Abstract
Integration of nanoparticles into polymeric materials enhances the optical properties and stability of the formed nano-composite materials. In this work, magnetic iron oxide nanoparticles Fe3O4NPs were prepared via chemical coprecipitation. In addition, it is polymerized in a host of polyvinylpyrrolidone PVP. The prepared iron oxide nano-size was about 14.0 nm as confirmed by Transmission Electron Microscopy, A vibrating sample magnetometer VSM studies showed Fe3O4NPs possess superparamagnetic behavior. Energy-dispersive X-ray spectroscopy, and X-ray diffraction confirmed the structure and composition of Fe3O4NPs. Open aperture Z-scan and optical limiting experiments were carried out at nanosecond pulses of wavelength 532 nm. The nonlinear absorption coefficients were calculated for the colloidal and polymeric iron oxide. Fe3O4NPs polymeric film exhibits enhanced nonlinear optical properties NLO, and superior optical limiting properties compared to Fe3O4NPs colloidal solution. That makes Fe3O4 nanocomposites a promising candidate for optical limiting applications.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
1 Introduction
Magnetic nanoparticles (MNPs) have potential applications such as catalysis, wastewater treatment, microwave devices, cancer therapy, magnetic pigments, and magnetic resonance imaging. Spinel ferrite nanoparticle materials, MFe2O4 (M = Zn2+, Fe2+, Cu2+, etc.), are highly preferred in numerous applications like information storage, biosensors, drug delivery, magnetic recording, drug delivery, and photonic applications [1, 2]. They crystallize in a cubic crystal structure and Fd3m space group [3]. The furthermost used magnetic nanomaterials are iron-based nanoparticles with low cost, nontoxicity, and high reactivity [4, 5].
Magnetic iron nanoparticles have gained significant attention in recent years due to their unique optical properties, particularly their nonlinear optical behavior. Nonlinear optical materials are essential for various applications, including optical limiting, imaging, and all-optical switching [2]. Nanomaterials with large NLO properties can be promising candidates for optical limiting. They have attracted considerable interest due to their use as imaging probes in multi-photon microscopy, optical switching, and optical limiters [4, 6, 7]. Recently there is a great development in the use of high-powered laser beams in daily life. The protection devices are necessary for protecting the eyes from high-power laser beams and for protecting optical devices like sensors from damage caused by interaction with high-power laser beams. The required materials for effective optical limiting have a strong linear optical transmittance, low optical limiting threshold, and high laser damage threshold [3].
Several studies have investigated the nonlinear optical properties of magnetic iron nanoparticles, including iron oxide nanoparticles [8,9,10,11], magnetic-plasmonic nanoparticles [9], and metamaterials [10] The third-order nonlinear optical properties of iron nanoparticles have been studied in water dispersions [11], and the nonlinear optical characteristics of magnetic nanoparticle colloidal suspensions have been explored using laser-induced clusters [12]. Noble metal nanoparticles, such as gold and silver, have also been investigated as nonlinear materials [13,14,15]. However, the use of magnetic iron nanoparticles as nonlinear optical materials is particularly attractive due to their ferrofluid properties and nonlinear optical activity [4, 11]. The synthesis and characterization of magnetic iron oxide nanoparticles have been extensively studied and their potential applications in optoelectronic devices have been explored [16, 17]. Z-scan technique has recently been used to detect a wide range of nonlinear optical materials [5]. The NLO properties of Fe3O4 nanoparticles are studied and the nonlinear parameters were calculated [18] using the standard model [19], Saravanan observed superior optical nonlinearity and optical limiting for zinc ferrite decorated reduced graphene oxide action under 532 nm continuous wave and 800 nm ultrafast laser [20]. Fe3O4 nanoparticles are recognized for their optical limiting effectiveness at nanosecond and femtosecond pulsed laser[21]. Some research articles have been published on the optical limiting of iron oxide nanomaterials, Abrinaei et al. and Gopal et al. illustrate reverse saturable absorption for PEGylated Fe3O4 ferrofluid nanoparticles.[22, 23]. Yang et al. has recorded switching from saturable absorption SA to reverse saturable absorption RSA in iron oxide nanoparticle suspension using picosecond lasers[24]. Mulenko observed high order third order nonlinearities for amorphous and crystalline Fe2O3 thin films prepared via pulsed laser deposition on silica substrates at temperatures (293 K and 800 K), respectively [25].
