ł, Horbanczuk OK, Nabavi SM, Mocan A. Pecan nuts: a review of reported bioactivities and health effects. Trends Food Sci Technol. 2018;71:246–57. https://doi.org/10.1016/J.TIFS.2017.10.019.
Hilbig J, Policarpi PDB, Grinevicius VMADS, Mota NSRS, Toaldo IM, Luiz MTB, Pedrosa RC, Block JM. Aqueous extract from pecan nut [Carya illinoinensis (Wangenh) C. Koch] shell show activity against breast cancer cell line MCF-7 and ehrlich ascites tumor in Balb-C mice. J Ethnopharmacol. 2018;211:256–66. https://doi.org/10.1016/j.jep.2017.08.012.
Ribas LE, Baravalle ME, Gasser FB, Renna MS, Addona S, Ortega HH, Savino GH, Van de Velde F, Hein GJ. Extraction of phenolic compounds from the shells of pecan nuts with cytotoxic activity through apoptosis against the colon cancer cell line HT-29. J Food Sci. 2021;86:5409–23. https://doi.org/10.1111/1750-3841.15950.
Bottari NB, Lopes LQS, Pizzuti K, Filippi dos Santos Alves C, Corrêa MS, Bolzan LP, Zago A, de Almeida Vaucher R, Boligon AA, Giongo JL, et al. Antimicrobial activity and phytochemical characterization of Carya illinoensis. Microb Pathog. 2017;104:190–5. https://doi.org/10.1016/j.micpath.2017.01.037.
Otto I. SAPPA: algemene jaarvergadering en inligtingsdag 2021. SA Pecan. 2021;89:2–3.
Achilonu CC, Gryzenhout M, Marais GJ, Ghosh S. Differential detection of Alternaria alternata haplotypes isolated from Carya illinoinensis using PCR-RFLP analysis of Alt a1 gene region. Genes. 2023;14:1115–29. https://doi.org/10.3390/genes14051115.
Achilonu CC, Marais GJ, Ghosh S, Gryzenhout M. Multigene phylogeny and pathogenicity trials revealed Alternaria alternata as the causal agent of black spot disease and seedling wilt of pecan (Carya illinoinensis) in South Africa. Pathogens. 2023;12:672–91. https://doi.org/10.3390/pathogens12050672.
Yang LN, He MH, Ouyang HB, Zhu W, Pan ZC, Sui QJ, Shang LP, Zhan J. Cross-resistance of the pathogenic fungus Alternaria alternata to fungicides with different modes of action. BMC Microbiol. 2019;19:1–10. https://doi.org/10.1186/s12866-019-1574-8.
Achilonu CC, Gryzenhout M, Ghosh S, Marais GJ. In vitro evaluation of azoxystrobin, boscalid, fentin-hydroxide, propiconazole, pyraclostrobin fungicides against Alternaria alternata pathogen isolated from Carya illinoinensis in South Africa. Microorganisms. 2023;11:1691. https://doi.org/10.3390/MICROORGANISMS11071691.
Bastos RW, Freitas GJC, Carneiro HCS, Oliveira LVN, Gouveia-Eufrasio L, Santos APN, Moyrand F, Maufrais C, Janbon G, Santos DA. From the environment to the host: how non-azole agrochemical exposure affects the antifungal susceptibility and virulence of Cryptococcus gattii. Sci Total Environ. 2019;681:516–23. https://doi.org/10.1016/j.scitotenv.2019.05.094.
Karim H, Boubaker H, Askarne L, Cherifi K, Lakhtar H, Msanda F, Boudyach EH, Ait Ben Aoumar A. Use of Cistus aqueous extracts as botanical fungicides in the control of citrus sour rot. Microb Pathog. 2017;104:263–7. https://doi.org/10.1016/j.micpath.2017.01.041.
Breda CA, Gasperini AM, Garcia VL, Monteiro KM, Bataglion GA, Eberlin MN, Duarte MCT. Phytochemical analysis and antifungal activity of extracts from leaves and fruit residues of Brazilian savanna plants aiming its use as safe fungicides. Nat Prod Bioprospect. 2016;6:195–204. https://doi.org/10.1007/s13659-016-0101-y.
Kalbende SP, Dalal LP. Effect of plant extracts on book deteriorated fungal species. Nat Prod Res. 2019;33:997–1005. https://doi.org/10.1080/14786419.2016.1180602.
El Hawary SS, Saad S, El Halawany AM, Ali ZY, El Bishbishy M. Phenolic content and anti-hyperglycemic activity of pecan cultivars from Egypt. Pharm Biol. 2016;54:788–98. https://doi.org/10.3109/13880209.2015.1080732.
