Abstract
Abstract
Agricultural, vector-control and industrial activities around Lake Hawassa pose a risk of organochlorine contamination of the lake biota. To assess organochlorine contamination, we measured levels of organochlorine pesticides (OCPs) and polychlorinated biphenyls (PCBs) in 3 species of carnivorous waterbird and 3 species of fish. A total of 50 samples of fish and bird species sampled from Lake Hawassa in 2019. We investigated factors influencing accumulation of OCPs and PCBs. Reproductive risk associated with tissue levels of 4,4’-dichloro-diphenyl-dichloro-ethylene (p,p’-DDE) is also estimated. Results show that dichloro-diphenyl-trichloroethane (DDT) is the dominant contaminant found in both bird and fish species. p,p’-DDE is the dominant DDT metabolite in both bird and fish species. Geometric mean of p,p’-DDE varied from 49.8–375.3 and 2.2–7.7 ng g−1 ww in birds and fish, respectively. Average p,p’-DDE level in birds is 33.3 times higher than in fish. p,p’-DDE constitutes 93.4–95.2% of total DDTs in bird species. Degree of exposure, chemical stability, and resistance to environmental and biological degradation could explain higher levels of p,p’-DDE both in bird and fish species. There is significant variation in p,p’-DDE levels among bird and fish species owing to differences in feeding habits, foraging habitat, and lipid content. An increase in DDT levels with increasing size is observed in both bird and fish species. A significant positive association between log-transformed p,p’-DDE, and stable nitrogen isotope ratio (δ15N) values is found. There is no reproductive health risk in bird species as a result of the current levels of p,p’-DDE.
Article Highlights
-
DDT is the dominant contaminant found in both bird and fish species
-
There is interspecies variation in accumulation of p,p’-DDE among fish and bird species
-
p,p’-DDE is biomagnified through food chain involving both bird and fish species
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Persistent organic pollutants (POPs) are synthetic chemicals with long environmental and biological half-lives, long-range transport capacity, and lipophilic property [1]. POPs accumulate in fatty tissues of organisms and biomagnify through the food chain reaching higher concentrations in organisms occupying higher trophic levels [2]. At present 30 POPs listed under the Stockholm convention including the first 12 legacy POPs [3]. Organochlorine pesticides (OCPs) and polychlorinated biphenyls (PCBs) constitute ten of the first 12 legacy pollutants listed under the Stockholm convention. Before they were banned by the Stockholm convention, OCPs and PCBs have been used for agricultural pest control and industrial purposes, respectively [4]. Despite their ban, OCPs and PCBs are still detected in virous environmental and biological matrices [5]. Dichloro-diphenyl-trichloroethane (DDT) is a prevalent environmental contaminant owing to its widespread use in the past as agricultural pest and disease vector control agent. DDTs have been shown to cause eggshell thinning that leads to reproductive failure in birds [6]. It also affects reproduction and growth in fish by interfering with the normal functioning of the endocrine system [7]. PCBs are known to cause impairments of reproductive behavior in birds, depressing courtship songs and nest attentiveness [8, 9].
Ethiopia signed and ratified the Stockholm convention in 2002 [10]. Even though the convention entered in to force in 2004 [4], it is only recently (in 2009) Ethiopia discontinued the use of DDT for agricultural purposes [11]. Moreover, the use of DDT is still continued for indoor residual spray (IRS) for the control of malaria vector mosquitoes [11]. OCPs environmental contamination also emanates from improperly stored obsolete pesticide stocks found distributed in different parts of the country [12]. Obsolete pesticide stocks are usually diverted to illegal distribution and use for agricultural purposes [12]. PCBs are also potential threats of environmental contamination due to the widespread presence of PCB-containing operational and worn-out high voltage transformers and capacitors [10]. Improper and open field storages of worn-out electrical equipment containing PCB are common in the country [13]
Aquatic systems are destinations to environmental pollutants through run-off water from agricultural lands and municipal waste dumping sites. Lake Hawassa is surrounded by both agricultural fields and Hawassa City. Run-off water from the surrounding vegetable, tobacco farms and municipal wastes could be sources of OCP and PCB contamination [14]. The lake is also surrounded by several industries releasing their effluents directly into the lake and into the tributary river. Referral hospital, breweries, beverages, and textile factories are some of the industries whose effluents end up into the lake [14,15,16]. Despite the presence of OCP and PCB exposure threats to the local biota, exposure and risk assessment studies are generally scarce and none for bird species.
Carnivorous waterbird species have been used as indicator species for biomonitoring of environmental POP contamination owing to their top trophic position [17]. Top-trophic level birds are susceptible for exposure and accumulation of POPs through biomagnification [18]. Declines in hatching success in carnivorous bird species as a result of high accumulation of 4,4’-dichloro-diphenyl-dichloro-ethylene (p,p’-DDE) have been documented [6]. In aquatic habitats and lake environments, the main route of contamination for carnivorous waterbird species is a diet of fish [19]. Fish as a possible source and route of DDT contamination to humans have been investigated [20,21,22]. However, fish as a key route for contamination of wildlife, particularly, carnivorous waterbird species have not been investigated from the present study site. To our knowledge, this is the first study to investigate levels of OCPs and PCB in carnivorous waterbird and fish species from Lake Hawassa.
The present study aims to assess the accumulation and risk associated with OCPs and PCB in carnivorous waterbird and fish species from Lake Hawassa. We test the following hypotheses: (1) Carnivorous waterbird species would accumulate higher OCP and PCB levels than fish species owing to their higher trophic position. (2) Birds and fish with larger sizes would accumulate higher levels of OCPs than those with smaller sizes.
In the next section, we described the study area, bird and fish sampling, sample preparation and chemical analysis methods. Section 3 shows the levels of OCPs and PCBs in muscle tissues of bird and fish species, and discusses factors affecting accumulation of OCPs, including reproductive risk associated with accumulation of p,p-DDE. In Sect. 4, we present the concluding remarks of the study. Finally, Sect. 5 shows the literature cited.
2 Materials and methods
The samples used in the present work have been treated by the same method of lipid content determination, stable isotope analysis, sample preparation, chemical analysis and quality control methods as described in the authors published work [23]. Here we present the summary of the methods involved. The adoption of the methods is based on the similarity of target analytes, and tissue type.