To the best of our knowledge, there are no reports for the optical limiting of iron oxide nanomaterial in polymeric form. For example, Yange et al. confirmed RSA for iron oxide nanoparticles colloidal. However, by depositing the particles as thin film RSA is no longer present[26]. This present work is important in two respects. It compares the nonlinear absorption and optical limiting of colloidal iron oxide and polymeric film of iron oxide nanoparticles. The optical limiting threshold and laser damage threshold are determined.
2 Materials and methods
2.1 Preparation of the magnetic iron oxide in colloidal and polymeric forms
2.1.1 Chemicals and reagents
Ferrous chloride tetrahydrate (FeCl2.4H2O)(Merck), Ferric chloride hexahydrate (FeCl3.6H2O) (Merck), Sodium Hydroxide (NaOH)(Merck), Hydrochloric acid (HCl)(MercK), Deionized water, Kerosine(Merck), Oleic acid extra pure(C18H34O2)(Loba Chemie), Ethylene Glycol(Fluka), Polyphenyl alcohol (PVA)(Fluka) and polyphenyl Propylene (PVP)(Fluka).
2.1.2 Synthesise of iron oxide
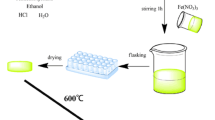
Fe3O4 magnetic ferrite nanoparticles have been prepared by the chemical co-precipitation method [27] as seen in Fig. 1. 4 mL of 1 M FeCl3 was mixed with 1 mL of 2 M FeCl2 4H2O during stirring. To eliminate particle-to-particle aggregation, 1 mL of Ethylene Glycol was added to the mixture during stirring. Afterward, 25 mL of 1 M NaOH was added slowly under vigorous stirring. Then, 0.5 mL of 0.1 M HCl was added. Black precipitation of the magnetite was produced as shown in the schematic presentation of Fe3O4 syntheses as seen in Fig. 1 where the product was separated by using a magnet. The resultant precipitate was washed by using deionized water twice and centrifuged at 1000 rpm for 15 min during that, the pH level was decreased from 12 to 7 approximately. Afterward, 1 mL of kerosene and deionized water were added to the product under stirring for 2 h at 30 °C. 5 mL of Oleic acid was added as a surfactant and coating material to the mixture.
To evaporate the liquid from the precipitation, the precipitated liquid was heated to 80 °C. after that, we cool it down to room temperature. We used distilled water for washing the precipitation to obtain chlorine and sodium-free particles and contaminants of the resulting and finally wash with ethanol for removing the excess surfactant. The beaker components were then centrifuged for 15 min at 4000 rpm to separate the supernatant liquid (ethanol plus water). After that, the supernatant liquid was decanted and centrifuged until only a black precipitate remained. To perform the XRD, a portion of this precipitate was dried at 100 °C for 10 h and ground into a fine powder.
2.1.3 Homogeneous Fe3O4- PVA colloidal solution
2.5 g of PVA was dissolved in 15 mL distilled water, then Fe3O4 NPs (0.001 g) were added to the PVA solution using ultrasonic agitation to ensure a homogenous distribution of particles through the colloidal. the open aperture Z-scan were studied for colloidal iron nanoparticles with concentation 0.07 mg/mL.
2.1.4 Fe3O4—PVP polymeric film fabrication
2.5 g of PVP was dissolved in 15 mL distilled water, then Fe3O4 NPs (0.005 g) were added to the PVP solution. The mixture solution was sonicated for 5 min. to ensure a homogenous distribution of particles. The mixture solution with concentration 0.33 mg/mL was dropped onto a piece of transparent glass to produce a Fe3O4-PVP film. The produced composite films were dried for 48 h at room temperature.