Abdallah HM, Salama MM, Abd-Elrahman EH, El-Maraghy SA. Antidiabetic activity of phenolic compounds from pecan bark in streptozotocin-induced diabetic rats. Phytochem Lett. 2011;4:337–41. https://doi.org/10.1016/j.phytol.2011.07.004.
Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. https://doi.org/10.1016/0022-1759(83)90303-4.
Da X, Nishiyama Y, Tie D, Hein KZ, Yamamoto O, Morita E. Antifungal activity and mechanism of action of Ou-Gon (Scutellaria root extract) components against pathogenic fungi. Sci Rep. 2019;9:1–12. https://doi.org/10.1038/s41598-019-38916-w.
Wianowska D, Garbaczewska S, Cieniecka-roslonkiewicz A, Dawidowicz AL, Typek R, Kielczewska A. Influence of the extraction conditions on the antifungal properties of walnut green husk isolates. Anal Lett. 2020;53:1970–81. https://doi.org/10.1080/00032719.2020.1725889.
Camacho-Fernández C, Hervás D, Rivas-Sendra A, Marín MP, Seguí-Simarro JM. Comparison of six different methods to calculate cell densities. Plant Methods. 2018;14:30–45. https://doi.org/10.1186/S13007-018-0297-4.
Wei QH, Cui DZ, Liu XF, Chai YY, Zhao N, Wang JY, Zhao M. In vitro antifungal activity and possible mechanisms of action of chelerythrine. Pesticide Biochem Physiol. 2020;164:140–8. https://doi.org/10.1016/j.pestbp.2020.01.007.
Abràmoff MD, Magalhães PJ, Ram SJ. Image processing with imageJ. Biophotonics Int. 2004;11:36–41. https://doi.org/10.1201/9781420005615.ax4.
Thabrew MI, Hughes RD, Mcfarlane IG. Screening of hepatoprotective plant components using a HepG2 cell cytotoxicity assay. J Pharm Pharmacol. 1997;49:1132–5. https://doi.org/10.1111/J.2042-7158.1997.TB06055.X.
R Core Team. R: a language and environment for statistical computing. R Foundation for Statistical Computing. 2020;2:1–3559.
RStudio Team. RStudio: integrated development environment for R. https://www.rstudio.com/. Accessed 17 June 2022.
De Mendiburu, F. Agricolae: statistical procedures for agricultural research. http://www.R-project.org. Accessed 27 June 2022.
Popat R, Banakara K. Doebioresearch: analysis of design of experiments for biological research. http://www.r-project.org/. Accessed 4 July 2021.
Achilonu CC, Davies A, Kanu OO, Noel CB, Oladele R. Recent Advances and Future Perspectives in Mitigating Invasive Antifungal-Resistant Pathogen Aspergillus Fumigatus in Africa. Curr Treat Options Infect Dis 2023, 15:1–20, https://doi.org/10.1007/s40506-023-00269-4.
Abubakar AR, Haque M. Preparation of medicinal plants: basic extraction and fractionation procedures for experimental purposes. J Pharm Bioallied Sci. 2020;12:1–10. https://doi.org/10.4103/jpbs.JPBS_175_19.
Altemimi A, Lakhssassi N, Baharlouei A, Watson DG, Lightfoot DA. Phytochemicals: extraction, isolation, and identification of bioactive compounds from plant extracts. Plants. 2017;6:42. https://doi.org/10.3390/plants6040042.
Saravanakumar D, Karthiba L, Ramjegathesh R, Prabakar K, Raguchander T. Characterization of bioactive compounds from botanicals for the management of plant diseases. Wallingford: Centre for Agriculture and Bioscience International (CABI); 2015. p. 1–18.
Mangwende E, Kritzinger Q, Truter M, Aveling TAS. Alternaria alternata: a new seed-transmitted disease of coriander in South Africa. Eur J Plant Pathol. 2018;152:409–16. https://doi.org/10.1007/s10658-018-1484-x.
Singh DP, Singh HB, Prabha R. Plant-microbe interactions in agro-ecological perspectives. Plant-Microbe Interact Agro-Ecol Perspect. 2017;2:1–763. https://doi.org/10.1007/978-981-10-6593-4.
Ambrico PF, Šimek M, Rotolo C, Morano M, Minafra A, Ambrico M, Pollastro S, Gerin D, Faretra F, De Miccolis Angelini RM. Surface dielectric barrier discharge plasma: a suitable measure against fungal plant pathogens. Sci Rep. 2020;10:3673–90. https://doi.org/10.1038/s41598-020-60461-0.