2.1 Study area
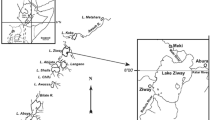
Lake Hawassa is one of the Ethiopian Rift Valley (ERV) lakes found in Sidama region, Ethiopia. It is located along the side of Hawassa city some 275 km away from the capital city Addis Ababa. The lake lies between 7.0313° N latitude and 38.4219° E longitude (Fig. 1). The lake is situated at an elevation of 1,680 m above sea level and has a surface area ranging from 85 to 90 km square. The Lake has a maximum and minimum depth of 22 and 11 m, respectively. The lake receives inflow from the Tikur Wuha River but has no surface outflow. The lake provides economic benefits to local people through ecotourism, recreation, and fishery.
Maps of Lake Hawassa (modified from Dsikowitzky [26])
Large numbers of waterbird species, including local and Palearctic migrant birds occur in the Lake. The lake is known for harboring the largest population of Marabou Stork (Leptoptilus crumeniferus) in Ethiopia [24]. Six species of fish occur in the lake [25]. Nile tilapia (Oreochromis niloticus), African sharp-tooth fish (Clarias gariepinus), and African big barb (Barbus intermedius) constitute the most common fish species.
2.2 Sampling
Permission was obtained for bird capture and sampling from Ethiopian Wildlife Conservation Authority (EWCA), Addis Ababa (Ref. No.: Wl. 32/318/2011). A total of 20 individual birds belonging to three species and 30 individuals of fish belonging to three species were sampled from the site. The bird species sampled were marabou stork (Leptoptilos crumeniferus), African sacred ibis (Threskiornis aethiopicus), and hamerkop (Scopus umbretta). All bird species are carnivorous, mainly consuming fish and fish scrapes in the local habitat [27,28,29]. The fish species sampled were African big barb (Barbus intermedius), Nile Tilapia (Oreochromis niloticus), and African sharp-tooth fish (Claris gariepinus). Birds were captured using traditional bird traps, then euthanized. Fish samples were purchased from the fresh catch of local fishermen. All bird and fish samples were transported to the laboratory for morphological measurement and excision of muscle samples. For birds, weight and wing chord length were recorded (Table 1). For fish, total length and weight measurements were recorded (Table 2). Bird morphological measurements were taken following the procedure described by Winker [30].
Individual bird and fish samples were dissected and 100 g of pectoral muscles and fillets were excised, respectively, for OCPs, PCBs, and stable isotope analysis. Excised bird and fish muscle samples were wrapped with aluminum foil, stored in a labeled zipper plastic bag and were frozen at − 18 °C. Then all bird and fish muscle samples were transported to the Norwegian Institute for Bioeconomy Research (NIBIO) laboratory for contaminant analysis.
2.3 Stable isotope analysis
The analysis of nitrogen (15N and 14N) and carbon (13C and 12C) isotopes was performed following method described in Ayele et al. [23]. The analysis method generally included homogenization of 1 g muscle tissue and freeze-drying. Freeze-dried sample was combusted in a Flash Elemental Analyzer and stable isotopes were determined by a continuous Flow-Infrared Mass Spectrometer. The isotopic ratios (15N/14N 13C/12C) were expressed in delta-values as follows: δ15N and δ13C(‰)\(=\left[\left(\mathrm{RSample}/\mathrm{RStandard}\right)-1\right]x1000\), where, R = 15N/14N for δ15N or R = 13C/12C for δ13C [21].
2.4 Lipid content determination
Lipid was extracted using SOXTEC Auto Lipid extractor as described in detail by Ayele et al. [23]. Summary of the extraction process described as follows. Lipid content was determined using pooled and homogenized five-gram sample. The sample was mixed with sodium sulfate powder and loaded in the extraction unit. The extraction solution was a mixture of ethyl acetate:cyclohexane with 1:1 ratio. After the completion of extraction lipid content was determined gravimetrically [23].
2.5 Reagents and chemicals
Mixture of POP standards, analytical grade acetonitrile and triphenyl phosphate were obtained from Dr. Ehrenstorfer GmbH Augsburg, Germany. Purified water was obtained from a Milli-Q Gradient A10 water system (Millipore, Bedford, MA, USA). Citrate buffering salts consist of 4 g magnesium sulfate, 1 g sodium chloride, 0.5 g sodium citrate dibasic sesquihydrate and 1 g sodium citrate tribasic dihydrate prepacked in a 15 mL tubes were supplied from Sigma-Aldrich GmbH, Germany. Primary Secondary Amine (PSA) clean-up tube consists of 150 mg of PSA and 900 mg MgSO4 prepacked in 15 mL centrifuge tubes were supplied from Sigma-Aldrich GmbH, Steinheim, Germany. Enhanced Matrix Removal-lipid tubes (EMR-Lipid tube) and EMR-Lipid polishing tubes consist of MgSO4 were supplied from Agilent technologies.
2.6 Sample preparation and chemical analysis
Sample preparation and chemical analysis were carried out at Norwegian Institute of Bioeconomy Research (NIBIO), Department of Pesticides and Natural Products Chemistry. Sample preparation was performed by the modification of the method described by Anastassiades et al. [31]. The modified method used is described in detail in Ayele et al. [23]. The summary of the method is as follows. 5 g of thawed muscle sample was measured into 50 mL extraction tube. 10 mL of Milli-Q water, 10 mL of acetonitrile and 50 ng (50µL) triphenyl phosphate internal standard were added. After homogenization, citrate buffering salt was added into the sample to aid phase separation between the tissue, water, and acetonitrile. Then the sample was cleaned using PSA clean-up tube and EMR-Lipid tube. Supernatant resulting from the cleaning processes was transferred to EMR-Lipid polishing tubed for the removal of traces of water from the acetonitrile. Finally, 15 µl of the aliquot of the supernatant was used for contaminant analysis using gas chromatography-mass spectrometry (GC-MS). The contaminants analyzed include DDTs (p,p’-DDT, p,p’-DDE, p,p’-DDD, o,p’-DDT, o,p’-DDE, o,p’-DDD), Oxychlordane, cis-chlordane, trans-Chlordane, Endosulpha-alpha, Endosulphan-beta, Endosulphan-sulphate, Aldrin and Dieldrin and PCBs (PCB-28, PCB-52, PCB-101, PCB-118, PCB-138, PCB-153, PCB180). The specifications and operating mode of the GC-MS used were as described in Ayele et al. [23].