2.2 Characterization techniques
2.2.1 linear and nonlinear optical characterization
Visible absorption spectroscopy of the samples was studied in the 400–700 nm range by using Perkin Elmer Lambda 35 spectrometer. Figure 2 shows an open aperture Z-scan experimental setup, the sample’s nonlinear absorption coefficient values, and signs were investigated. utilizing 532 nm (2.33 eV) frequency-doubled Q-switched Nd: YAG laser of 6 ns pulse duration, low-repetition rate (1 Hz)., the laser energy was varied By using various neutral density filters from 118 to 750 µJ. This range of energies is below the optical breakdown threshold. The laser beam was focused using a 15 cm focusing lens, The sample was scanned through the focal field using a motorized stage (LTS 150 M THORLABS). Photo-diode detectors (S142C Thorlabs-PM320 E Thorlabs) were used to measure the transmittance and the reference beams.
The nonlinear absorption coefficient β can be determined by mathematical fitting of the experimental results of the transmittance signal via the open aperture as a function of sample location, Z, relative to the focal plane [19, 28].
where T is Normalized transmittance,
and Leff is the effective interaction length with the sample thickness L,
where αo is the linear absorption coefficient.
The imaginary part of third-order susceptibility χ (3) is calculated by the following equation [29] using the value of the nonlinear absorption coefficient:
where εo is the permittivity of free space, no is the linear refractive index of the material, λ is the laser wavelength, β is the nonlinear absorption coefficient, and c is the speed of light.
Intensity-dependent, Open Aperture Z -scan experiment (I-scan) was carried out by placing the samples at the focal point (Fig. 3). The relation between laser input energy and normalized transmittance gives for absorptive optical limiting OL characteristics.
2.2.2 Structure characterization
High-Resolution Transmission (HR-TEM, Tecnai G20, Super twin, double tilt, FEI, Netherland.) was used for imaging and size calculations. Investigations of X-ray diffraction (XRD) were verified at room temperature with Philips X-ray diffractometer (λ = 1.54056 Aº). Energy-dispersive X-ray spectroscopy (EDX) was used for extracting some information about a specific sample composition. EDX detector was installed as an integrated part of SEM. The used SEM model for this purpose was Qunta 250 FEG attached to the EDX unit. Using the scanning capability of the SEM, it is now able to create an elemental composition map. An EDX graph is often depicted as a count of X-rays as a function of energy in KeV. The graph identifies elements by their small peaks at specific energies.
2.2.3 Magnetic properties
A vibrating sample magnetometer (VSM) Lake Shore Model 7410 was used to analyze the magnetization of the synthesized nanoparticles. The magnetic characteristics of iron oxide nanoparticles were obtained from its hysteresis loop.
3 Results and discussion
For linear optical absorbance of magnetic ferrite nanoparticles Fe3O4 NPs; the supreme absorption was recorded at 300 nm wavelength as shown in Fig. 4a for colloidal and polymeric forms of Fe3O4 NPs. This absorbance corresponds to the crystal field d-d transition of Fe3+ [4] moreover, the linear absorption becomes minor or non-existent as the wavelength increases from 450 to 800 nm.
The TEM image of the Fe3O4 NPs shows that the sample is nearly spherical morphology with an average diameter of about 14.0 nm as shown in Fig. 4b. Energy-dispersive X-ray (EDX) analysis was used to determine the chemical compositions of iron oxide nanoparticles, Fig. 5 indicates the existence of iron as a significant metallic element. The presence of iron and oxide was evaluated using the EDX spectrum, where the weight percentage of iron and oxide were 62.48% and 37.51%, respectively as seen in Table 1.
