Abstract
Heavy metals contamination in freshwater ecosystems has drawn attention worldwide. It is necessary to investigate heavy metals content and assess their ecological risk in order to protect the aquatic ecosystems. In this study, we collected surface sediment samples from the freshwater ecosystem of the city of Zhenjiang, in China, in both winter and summer. Then, we analyzed the seasonal and spatial distribution patterns of lead (Pb), chromium (Cr), cadmium (Cd), zinc (Zn), and copper (Cu). The contamination factor (CF), enrichment factor (EF), geo-accumulation index (Igeo), and potential ecological risk (Eri) were jointly used to assess the pollution degree and the ecological risk posed to the freshwater ecosystem by the aforementioned elements. Multivariate statistical analysis, including Pearson’s correlation and principal component analysis and cluster analysis, were used to identify potential sources of the investigated metals in this research area. Study results showed that: (1) the average concentrations values were 1.81 mg/kg and 1.15 mg/kg for Cd, 55.3 mg/kg and 62.2 mg/kg for Cu, 88.0 mg/kg and 52.5 mg/kg for Cr, 27.3 mg/kg and 22.8 mg/kg for Pb, 87.0 mg/kg and 271 mg/kg for Zn, in winter and summer, respectively. Amongst the investigated elements, the average concentrations of Cd, Cu, Cr, and Pb, were above the local background values in winter, whereas, Cd, Cu and Zn concentrations were higher than the background values in Zhenjiang; (2) The CF and EF indicated that Cd had a high contamination degree and a significant enrichment compare to others investigated metals in the surface sediment of in this research area. (3) Cd posed moderate, considerable, or very high ecological risks in different sites, while the other elements (i.e., Cu, Cr, Pb, and Zn) presented a low degree of ecological risk. (4) Multivariate statistical analyses results indicated Pb, Cu, and Zn had similar geochemical characteristics, while, Cd and Cr had significant differences with the above elements. Therefore, Pb, Cu, and Zn probably originate from the same sources, while Cd and Cr might have mixed sources, including both natural sources and human activities. Overall, more attention should be paid to Cd for risk assessment in the current study area. The findings of this study provide fundamental information for the evaluation and management of the heavy metals investigated in the freshwater ecosystem of Zhenjiang.
Article highlights
-
Five heavy metals concentrations displayed a wide seasonal and spatial variation. The mean concentration was Cr ˃ Zn ˃ Cu ˃ Pb ˃ Cd in winter and Zn ˃ Cu ˃ Cr ˃ Pb ˃ Cd in summer.
-
The average CF value was Cd ˃ Cr ˃ Pb ˃ Cu ˃ Zn in winter and Cd ˃ Cu ˃ Zn ˃ Pb ˃ Cr in summer, respectively. The average EF value was Cd, followed by Cu, Pb, Cr, and Zn in winter and Cd, Zn, Cu, Pb, and Cr in summer, from highest to lowest, respectively.
-
Contamination caused by Cd was more severe than for other heavy metals in both winter and summer.
-
Cd was the major contributor to the potential ecological risk in the study region.
-
Multivariate statistical analyses results showed Cd and Cr was more distinct from other element in winter and summer, indicate that Cd and Cr have a mixed source include both anthropogenic and lithogenic sources.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Recently, an alarming level of heavy metals have been reported in water bodies globally [1]. Heavy metals have become one of the most serious contaminants in freshwater ecosystems due to their environmental persistence, toxic effects, the ability of bioaccumulation in organisms, and biomagnification in food webs [2, 3]. These metals were finally assimilated by consumers resulting in a significant threat to the human health [4, 5]. Therefore, heavy metal pollution in freshwater systems had been paid more and more attention at present [6, 7].
Previous studies reported that less than 1% of heavy metals entering the aquatic environment can dissolve in water, and the remaining 99% is stored in the sediment [8], which acts as a major sink for heavy metals accumulation [9]. Moreover, once the hydrological or environmental conditions in the water–sediment interface change due to various biotic and abiotic processes [10, 11], the heavy metals accumulated in the sediment may be re-activated and easily released into the overlying water, resulting in serious ecological risk to aquatic organisms [12]. Therefore, it is necessary and important to investigate heavy metals pollution in sediments in order to assess their potential risks and toxicity levels to the aquatic ecosystems.