2.7 Quality control
Quality control has been carried out following the method described in Ayele et al. [23]. Reagent blanks and spiked blanks analysis were performed for quality control. A mixture of 10 mL Milli-Q water and 10 mL acetonitrile, serving as reagent blank, treated through all of the analysis procedure. Analysis results showed no target analytes in the blank samples. A multi-level calibration curve was made by spiking a mixture of POP standards to a blank matrix of 5 g chicken and 5 g cod muscle. The coefficient of determination (r2) for the calibration curve was ≥ 0.99. Five grams of cod and 5 g chicken muscles spiked at 10 ng g−1 of a mixture of POP standards showed recovery ranged from 80–100%, and 74–103% for all OCPs, respectively. Recovery for PCBs in cod and chicken muscle ranged from 70–88.4% and 62.1–82.2%, respectively. Relative standard deviation (RSD) was less than 14 for all contaminants investigated in both cod and chicken. The limit of detection (LOD) was 0.3 ng g−1 ww.
2.8 Risk assessments
Sublethal toxic effects from exposure to organochlorine pollutants are common and more important causes of wild avian population decline [32]. We performed reproductive health risk assessment in birds by comparing the present DDTs and PCBs tissue concentrations with minimum toxic threshold concentrations to cause reproductive failure in bird species from literature [19]. Due to the lack of toxic threshold data for the bird species investigated in the present study, toxic threshold concentrations recorded for other bird species were used. Care must also be taken during comparison due to differences in tissues types, sex, and developmental stages of birds investigated. Despite these shortcomings, the present avian reproductive risk assessment would provide a general reproductive risk estimation.
2.9 Statistical analysis
Comparison of mean levels of contaminants among species was performed using one-way analysis of variance (ANOVA) with Tukey (Kramer) HSD (Honestly Significant Difference) post hoc test. Pearson correlation was used to determine the association of DDT residue levels with morphological measurements in birds and fish. The association between log-transformed concentrations of sum DDTs (∑DDTs) and δ15N was used to determine the occurrence of biomagnification. The value of the slope of the regression line was used as a measure of the magnitude of bioaccumulation. Statistical analyses were performed at 0.05 significance level. SPSS statistical software (SPSS 20) was used in data analysis.
3 Results and discussion
3.1 Body measurements and lipid contents
Mean weight of birds varied from 520 g (S. umbretta) to 5164 g (L. crumeniferus). Mean wing chord length varied from 31.6 to 68.8 cm (Table 1). There was a strong statistically significant association between weight and wing chord length (r = 0.99; p < 0.05) suggesting both measurements could be used for gross determination of bird size [33]. Percent lipid contents for bird species were varied from 2.83 to 5.31. The maximum percent lipid content was recorded for S. umbretta followed by T. aethiopicus (2.90%). The mean total lengths of fish were varied from 20.2 to 37.3 cm. Mean weight of fish varied between 145 and 390 g. There was a significant association between total length and weight (r = 0.9; p < 0.05). Lipid contents of fish muscle samples varied from 1.05 to 1.72%. The maximum lipid content was recorded for C. gariepinus (Table 3).
3.2 OCPs and PCB tissue concentrations
Among the investigated OCPs and PCBs, DDT was the dominant contaminant detected in all bird and fish muscle samples. Except for dieldrin and PCBs, which were quantified only in one individual bird, the rest of the OCPs analyzed (Cis-, trans- and oxy-chlordane, Endosulfan-alfa, endosulfan-beta, endosulfan sulfate, and aldrin) were below the detection limit in all the samples (Table 3). The present finding of a higher proportion of DDTs is consistent with findings from previous studies [34, 35]. The predominance of DDTs in the present finding could be due to the widespread presence of DDT in the local environment [21]. DDT is widely used in the region for indoor residual spray (IRS) for malaria vector control [36]. There are also reports of illegal use of DDT for agricultural purposes especially by small-scale farmers [37] that could potentially contaminate the lake and the biota therein.
Among the DDT metabolites, p,p’-DDE was detected in all bird and fish species. 4,4’- dichloro-diphenyl-dichloro-ethane (p,p’-DDD) was detected in all bird species and one species of fish. 4,4’- dichloro-diphenyl-trichloro-ethane (p,p’-DDT) and 2,4’- dichloro-diphenyl-trichloro-ethane (o,p’-DDT) were not detected in all fish and bird muscle samples. The absence of p,p’-DDT may suggest the source of current environmental DDT exposure could be from the historic application. This is also substantiated by the value of the ratio of p,p’-DDE/p,p’-DDT. The ratio of p,p’-DDE/p,p’-DDT greater than 1.0 indicates past input, and the ratio less than 1.0 indicates fresh input [19]. The value of the ratio of p,p’-DDE/p,p’-DDT for both bird and fish samples was greater than 1.0 indicating historic use.
p,p’-DDE accounts for about 95.2% and 99.2% of all DDT metabolites in bird and fish species, respectively. The higher proportion of p,p’-DDE could result from its greater resistance to environmental transformation and high biomagnification potential [32]. Moreover, it could also result from the efficient biotransformation of parent p,p’-DDT [38] and long half-life of p,p’-DDE in fish tissue (7 years) [22]. The present finding of the predominance of p,p’-DDE is consistent with previous studies in birds [19], and in fish [21] from the same region.