The powder X-ray diffractogram of the magnetite powders used as cores, along with the labeling of the respective Miller indices. As shown in Fig. (6), the diffraction peaks at 18.4° (111), 30.2° (220), 35.5° (311), 43.1° (400), 53.6° (422), 57.1° (511), and 62.7° (440) are consistent with the presence of magnetite with cubic inverse spinel structure. The strongest peak in the XRD pattern correlates to the (311) crystallographic orientation of the spinel phase of Fe3O4 magnetic nanoparticles[30]. The observed miller indicators are in good match with the standard data of card numbers (ICDDPDF card number 04-006-6550). Table 2 shows the XRD results of iron oxide nanoparticles including the planes, d-spacing, and relative peak intensities.
By using a vibrating sample magnetometer (VSM), the magnetic characteristics of iron oxide nanoparticles were obtained from the hysteresis loop [18]. Figure 7 shows the magnetization curves for Fe3O4 NPs with a magnetic field ranging from -2000 to 2000 Oe. The hysteresis loop demonstrates ferromagnetic behavior with the saturation magnetization (Ms) value of about 54.929 emu/g, Coercivity (Hci) of 30.149 G, Retentivity (Mr) of 2.4763 emu/g, and the slope at Hc is about 80.853E-3 emu/(g G). The measurement was performed at room temperature. This demonstrates the superparamagnetic nature of magnetite nanoparticles. In our study, the value of saturation magnetization is consistent with the results of Wei et al., [31], who reported that the values of Fe3O4 saturation magnetization were 50.61, 61.36, 56.05, and 55.43 emu/g. In our study, The squareness ratio SQR (Mr/Ms) is 0.045, This squareness ratio value suggests the formation of a multi-domain structure with uniaxial anisotropy (SQR > 0.5) [1].
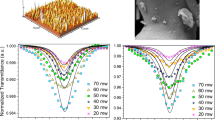
An open aperture Z- scan arrangement was performed for 14 nm iron oxide NPs in two forms, Fe3O4 colloidal solution and Fe3O4NPs polymeric film. The experiment was carried out for the Fe3O4 NPs colloidal sample at laser energies of 180, 223, 286, and 366 µJ, with peak intensities ranging from 2.84 × 1012 to 6.93 × 1012 W/m2 and for Fe3O4 NPs polymeric film at laser energies at 286,366 µJ with peak intensities 5.4 × 1012, 6.93 × 1012 W/m2. The nonlinear absorption measurements for the distilled water and polymeric film of pure PVP was conducted by using open aperture z- scan (Online Appendix 1), the results indicates that the reference materials did not exhibit any nonlinear optical absorptions.
When the sample was far from the focus, the transmittance is normalized to be equal unity and transmission is minimum at the focal point. The far-field normalized transmission was plotted as a function of the sample’s displacement around the focal point as shown in Fig. (8). The nonlinearity responses improve as the laser incident intensity increases. The open-aperture curve shows that transmittance is lowest at the focal point and gradually increases on both sides of the focus, showing the existence of reverse saturable absorption (RSA) in the sample. Figure 8a–d shows the nonlinear absorption of colloidal iron nanoparticles. It is clear that the depth of the valley increases as the energy is increased from 180 to 366 µJ. Furthermore, the nonlinear absorption of Fe3O4 NPs—PVP polymeric film shows the depth of the valley increases with energy from 286 to 366 µJ as presented in Fig. 8e,f). The data obtained from the fitting of the normalized transmittance by using Eq. (1) yielded the nonlinear absorption coefficient β(m/W). It is noted that β is increased by increasing the intensity of the incident laser as seen in Tables 3 and 4. This nonlinear behavior was compatible with Abrinaei et al. for iron oxide and PEGylated Fe3O4 nanoparticles nonlinear behavior [22].