Otibi FA, Rizwana H. Chemical composition, FTIR studies, morphological alterations, and antifungal activity of leaf extracts of Artemisia sieberi from Saudi Arabia. Int J Agric Biol. 2019;21:1241–8. https://doi.org/10.17957/IJAB/15.1017.
Rizwana H, Bokahri NA, Alsahli SA, Al Showiman AS, Alzahrani RM, Aldehaish HA. Postharvest disease management of Alternaria spots on tomato fruit by Annona muricata fruit extracts. Saudi J Biol Sci. 2021;28:2236–44. https://doi.org/10.1016/j.sjbs.2021.01.014.
Pacheco-Palencia LA, Mertens-Talcott S, Talcott ST. Chemical composition, antioxidant properties, and thermal stability of a phytochemical enriched oil from Açai (Euterpe oleracea Mart.). J Agric Food Chem. 2008;56:4631–6. https://doi.org/10.1021/jf800161u.
Rababah TM, Hettiarachchy NS, Horax R. Total phenolics and antioxidant activities of fenugreek, green tea, black tea, grape seed, ginger, rosemary, gotu kola, and ginkgo extracts, vitamin E, and tert-butylhydroquinone. J Agric Food Chem. 2004. https://doi.org/10.1021/jf049645z.
Palacios I, Lozano M, Moro C, D’Arrigo M, Rostagno MA, Martínez JA, García-Lafuente A, Guillamón E, Villares A. Antioxidant properties of phenolic compounds occurring in edible mushrooms. Food Chem. 2011;128:674–8. https://doi.org/10.1016/j.foodchem.2011.03.085.
Chao C-Y, Yin M-C. Antibacterial effects of Roselle Calyx extracts and protocatechuic acid in ground beef and apple juice. Foodborne Pathog Dis. 2009;6:201–6. https://doi.org/10.1089/fpd.2008.0187.
Aree T. β-Cyclodextrin inclusion complexes with catechol-containing antioxidants protocatechuic aldehyde and protocatechuic acid—an atomistic perspective on structural and thermodynamic stabilities. Molecules. 2021;26:3574. https://doi.org/10.3390/MOLECULES26123574.
Naveed M, Hejazi V, Abbas M, Kamboh AA, Khan GJ, Shumzaid M, Ahmad F, Babazadeh D, FangFang X, Modarresi-Ghazani F, et al. Chlorogenic acid (CGA): a pharmacological review and call for further research. Biomed Pharmacother. 2018;97:67–74. https://doi.org/10.1016/J.BIOPHA.2017.10.064.
Taş NG, Gökmen V. Phenolic compounds in natural and roasted nuts and their skins: a brief review. Curr Opin Food Sci. 2017;14:103–9. https://doi.org/10.1016/j.cofs.2017.03.001.
Gwinn KD. Bioactive natural products in plant disease control. In: Studies in natural products chemistry, vol. 56. Amsterdam: Elsevier; 2018. p. 229–46.
Robbins KS, Gong Y, Wells ML, Greenspan P, Pegg RB. Investigation of the antioxidant capacity and phenolic constituents of US pecans. J Funct Foods. 2015;18:1002–13. https://doi.org/10.1016/j.jff.2015.05.026.
Feduraev P, Chupakhina G, Maslennikov P, Tacenko N, Skrypnik L. Variation in phenolic compounds content and antioxidant activity of different plant organs from Rumex crispus L. and Rumex obtusifolius L. at different growth stages. Antioxidants. 2019;8:237. https://doi.org/10.3390/ANTIOX8070237.
Popović Z, Milošević DK, Stefanović M, Vidaković V, Matić R, Janković J, Bojović S. Variability of six secondary metabolites in plant parts and developmental stages in natural populations of rare Gentiana pneumonanthe. Plant Biosyst. 2020;155:816–22. https://doi.org/10.1080/11263504.2020.1785966.
Eloff JN. Which extractant should be used for the screening and isolation of antimicrobial components from plants? J Ethnopharmacol. 1998;60:1–8. https://doi.org/10.1016/S0378-8741(97)00123-2.
Yang J, Zhou F, Xiong L, Mao S, Hu Y, Lu B. Comparison of phenolic compounds, tocopherols, phytosterols and antioxidant potential in Zhejiang pecan [Carya cathayensis] at different stir-frying steps. LWT Food Sci Technol. 2015;62:541–8. https://doi.org/10.1016/j.lwt.2014.09.049.
AL Zahrani NA, El-Shishtawy RM, Asiri AM. Recent developments of gallic acid derivatives and their hybrids in medicinal chemistry: a review. Eur J Med Chem. 2020;204: 112609. https://doi.org/10.1016/j.ejmech.2020.112609.