The geometric mean of p,p’-DDE ranges from 49.8–375.3 and 2.2–7.7 ng g−1 ww in birds and fish, respectively. The geometric mean level of p,p’-DDD in birds ranges from 2.5 to 17.5 ng g−1 ww. The maximum mean levels of p,p’-DDE in birds is 48.6 to 169 times (average 33 times) higher than levels in fish. The relatively higher levels of p,p’-DDE in birds could be a result of its biomagnification in the local food web [18]. p,p’-DDE is the most resistant metabolite due to its long environmental and biological half-lives [32]. Its persistence together with its lipophilic property could contribute to the higher levels of p,p’-DDE in carnivorous waterbird species occupying high trophic position [18]. Accumulation of p,p’-DDE’s in predator birds several folds (5–15 folds) higher than their prey have been documented [32]. Moreover, the predator bird’s poorly developed oxidative detoxification system could also be responsible for high-level accumulation of lipophilic contaminants such as p,p’-DDE’s. [35].
Statistically significant differences in mean levels of p,p’-DDE [F(2,17) = 28.1; p < 0.05] and p,p’-DDD [F(2,17) = 26.3; p < 0.05] were found among bird species. Maximum geometric mean p,p’-DDE level was recorded in S. umbretta. The variation in DDTs, particularly, p,p’-DDE (the main contributor to total DDTs) levels among bird species could be explained by lipid content [35], feeding habit [39], and foraging habitat [40, 41]. The high levels of ∑DDT in S. umbretta could be attributed to its highest mean lipid content [35]. In addition to that, high levels of p,p’-DDE in S. umbretta could be attributed to the bird species’ preference to the diet of fish and fish scraps [29] and frequent foraging in aquatic habitats which allows a higher degree of exposure [18]. In the present study, S. umbretta accumulated 2.8 and 7.5 times higher p,p’-DDE than T. aethiopicus and L. crumeniferus and respectively. This finding is substantiated by the relatively narrower δ13C value for the species with respect to the other two. On the other hand, the relatively lower levels of p,p’-DDE in L. crumeniferus and T. aethiopicus could be due to the inclusion of diets from terrestrial origins [28] and lower lipid contents.
Regarding fish species, there was a statistically significant difference in mean p,p’-DDE among fish species [F(2,27) = 10.6; p < 0.05] that could be due to difference in feeding habits [42], trophic position [43], lipid content [44] and specific habitats [39]. O. niloticus, B. intermedius, and C. gariepinus from ERV lakes have been shown to be herbivore, omnivore, and carnivore, respectively [21, 44, 45]. The highest p,p’-DDE level was found in C. gariepinus that could be attributed to its top trophic position, carnivorous feeding habit, and high lipid content than the rest species of fish. This finding is consistent with findings from earlier studies [22, 43, 44]. Moreover, C. gariepinus’ bottom-dwelling habit predisposes it to a higher degree of exposure to DDTs associated with sediments [38]. O. niloticus had the lowest p,p’-DDE levels that could be explained by its low trophic position and herbivorous feeding habits [44].
3.3 Comparison with other studies
Muscle tissue concentrations of DDTs in the present study were compared with muscle tissue concentrations of birds from other studies around the world to determine the relative status of the present contamination. The concentrations of ∑DDT in the present study in birds (24.9–799 ng g−1 ww [880–15,051 ng g−1 lw]) were greater than values in herring gulls (Larus argentatus) from the Polish coastal zone of the Southern Baltic Sea (mean total DDT 0.1 ng g−1 ww) [22], in piscivorous birds from Argentina (1069–6480 ng g−1 lw) [46] and in Asian Openbill (Anastomus oscitans) from India (mean 96 ng g−1 ww) [47]. However, they were lower than values reported in piscivorous waterbird species from Ethiopia (3.7–148.3 µg g−1 lw) [19], in bird species from Iran (0.5–9040 ng g−1 ww) [35], in white-tailed eagles (Haliaeetus albicilla) from West Greenland (0.7–530 µg g−1 lw) [48], in predator birds from Spain (141.6–24,772 ng g−1 ww) [17] and in Catharacta spp. of birds from Antarctica (287–1028 ng g−1 ww) [49]. However, the differences in sample size, study years, types of bird species in these studies make comparison difficult.
The tissue concentrations of ∑DDT in the present study in fish were greater than values reported in fish from Antarctica (up to 3 ng g−1) [49], from Lake Ziway, Ethiopia (0.1–10.6 ng g−1 lw) [42] and in fish from Brazil (mean ∑DDT 2 ng g−1 ww) [22]. However, the levels in the present study were lower than values reported from Lake Hawassa, Ethiopia (7.8–172 ng g−1 ww, 6.82–73.3 ng g−1 ww) [21, 50], and in fish from Pakistan (16.9–402 ngg−1 ww) [20]. The present levels were also lower than values in fish from Lake Ziway and Lake Koka (0.9–172 ng g−1 ww, 0.1 to 72.5 ng g−1 ww), respectively [43, 44], and from South African Lakes (73 and 893 ng g−1 wet mass) [38], (1.9 to 5643 ng g−1 ww) [51], (< LOQ–61 ng g−1 ww) [52].
3.4 Association of DDT tissue concentration with bird and fish size
Accumulation of OCPs depends on age and size of birds [35, 53]. Due to difficulty in determination of the age of birds in the wild, we use morphological measurements as a gross representation of size. Since the weights of birds vary depending on gut fullness, we use wing chord length as an index of bird size [33]. The investigation of the relationship between wing chord length (cm) and log-transformed ∑DDTs (ng g−1 ww) showed a positive association for all species of birds (Fig. 2). However, all associations were not statistically significant. Contrary to the general rule, the absence of significant association between bird size and accumulation of DDTs among individuals of each of T. aethiopicus and S. umbretta species could result from the small variation in wing chord length (range = 31.1–37.3, 30.3–32.7, respectively) that could lead to similar periods of exposure to DDT (Table 1). In individuals of L. crumeniferus, however, there was a relatively large difference between the minimum and maximum wing chord length (range = 62.6–75.5). The non-significant association could result from the higher proportion of juveniles (personal observation & implication from wing chord length) with relative lower percent lipid content, shorter periods of exposure [34]. In addition to the above, small sample size may play a role in the non-significant associations found. The positive influence of age and lipid content on the accumulation of DDT have been documented [35, 53].