The nonlinear absorption coefficient, and imaginary part of χ(3) for iron oxide NPs, where the average particle size was 14 nm were shown in Table 4. Several types of nanomaterials and the parameters of the Z-Scan experiment as collected from the literature were illustrated in Table 5. To understand the nonlinear absorption process of the prepared samples, realistic two photo absorption 2PA happens when nonlinear absorption coefficient remains constant for varying input intensity as weak 2PA cannot reduce the population of electrons in the ground state. Excited State absorption ESA is a nonlinear absorption process, which means that the rate of absorption is influenced by the incident light intensity [32]. However, in the ESA process, due to substantial depletion of ground-state population, the nonlinear absorption coefficient will either increase or decrease as a function of peak intensity. Reverse saturable absorption RSA happens as the absorption cross-section of the excited states rises relative to the ground state. Saturable absorption SA, on the other hand, occurs when the excited state's cross-section is smaller than that of the ground state. When pumped with a powerful laser beam, the system's transmittance will increase [33].
Singh et al. performed Z-scan study for iron oxide nanoparticles with nano and pico-second 532 nm laser pulses to discriminate between the electronic and thermal nonlinearity [34]. The damage threshold of a material is larger for picosecond pulses than for nanosecond pulses. Additionally, compared to nano-second pulses, pico-second pulses can achieve higher intensities with a smaller fluence. iron oxide nanoparticles’ RSA is strongly dependent on the laser pulse duration. Shorter pulse durations can lead to a higher degree of RSA, as the nanoparticles have less time to relax between the absorption of photons.
The samples were located at the center of the focal plane and exposed to a 532 nm nanosecond pulsed laser with different powers to obtain the optical limiting measurements as shown in Fig. 9. The linear transmittance of 27.7, 29.3% were found for colloidal and polymeric iron nanoparticles at low laser energies while polymeric Fe3O4 NPs exhibits enhanced optical limiting effect at higher laser energies, compared with colloidal Fe3O4 NPs.The incident energy level at which optical limiting activity starts is referred to as the optical limiting threshold, the optical limiting threshold is clearly found at 150, 230 µJ for colloidal and polymeric iron nanoparticles, respectively.Fe3O4–PVP polymeric film exhibits enhanced NLO and superior optical limiting behavior compared to Fe3O4–PVA colloidal solution. where β for the polymeric Fe3O4–PVP is enhanced. That makes Polymeric Fe3O4–PVP a promising nonlinear optical material due to its stability. It can be used in photonic applications.
4 Conclusion
Preparing magnetic iron oxide nanoparticles in various shapes for photonic applications is the major objective of the current study. The present study was designed to prepare magnetic Fe3O4NPS by chemical co-precipitation method; characterize the prepared materials; and assess their potential as optical limiting materials. The iron oxide NPs were prepared in two different forms: Fe3O4NPs-PVA colloidal solution and Fe3O4NPs-PVP polymeric film. For the prepared samples, the findings indicate that Fe3O4NPs have optical limiting features. Furthermore, the most obvious is the polymeric film of Fe3O4NPs has superior optical limiting performance. Future research should concentrate on customizing nanocomposites to improve photonic applications.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Almessiere MA et al (2019) Effect of Nb3+ substitution on the structural, magnetic, and optical properties of Co 0.5 Ni 0.5 Fe2O4 nanoparticles. Nanomaterials. https://doi.org/10.3390/nano9030430