El-Nagar A, Elzaawely AA, Taha NA, Nehela Y. The antifungal activity of gallic acid and its derivatives against Alternaria solani, the causal agent of tomato early blight. Agronomy. 2020;10:1402. https://doi.org/10.3390/AGRONOMY10091402.
Zhao J, Khan IA, Fronczek FR. Gallic acid. Acta Crystallogr Sect E Struct Rep Online. 2011;67:1–24. https://doi.org/10.1107/S1600536811000262.
Lei Z, Kranawetter C, Sumner BW, Huhman D, Wherritt DJ, Thomas AL, Rohla C, Sumner LW. Metabolomics of two pecan varieties provides insights into scab resistance. Metabolites. 2018;8:56. https://doi.org/10.3390/metabo8040056.
Al-Huqail AA, Behiry SI, Salem MZM, Ali HM, Siddiqui MH, Salem AZM. Antifungal, antibacterial, and antioxidant activities of Acacia saligna (Labill.) H. L. Wendl. flower extract: HPLC analysis of phenolic and flavonoid compounds. Molecules. 2019;24:714. https://doi.org/10.3390/MOLECULES24040700.
El-hefny M, Salem MZM, Behiry SI. The potential antibacterial and antifungal activities extract, and the phenolic, caffeine, and flavonoid. Processes. 2019;8:1–13.
Attard E. A rapid microtitre plate Folin–Ciocalteu method for the assessment of polyphenols. Cent Eur J Biol. 2013;8:48–53. https://doi.org/10.2478/s11535-012-0107-3.
Porto LCS, Silva JD, Sousa K, Ambrozio ML, De Almeida A, Dos Santos CEI, Dias JF, Allgayer MC, Dos Santos MS, Pereira P, et al. Evaluation of toxicological effects of an aqueous extract of shells from the pecan nut Carya illinoinensis (Wangenh.) K. Koch and the possible association with its inorganic constituents and major phenolic compounds. Evid-based Complement Altern Med. 2016;2016:1–9. https://doi.org/10.1155/2016/4647830.
De la Rosa LA, Vazquez-Flores AA, Alvarez-Parrilla E, Rodrigo-García J, Medina-Campos ON, Ávila-Nava A, González-Reyes S, Pedraza-Chaverri J. Content of major classes of polyphenolic compounds, antioxidant, antiproliferative, and cell protective activity of pecan crude extracts and their fractions. J Funct Foods. 2014;7:219–28. https://doi.org/10.1016/j.jff.2014.02.008.
Guillén-Meléndez GA, Soto-Domínguez A, Loera-Arias MJ, Castillo-Velázquez U, Villa-Cedillo SA, Piña-Mendoza EI, Estrada-Castillón E, Chávez-Montes A, González-Alcocer A, Becerra-Verdín EM, et al. Effect of methanolic extract of Mimosa malacophylla A.Gray in Vero and HEK-293 cell lines, and in the morphology of kidney and bladder of rats with induced urolithiasis. J Ethnopharmacol. 2022;297: 115552. https://doi.org/10.1016/J.JEP.2022.115552.
Acknowledgements
The authors thank the South African Pecan Nut Producers Association (SAPPA) for financial support of this study. We also thank the Department of Genetics and the Department of Plant Sciences at the University of the Free State, South Africa, and the Department of Biochemistry and Nutrition at Ain Shams University, Egypt, for providing the laboratory facilities for the studies.
Plant guidelines
The authors confirm that the use of plants in the present study complies with international, national and/or institutional guidelines. Permissions to collect the plants/plant parts All plant specimens collected with permissions. Source of the plant used in our study All plants seeds name and the source of them are in Sect. 2.
Funding
This research was funded by the South African Pecan Nut Producers Association, (Grant No. UFS-ESD2020/0043/0611), affiliated to the University of the Free State, South Africa.
Author information
Authors and Affiliations
Contributions
CCA: conceptualization, wrote and edited the main manuscript text. CCA and DJ: investigator, methodology, visualiser, and data analyser. MG, MJG, SOH, and SG: read and edited the manuscript. MJG: project administration and funding acquisition. The authors approved the manuscript for publishing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Achilonu, C.C., Gryzenhout, M., Marais, G.J. et al. Antifungal activity of Carya illinoinensis extracts against Alternaria alternata pathogen and their cytotoxicity effects on HEK-293T cells: HPLC analysis of bioactive compounds. Discov Appl Sci 6, 67 (2024). https://doi.org/10.1007/s42452-024-05721-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-024-05721-8