The association between log-transformed p,p’-DDE (ng g−1 ww), and total length in fish species was investigated to determine the influence of age on p,p’-DDE accumulation. Moderate (r = 0.62) and weak (r = 0.15) positive associations were found in C. gariepinus and B. intermedius, respectively. In O. niloticus the association was negative (r = − 0.43; p > 0.05). All associations between p,p’-DDE and total length were not statistically significant (Fig. 3). Despite the presence of greater variation in total length among individuals of each of C. gariepinus (range = 29.1–50.7) and B. intermedius (range = 25.1–33.6), the absence of significant positive association is most likely a result of exposure to constant levels and shorter time period p,p’-DDE took to reach a steady-state tissue concentration as a result of its higher biomagnification potential [32]. Once a steady-state is reached, extended exposure periods would bring an insignificant increase in p,p’-DDE levels. To be certain, however, this needs further investigation. The negative association in O. niloticus could be a result of biodilution that in turn could be caused by faster growth in tropical fish [54]. The phenomenon of biodilution of contaminants in fish from Lake Hawassa was documented [50]. In addition to that, the negative association in O. niloticus could result from the combined effect of very small variation in total lengths (range = 19.5–21.5) [43] and small sample size.
3.5 Stable isotopes analyses
Trophic position and carbon sources of bird and fish species were determined using carbon and nitrogen stable isotopes. Trophic relationships among species of birds and fish are given in Fig. 4. In all investigated bird species, the mean stable nitrogen isotope ratio (δ15N) and stable carbon isotope ratio (δ13C) values varied from 9.6 to 12.6‰ and − 23.1 to − 22.3‰, respectively. In fish species, the mean δ15N and δ13C values varied from 0.6–5.8‰ and − 25.1 to − 23.9‰, respectively. The level of δ15N values in bird species in the present study generally correspond to birds utilizing diets of aquatic origin (preys of planktivorous and piscivores fish) [55]. The δ15N values in birds were significantly higher than values in all the fish species [F(1,22) = 23.7; p < 0.05], indicating their higher trophic position relative to fish species. Considering the trophic fractionation factor of 3‰ [56], the difference between the maximum and minimum mean δ15N suggests all species of birds occupy the same trophic position. In fish species, the difference of 5.2‰ between the maximum and minimum mean δ15N values indicates that the fish species occupy two trophic levels. The carnivorous fish C. gariepinus occupies the highest trophic position relative to the other two fish species. The lowest δ15N values were recorded for O. niloticus and B. intermedius indicating their lowest trophic position in the local food web. The low trophic position of O. niloticus and B. intermedius is in agreement with their herbivore and omnivore diets, respectively [42, 44].
Birds also have shown significantly higher and wider δ13C values than values recorded for all species of fish [F(1,22) = 49.7; p < 0.05]. The higher δ13C values in birds indicate that the birds are generalist in their feeding habit as a result of the utilization of a wide range of aquatic food sources [27, 28]. Moreover, fish scrape (that is composed of different species) constitute diets of the studied bird species at Lake Hawassa that could diversify prey items. B. intermedius and O. niloticus have shown the lowest and narrowest δ13C values implying utilization of a narrow range of food sources from the pelagic zone [21]. With respect to B. intermedius and O. niloticus, C. gariepinus showed relatively higher value of δ13C ratio. This could suggest the utilization of carbon sources from the littoral zone.
Biomagnification of p,p’-DDE through the local food chain involving fish and bird species was determined from a regression line between log-transformed p,p’-DDE concentrations against δ 15N values (Fig. 5). The outcome of the regression analysis indicates a significant positive association between p,p’-DDE and δ15N [F(1,22) = 27.9; p < 0.05] suggesting the occurrence of biomagnification of p,p’-DDE through the local food web. The value of the slope of the regression line (slope = 0.11; R2 = 0.56) indicates rate of biomagnification of p,p’-DDE ([logp,p’-DDE] = 0.11*δ15N + 0.65). Biomagnification of DDTs in the food web involving fish [50] and bird and fish species were documented [19]. The order of p,p’-DDE levels among fish and birds (S. umbretta > T. aethiopicus > L. crumeniferus > C. gariepinus > B. intermedius > O. niloticus) substantiates the variation in p,p’-DDE levels was a result of the difference in trophic levels. The biomagnification of DDTs in a food web involving fish and birds is consistent with other findings [2].
3.6 Risk assessments
DDTs, particularly, p,p’-DDE, have been known to affect bird reproduction and survival of young [6, 57]. The risk associated with the levels of p,p’-DDE in birds was assessed by comparing tissue concentrations with toxicity thresholds from literature. Considering lipid normalized p,p’-DDE muscle tissue concentrations are comparable with liver concentrations [19], the maximum p,p’-DDE levels in the present study (3614–14,172 ng g−1 lw) were below the minimum threshold average concentration (20,000 g g−1 lw) in liver, which was linked with impairment in individual bird reproduction [58]. Assuming 20% maternal DDT transfer rate into eggs [59], the current levels of p,p’-DDE were also below the minimum threshold concentration of 3 µg g−1 ww that was suggested to cause reproductive failure in brown pelican (Pelecanus occidentalis) [57]. However, caution must be taken due to the presence of interspecies differences in sensitivity to p,p’-DDE [60].
4 Conclusions
The carnivorous waterbird species have accumulated an average of 33 times higher levels of p,p’-DDE than the fish species. Generally, larger individuals of each species of birds and fish have accumulated relatively higher levels of p,p’-DDE than those with smaller sizes. p,p’-DDE constitute the main exposure threat in Lake Hawassa. Trophic position, feeding habits, and lipid contents are the main factors influencing the accumulation of p,p’-DDE in both fish and carnivorous waterbird species. There is no reproductive health risk associated with the current levels of p,p’-DDE in carnivorous waterbird species. The presence of organochlorine exposure threats in the area suggests the need for further investigation of contamination in other waterbird species. The present study may serve as a baseline for further investigation of organochlorine contamination in waterbird and fish species from the ERV lakes.
Data availability
The datasets analyzed during the current study are available from the corresponding author on reasonable request.