Slimani Y et al (2019) Effect of bimetallic (Ca, Mg) substitution on magneto-optical properties of NiFe2O4 nanoparticles. Ceram Int 45(5):6021–6029. https://doi.org/10.1016/j.ceramint.2018.12.072
Slimani Y (2019) Microstructural, optical, and magnetic properties of vanadium-substituted nickel spinel nanoferrites. J Supercond Novel Magn 32:1057–1065
Ahmadi N, Poursalehi R, Kirilyuk A, Moravvej-farshi MK (2019) Applied surface science E ff ect of gold plasmonic shell on nonlinear optical characteristics and structure of iron based nanoparticles. Appl Surf Sci 479(January):114–118. https://doi.org/10.1016/j.apsusc.2019.02.073
Saravanan M, Girisun TCS, Venugopal Rao S (2017) Super-paramagnetic and unusual nonlinear absorption switching behavior of an: In situ decorated CdFe2O4-rGO nanocomposite. J Mater Chem C 5(38):9929–9942. https://doi.org/10.1039/c7tc02929b
Zhang XL et al (2011) Nonlinear optical and optical limiting properties of graphene oxide-Fe3O4 hybrid material. J Opt. https://doi.org/10.1088/2040-8978/13/7/075202
Zheng X, Feng M, Zhan H (2013) Giant optical limiting effect in Ormosil gel glasses doped with graphene oxide materials. J Mater Chem C 1(41):6759–6766. https://doi.org/10.1039/c3tc31314j
Singh CP, Bindra KS, Bhalerao GM, Oak SM (2008) Investigation of optical limiting in iron oxide nanoparticles. Opt Express. https://doi.org/10.1364/oe.16.008440
Brennan G, Ryan SM, Soulimane T, Tofail SAM, Silien C (2021) Dark field and coherent anti-stokes Raman (DF-CARS) imaging of cell uptake of core-shell, magnetic-plasmonic nanoparticles. Nanomaterials. https://doi.org/10.3390/nano11030685
Kruk S et al (2015) Enhanced magnetic second-harmonic generation from resonant metasurfaces. ACS Photonics. https://doi.org/10.1021/acsphotonics.5b00215
S. Couris (2009) Nonlinear optical response of water dispersions of iron oxide nanoparticles. doi: https://doi.org/10.1109/icton.2009.5185053.
Yang X, Li Q, Meng X, Li D (2020) Evolution of nonlinear optical characteristics of magnetic nanoparticle colloidal suspensions after laser-induced clusters. ACS Omega. https://doi.org/10.1021/acsomega.0c00609
Z. Zhou et al., (2010) Silver nanocrystals incorporated tellurite glass for highly nonlinear fiber Development. doi: https://doi.org/10.1109/acp.2010.5682559.
D. R. S. Dores et al., (2019) Effect of thermal treatment on morphological properties of borosilicates glass doped with silver. doi: https://doi.org/10.5151/ecfa2019-03.
Salah A, Mansour AS, Mohamed MB, Hassab-Elnaby S (2019) Nonlinear absorption and optical limiting of Ag–CdSe nano-hybrids of different growth times. Optik (Stuttg) 181:278–286. https://doi.org/10.1016/j.ijleo.2018.12.092
Romero-Jaime AK, del Acosta-Enríquez M C, Vargas-Hernández D, Tánori-Córdova JC, Pineda-León HA, and Castillo SJ (2021) Synthesis and characterization of silica-lead sulfide core shell nanospheres for applications in optoelectronic devices. doi: https://doi.org/10.21203/rs.3.rs-237861/v1.
Schwaminger SP, Syhr C, Berensmeier S (2020) Controlled synthesis of magnetic iron oxide nanoparticles: magnetite or maghemite? Crystals. https://doi.org/10.3390/cryst10030214
Nadafan M, Sabbaghan M, Sofalgar P, Anvari JZ (2020) Comparative study of the third-order nonlinear optical properties of ZnO/Fe3O4 nanocomposites synthesized with or without Ionic Liquid. Opt. Laser Technol. 131:106435. https://doi.org/10.1016/j.optlastec.2020.106435
Sheik-Bahae M, Said AA, Wei TH, Hagan DJ, Van Stryland EW (1990) Sensitive measurement of optical nonlinearities using a single beam. IEEE J Quantum Electron 26(4):760–769. https://doi.org/10.1109/3.53394
Saravanan M, Sabari Girisun TC, Vinitha G, Venugopal Rao S (2016) Improved third-order optical nonlinearity and optical limiting behaviour of (nanospindle and nanosphere) zinc ferrite decorated reduced graphene oxide under continuous and ultrafast laser excitation. RSC Adv 6(94):91083–91092. https://doi.org/10.1039/c6ra21428b