References
Naso B, Perrone D, Ferrante MC, Zaccaroni A, Lucisano A (2003) Persistent organochlorine pollutants in liver of birds of different trophic levels from coastal areas of Campania, Italy. Arch Environ Contam Toxicol 45(3):407–414. https://doi.org/10.1007/s00244-003-2201-z
Kim JT, Choi YJ, Barghi M, Kim JH, Jung JW, Kim K, Kang JH, Lammel G, Chang YS (2021) Occurrence, distribution, and bioaccumulation of new and legacy persistent organic pollutants in an ecosystem on King George Island, maritime Antarctica. J Hazard Mater 405:124141. https://doi.org/10.1016/j.jhazmat.2020.124141
Stockholm convention on POPs (2019) New POPs under Stockholm convention. http://chm.pops.int/TheConvention/ThePOPs/TheNewPOPs/tabid/2511/Default.aspx. Accessed 12 Oct 2021
Stockholm Convention (2010) Ridding the world of POPs: a guide to the Stockholm Convention on Persistent Organic Pollutants. United Nations Environmental Program (UNEP), Geneva
Ashraf MA (2017) Persistent organic pollutants (POPs): a global issue, a global challenge. Environ Sci Pollut Res 24:4223–4227. https://doi.org/10.1007/s11356-015-5225-9
Findholt SL, Trost CH (1985) Organochlorine pollutants, eggshell thickness, and reproductive success of Black-crowned Night-Herons in Idaho, 1979. Colon Waterbirds. https://doi.org/10.2307/1521192
Marchand MJ, Pieterse GM, Barnhoorn IE (2008) Preliminary results on sperm motility and testicular histology of two feral fish species, Oreochromis mossambicus and Clarias gariepinus, from a currently DDT-sprayed area, South Africa. J Appl Ichthyol 24(4):423–429. https://doi.org/10.1111/j.1439-0426.2008.01141.x
Bustnes JO, Bakken V, Erikstad KE, Mehlum F, Skaare JU (2001) Patterns of incubation and nest-site attentiveness in relation to organochlorine (PCB) contamination in glaucous gulls. J Appl Ecol 38:791–801. https://doi.org/10.1046/j.1365-2664.2001.00633.x
DeLeon S, Halitschke R, Hames RS, Kessler A, DeVoogd TJ, Dhondt AA (2013) The effect of polychlorinated biphenyls on the song of two passerine species. PLoS ONE 8:734–771. https://doi.org/10.1371/journal.pone.0073471
Federal Democratic Republic of Ethiopia-National Implementation Plan (FDRE-NIP) (2006) National implementation plan for the Stockholm Convention. http://chm.pops.int/implementation/NationalImplementationPlans/NIPTransmission/tabid/253/default.aspx. Accessed 14 Aug 2021
Van Den Berg H, Manuweera G, Konradsen F (2017) Global trends in the production and use of DDT for control of malaria and other vector-borne diseases. Malar J 16(1):1–8. https://doi.org/10.1186/s12936-017-2050-2
Haylamicheal I, Dalvie M (2009) Disposal of obsolete pesticides, the case of Ethiopia. Environ Int 35:667–673. https://doi.org/10.1016/j.envint.2008.11.004
Debela SA, Sheriff I, Wu J, Hua Q, Zhang Y, Dibaba AK (2020) Occurrences, distribution of PCBs in urban soil and management of old transformers dumpsite in Addis Ababa, Ethiopia. Sci Afr 8:e00329. https://doi.org/10.1016/j.sciaf.2020.e00329
Mereta ST, Ambelu A, Ermias A, Abdie Y, Moges M, Haddis A, Hailu D, Beyene H, Kebede B, Mulat WL (2020) Effects of untreated industrial effluents on water quality and benthic macroinvertebrate assemblages of Lake Hawassa and its tributaries, Southern Ethiopia. Afr J Aquat Sci 45(3):285–295. https://doi.org/10.2989/16085914.2019.1671166
Gebretsadik T, Mereke K (2017) Threats and opportunities to major Rift Valley Lakes wetlands of Ethiopia. Agric Res Technol 9:1–6. https://doi.org/10.19080/ARTOAJ.2017.09.555751
Tafesse TB, Yetemegne AK, Kumar S (2015) The physico-chemical studies of wastewater in hawassa textile industry. J Environ Anal Chem 2(153):2380–2391. https://doi.org/10.4172/2380-2391.1000153
Luzardo OP, Ruiz-Suárez N, Henríquez-Hernández LA, Valerón PF, Camacho M, Zumbado M, Boada LD (2014) Assessment of the exposure to organochlorine pesticides, PCBs and PAHs in six species of predatory birds of the Canary Islands, Spain. Sci Total Environ 472:146–153. https://doi.org/10.1016/j.scitotenv.2013.11.021
Huertas D, Grimalt JO, Jover L, Sanpera C (2016) Influence of diet in the accumulation of organochlorine compounds in herons breeding in remote riverine environments. Chemosphere 145:438–444. https://doi.org/10.1016/j.chemosphere.2015.11.101
Yohannes YB, Ikenaka Y, Nakayama SMM, Mizukawa H, Ishizuka M (2014) Organochlorine pesticides in bird species and their prey (fish) from the Ethiopian Rift Valley region, Ethiopia. Environ Pollut 192:121–128. https://doi.org/10.1016/j.envpol.2014.05.007
Aamir M, Khan S, Nawab J, Qamar Z, Khan A (2016) Tissue distribution of HCH and DDT congeners and human health risk associated with consumption of fish collected from Kabul River, Pakistan. Ecotoxicol Environ Saf 125:128–134. https://doi.org/10.1016/j.ecoenv.2015.12.005
Deribe E, Rosseland BO, Borgstrøm R, Salbu B, Gebremariam Z, Dadebo E, Skipperud L, Eklo OM (2014) Organochlorine pesticides and polychlorinated biphenyls in fish from Lake Awassa in the Ethiopian Rift Valley: human health risks. Bull Environ Contam Toxicol 93(2):238–244. https://doi.org/10.1007/s00128-014-1314-6
Ferreira VB, Estrella LF, Alves MGR, Gallistl C, Vetter W, Silva TTC, Malm O, Torres JPM, Abadio Finco FDB (2020) Residues of legacy organochlorine pesticides and DDT metabolites in highly consumed fish from the polluted Guanabara Bay, Brazil: distribution and assessment of human health risk. J Environ Sci Health B 55(1):30–41. https://doi.org/10.1080/03601234.2019.1654808
Ayele S, Mamo Y, Deribe E, Eklo OM (2022) Levels of organochlorine pesticides in five species of fish from Lake Ziway, Ethiopia. Sci Afr 16:e01252. https://doi.org/10.1016/j.sciaf.2022.e01252
Desta Z (2003) Challenges and opportunities of Ethiopian wetlands: the case of Lake Awassa and its feeders. In: Abebe YD, Geheb K (eds) Wetlands of Ehiopia. Procedings of a seminar on the resourrces and status of Ethiopia’s Wetlands. IUCN, Addis Ababa, p 67
Pattnaik DB (2014) Species diversity of lake Hawassa, Ethiopia. Int J Sci Res 3(11):33–35
Dsikowitzky L, Mengesha M, Dadebo E, de Carvalho CEV, Sindern S (2013) Assessment of heavy metals in water samples and tissues of edible fish species from Awassa and Koka Rift Valley Lakes, Ethiopia. Environ Monit Assess 185(4):3117–3131. https://doi.org/10.1007/s10661-012-2777-8
Chane M, Balakrishnan M (2016) Population structure, feeding habits and activity patterns of the African Sacred ibis (Threskiornis aethiopicus) in Dilla Kera area, southern Ethiopia. Ethiop J Biol Sci 15(1):93–105
Datiko D, Bekele A (2012) Population and feeding ecology of the Marabou stork (Leptoptilos crumeniferus) around Lake Ziway, Ethiopia. Ethiop J Biol Sci 11(2):181–191
Mengistu S, Bekele A (2015) Population status and activity pattern of hamerkop (Scopus umbretta) in Lake Hora-Arsedi, Bishoftu, Ethiopia. Ethiop J Biol Sci 14(2):185–200
Winker K (1998) Suggestions for measuring external characters of birds. Ornitol Neotrop 9:23–30
Anastassiades M, Lehotay SJ, Štajnbaher D, Schenck FJ (2003) Fast and easy multiresidue method employing acetonitrile extraction/partitioning and “dispersive solid-phase extraction” for the determination of pesticide residues in produce. J AOAC Int 86(2):412–431. https://doi.org/10.1093/jaoac/86.2.412
Walker CH, Sibly RM, Peakall DB (2012) Principles of ecotoxicology, 4th edn. CRC Press, New York
Bollinger EK, Gavin TA (1989) The effects of site quality on breeding-site fidelity inn Bobolinks. Auk 106:584–594. https://doi.org/10.2307/4087663
Falkowska L, Reindl AR, Grajewska A, Lewandowska AU (2016) Organochlorine contaminants in the muscle, liver and brain of seabirds (Larus) from the coastal area of the Southern Baltic. Ecotoxicol Environ Saf 133:63–72. https://doi.org/10.1016/j.ecoenv.2016.06.042
Rajaei F, Sari AE, Bahramifar N, Savabieasfahani M, Ghasempouri M (2011) Persistent organic pollutants in muscle and feather of ten avian species from Māzandarān Province of Iran, on the coast of the Caspian Sea. Bull Environ Contam Toxicol 87(6):678–683. https://doi.org/10.1007/s00128-011-0420-y
Biscoe ML, Mutero C, Kramer RA (2005) Current policy and status of DDT use for malaria control in Ethiopia, Uganda, Kenya and South Africa. International Water Management Institute (IWMI). Colombo
Negatu B, Kromhout H, Mekonnen Y, Vermeulen R (2016) Use of chemical pesticides in ethiopia: a cross-sectional comparative study on knowledge, attitude and practice of farmers and farm workers in three farming systems. Ann Occup Hyg 60:551–566. https://doi.org/10.1093/annhyg/mew004
Pheiffer W, Wolmarans NJ, Gerber R, Yohannes YB, Ikenaka Y, Ishizuka M, Smit NJ, Wepener V, Pieters R (2018) Fish consumption from urban impoundments: what are the health risks associated with DDTs and other organochlorine pesticides in fish to township residents of a major inland city. Sci Total Environ 628:517–527. https://doi.org/10.1016/j.scitotenv.2018.02.075
Thomas M, Lazartigues A, Banas D, Brun-Bellut J, Feidt C (2012) Organochlorine pesticides and polychlorinated biphenyls in sediments and fish from freshwater cultured fish ponds in different agricultural contexts in north-eastern France. Ecotoxicol Environ Saf 77:35–44. https://doi.org/10.1016/j.ecoenv.2011.10.018
Holoubek I, Dušek L, Sáňka M, Hofman J, Čupr P, Jarkovský J, Zbíral J, Klánová J (2009) Soil burdens of persistent organic pollutants–their levels, fate and risk. Part I. Variation of concentration ranges according to different soil uses and locations. Environ Pollut 157(12):3207–3217. https://doi.org/10.1016/j.envpol.2009.05.031
Lèche A, Gismondi E, Martella MB, Navarro JL (2021) First assessment of persistent organic pollutants in the Greater rhea (Rhea americana), a near-threatened flightless herbivorous bird of the Pampas grasslands. Environ Sci Pollut Res 28(22):7681–27693. https://doi.org/10.1007/s11356-021-12614-5
Yohannes YB, Ikenaka Y, Saengtienchai A, Watanabe KP, Nakayama SM, Ishizuka M (2014) Concentrations and human health risk assessment of organochlorine pesticides in edible fish species from a Rift Valley Lake—Lake Ziway, Ethiopia. Ecotoxicol Environ Saf 106:95–101. https://doi.org/10.1016/j.ecoenv.2014.04.014
Deribe E, Rosseland BO, Borgstrøm R, Salbu B, Gebremariam Z, Dadebo E, Skipperud L, Eklo OM (2013) Biomagnification of DDT and its metabolites in four fish species of a tropical lake. Ecotoxicol Environ Saf 95:10–18. https://doi.org/10.1016/j.ecoenv.2013.03.020
Deribe E, Rosseland BO, Borgstrøm R, Salbu B, Gebremariam Z, Dadebo E, Norli HR, Eklo OM (2011) Bioaccumulation of persistent organic pollutants (POPs) in fish species from Lake Koka, Ethiopia: the influence of lipid content and trophic position. Sci Total Environ 410:136–145. https://doi.org/10.1016/j.scitotenv.2011.09.008