Nair SS, Thomas J, SuchandSandeep CS, Anantharaman MR, Philip R (2008) An optical limiter based on ferrofluids. Appl. Phys. Lett. 92(17):171908. https://doi.org/10.1063/1.2919052
Abrinaei F, Naseroleslami M (2018) Nonlocal nonlinear optical response of PEGylated superparamagnetic Fe3O4 nanoparticles. Opt Laser Technol 106:327–334. https://doi.org/10.1016/j.optlastec.2018.04.033
Gopal SV, Chitrambalam S, Joe IH (2018) Coherent source interaction, third-order nonlinear response of synthesized PEG coated magnetite nanoparticles in polyethylene glycol and its application. Opt Laser Technol 98:84–91. https://doi.org/10.1016/j.optlastec.2017.07.047
Yang X, Li D, Meng X, Li Q, Zhang H, Peng J (2021) Tunable nonlinear optical properties in magnetic nanoparticles suspension under picosecond laser excitation. Opt Laser Technol 142:107260. https://doi.org/10.1016/j.optlastec.2021.107260
Mulenko SA, Rudenko VI, Liakhovetskyi VR, Brodin AM, Stefan N (2016) Large third-order optical nonlinearities in iron oxide thin fi lms synthesized by reactive pulsed laser deposition. Opt Mater (Amst) 60:123–127. https://doi.org/10.1016/j.optmat.2016.07.017
Yang X, Li Q, Meng X, Li D (2020) “Evolution of nonlinear optical characteristics of magnetic nanoparticle colloidal suspensions after laser-induced clusters. ACS Omega. https://doi.org/10.1021/acsomega.0c00609
Wan J, Li H, Chen K (2009) Synthesis and characterization of Fe3O4@ZnO core-shell structured nanoparticles. Mater Chem Phys 114(1):30–32. https://doi.org/10.1016/j.matchemphys.2008.10.039
Sheik-Bahae M, Hasselbeck MP (2000) Third Order Optical Nonlinearities. Handb Opt 4:16
Kinsey N et al (2015) Effective third-order nonlinearities in metallic refractory titanium nitride thin films. Opt Mater Express 5(11):2395. https://doi.org/10.1364/ome.5.002395
Venkateswarlu S, Natesh Kumar B, Prasad CH, Venkateswarlu P, Jyothi NVV (2014) Bio-inspired green synthesis of Fe3O4 spherical magnetic nanoparticles using Syzygium cumini seed extract. Phys B Condens. Matter 449:67–71. https://doi.org/10.1016/j.physb.2014.04.031
Wei Y, Han B, Hu X, Lin Y, Wang X, Deng X (2012) Synthesis of Fe3O4 nanoparticles and their magnetic properties. Procedia Eng 27(2011):632–637. https://doi.org/10.1016/j.proeng.2011.12.498
Fang LB, Pan W, Zhong SH, Shen WZ (2017) Nonresonant and resonant nonlinear absorption of CdSe-based nanoplatelets. Chinese Phys Lett 34(9):5–10. https://doi.org/10.1088/0256-307X/34/9/098101
Salah A, Mansour A, Mohamed MB, Azzouz IM, Elnaby S, Badr Y (2015) Effects of nanoparticles size and concentration and laser power on nonlinear optical properties of Au and Au–CdSe nanocrystals. Appl Surf Sci 353:112–117. https://doi.org/10.1016/j.apsusc.2015.06.060
Singh CP, Bindra KS, Bhalerao GM, Oak SM (2008) Investigation of optical limiting in iron oxide nanoparticles. Opt Express 16(12):8440. https://doi.org/10.1364/oe.16.008440
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). The authors did not receive support from any organization for the submitted work.
Author information
Authors and Affiliations
Contributions
Conceptualization: [YB], Materials’ preparation and Investigation: [SH], measurement of the nonlinear absorption and analysis: [SH], Writing—original draft preparation: [AS, SH]; Writing—review and editing: [AS], supervision: [YB, SH-E, AS]; Resources: [SH-E, AS], All authors reviewed and edited the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Helayl, S., Hassab-Elnaby, S., Badr, Y. et al. Nonlinear optical absorption and optical limiting of magnetic iron oxide nanomaterials. SN Appl. Sci. 5, 229 (2023). https://doi.org/10.1007/s42452-023-05449-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-023-05449-x