Dadebo E, Tesfahun A, Teklegiorgis Y (2013) Food and feeding habits of the African big barb Labeobarbus intermedius (Rüppell, 1836) (Pisces: Cyprinidae) in Lake Koka, Ethiopia. E3 J Agric Res Dev 3(4):49–58
Cid FD, Antón RI, Caviedes-Vidal E (2007) Organochlorine pesticide contamination in three bird species of the Embalse La Florida water reservoir in the semiarid midwest of Argentina. Sci Total Environ 385(1–3):86–96. https://doi.org/10.1016/j.scitotenv.2007.07.004
Jayakumar S, Muralidharan S, Dhananjayan V (2020) Organochlorine pesticide residues among colonial nesting birds in Tamil Nadu, India: a maiden assessment from their breeding grounds. Arch Environ Contam Toxicol 78(4):555–567. https://doi.org/10.1007/s00244-020-00709-y
Jaspers VLB, Sonne C, Soler-Rodriguez F, Boertmann D, Dietz R, Eens M, Rasmussen LM, Covaci A (2013) Persistent organic pollutants and methoxylated polybrominated diphenyl ethers in different tissues of white-tailed eagles (Haliaeetus albicilla) from West Greenland. Environ Pollut 175:137–146. https://doi.org/10.1016/j.envpol.2012.12.023
Cipro CV, Colabuono FI, Taniguchi S, Montone RC (2013) Persistent organic pollutants in bird, fish and invertebrate samples from King George Island, Antarctica. Antarct Sci 25(4):545–552. https://doi.org/10.1017/S0954102012001149
Yohannes YB, Ikenaka Y, Nakayama SM, Saengtienchai A, Watanabe K, Ishizuka M (2013) Organochlorine pesticides and heavy metals in fish from Lake Awassa, Ethiopia: insights from stable isotope analysis. Chemosphere 91(6):857–863. https://doi.org/10.1016/j.chemosphere.2013.01.047
Govaerts A, Verhaert V, Covaci A, Jaspers VL, Berg OK, Addo-Bediako A, Jooste A, Bervoets L (2018) Distribution and bioaccumulation of POPs and mercury in the Ga-Selati River (South Africa) and the rivers Gudbrandsdalslågen and Rena (Norway). Environ Int 121:1319–1330. https://doi.org/10.1016/j.envint.2018.10.058
Verhaert V, Newmark N, D’Hollander W, Covaci A, Vlok W, Wepener V, Addo-Bediako A, Jooste A, Teuchies J, Blust R, Bervoets L (2017) Persistent organic pollutants in the Olifants River Basin, South Africa: bioaccumulation and trophic transfer through a subtropical aquatic food web. Sci Total Environ 586:792–806. https://doi.org/10.1016/j.scitotenv.2017.02.057
Shlosberg A, Wu Q, Rumbeiha WK, Lehner A, Cuneah O, King R, Hatzofe O, Kannan K, Johnson M (2012) Examination of Eurasian griffon vultures (Gyps fulvus fulvus) in Israel for exposure to environmental toxicants using dried blood spots. Arch Environ Contam Toxicol 62(3):502–511. https://doi.org/10.1007/s00244-011-9709-4
Deribe E, Masresha AE, Gade PA, Berger S, Rosseland BO, Borgstrøm R, Dadebo E, Gebremariam Z, Eklo OM, Skipperud L, Salbu B (2014) Bioaccumulation of mercury in fish species from the Ethiopian Rift Valley Lakes. Int J Environ Protect 4(1):15–22. https://doi.org/10.2989/16085914.2003.9626604
Hebert CE, Shutt JL, Hobson KA, Weseloh DC (1999) Spatial and temporal differences in the diet of Great Lakes herring gulls (Larus argentatus): evidence from stable isotope analysis. Can J Fish Aquat Sci 56(2):323–338. https://doi.org/10.0039/f98-189
Dionne K, Dufresne F, Nozais C (2016) Variation in δ 13 C and δ 15 N trophic enrichment factors among Hyalella azteca amphipods from different lakes. Hydrobiologia 781(1):217–230. https://doi.org/10.1007/s10750-016-2846-z
Blus LJ (1982) Further interpretation of the relation of organochlorine residues in brown pelican eggs to reproductive success. Environ Pollut Ser A 28(1):15–33. https://doi.org/10.1016/0143-1471(82)90042-3
Tanabe S, Senthilkumar K, Kannan K, Subramanian AN (1998) Accumulation features of polychlorinated biphenyls and organochlorine pesticides in resident and migratory birds from South India. Arch Environ Contam Toxicol 34(4):387–397. https://doi.org/10.1007/s002449900335
Bargar TA, Scott GI, Cobb GP (2001) Maternal transfer of contaminants: case study of the excretion of three polychlorinated biphenyl congeners and technical-grade endosulfan into eggs by white leghorn chickens (Gallus domesticus). Environ Toxicol Chem: Int J 20(1):61–67. https://doi.org/10.1002/etc.5620200106
Blus LJ, Wiemeyer SN, Henny CJ (1996) Organochlorine pesticides. In: Fairbrother A, Locke LN, Hoff GL (eds) Noninfectious diseases in wildlife, 2nd edn. Iowa State University Press, Ames, pp 61–70
Acknowledgements
I would like to thank the Institutional collaboration between Hawassa University and Norwegian University of Life Sciences for the financial support. I would also like to thank Hans Ragnar Norli for his cooperation during laboratory work.
Funding
This study was funded by the Institutional collaboration between Hawassa University and Norwegian University of Life Sciences. Project Number 4.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by SA. The first draft of the manuscript was written by SA and all authors commented on the previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Research involving human and animals participants
International and national guidelines for the care and use of animals were followed during the present research.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ayele, S., Mamo, Y., Deribe, E. et al. Organochlorine pesticides and polychlorinated biphenyls in carnivorous waterbird and fish species from Lake Hawassa, Ethiopia. SN Appl. Sci. 4, 285 (2022). https://doi.org/10.1007/s42452-022-05177-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-022-05177-8