In recent years, numerous investigations studies about heavy metal concentrations have been carried out in many lakes or rivers sediment all over the world [6, 7, 13]. In these previous studies, the contamination status and their ecological risk of heavy metals in sediment were usually comprehensively evaluated by a variety of indices, including contamination factor (CF), enrichment factor (EF), geoaccumulation index (Igeo) and potential ecological risk index (Eir) [14, 15]. Furthermore, multivariate statistical methods, including cluster analysis (CA) and principal component analysis (PCA), were also widely used to describing the potential natural and anthropogenic sources of heavy metals [14]. For example, Zhang et al. [16] found that Cd posed a significant potential ecological risk to the tributaries of the Han River. Lin et al. [13] found that Fengshan River sediments, an industrialized urban river, were extremely contaminated with heavy metal of Hg, Cr, Ni, and Zn. The previous studies have shown that the pollution level, distribution pattern, and ecological risk associated with heavy metals in sediment are very uneven [12, 17], which often depends on both natural factors, such as the type of aquatic ecosystem [18] and differences in heavy metals local background values [16], and anthropogenic activities [19]. Even in a same lake or river, the content of heavy metals will greatly vary due to differences in sampling time, sites, depth, etc. [20]. Indeed, distribution characteristics of heavy metals in sediments are complex and diverse across different regional environments. Thus, the results applied to determine the pollution level of sediment were not precise for other regions. Therefore, regional lakes and river should be chosen and investigated in order to providing more accurate and comprehensive information for ecological risk assessment of heavy metals.
Zhenjiang is an important city in the east of China, characterized by abundant water resources. In recent decades, water pollution has frequently occurred owing to rapid urbanization, industrialization and agricultural developments, and human exploitation. In order to prevention and control water pollution, the State Council of China issued the Action Plan for Water Pollution Prevention and Control in April 2015. And the Jiangsu Provincial People's Government proposed work plans on December, 2016. In this study, we selected representative lakes and rivers and collected surface sediment samples in winter and summer to assess heavy metals pollution in the Zhenjiang freshwater ecosystem. The primary objectives of the present study were to: (1) identify the spatial and seasonal distributions of heavy metals (Pb, Cr, Cd, Zn, and Cu) in surface sediments in the freshwater ecosystem of Zhenjiang; (2) evaluate the contamination, pollution degree, and potential ecological risks posed to the ecosystem by the aforementioned elements, by calculating the contamination factor (CF), enrichment factor (EF), the geo-accumulation index (Igeo), and the potential ecological risk (Eri); (3) identify the potential natural and anthropogenic sources of these elements, by employing multivariate statistical techniques. The results may provide fundamental information for the evaluation and management of heavy metals in surface sediments in the freshwater ecosystem of Zhenjiang city, China in Zhenjiang.
2 Materials and methods
2.1 Study area
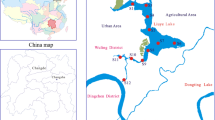
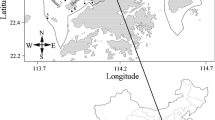
The study area is the city of Zhenjiang in Jiangsu Province, China. In this area, two of the most famous watercourses of China, namely the Yangtze River and the Beijing-Hangzhou Grand Canal, meet together and create an environment that is rich in water resources. Jinshan Lake is the largest freshwater shallow lake in Zhenjiang, accounting for more than 80% of all surface waters in this area. Moreover, the Guyun River is the longest and oldest artificial river in Zhenjiang city, with a length of approximately 17 km, a watershed area of 81 km2, and an average water depth of 4–6 m. The Yangtze River supplies water to the Jinshan Lake, which in turn supplies water to the Guyun River. Our earlier study [21] has illuminated further information and parameters description on Jinshan Lake and the Guyun River.
A total of 21 sampling sites were chosen in this study: 10 in Jinshan Lake (i.e., S1–S10); 5 in the main stream of Guyun River (i.e., S16, S17, S18, S19, and S20); and one for each of the 6 main tributaries (i.e., Hongqiaogang: S11; Yudai River: S12; Tuanjie River: S13; Siming River: S14; Zhoujia River: S15; and Yunliang river: S21). The details of these sampling sites have been described in our previous study [21].
2.2 Sample collection
Surface sediment samples with the depth of 0–25 cm in three replicates were randomly collected using a Petersen grab sampler on January (winter) and July (summer) 2019. The collected samples were adequately mixed to form a composite sample for each sampling site; then, they were sealed in self-sealing polyethylene bags and immediately transported to the laboratory. There, the samples were air dried, powdered, sieved through a 100-mesh nylon sieve, and stored at 4 ℃ to perform the analyses of heavy metal concentrations.
2.3 Sample analyses and quality control
The sediment samples were digested with a mixture of HNO3–HF–HClO4 in Teflon tubes to assess the total heavy metals concentration. The digested solutions were analyzed using inductively coupled plasma optical emission spectrometry (ICPE-9820, Shimadzu, Japan). Quality assurance and quality control were ensured through the analysis of reagent blanks, replicates samples, and standard reference materials. The precision and accuracy of heavy metals analyses were verified using standard reference materials from the National Research Center for Geo Analysis of China [sediment, GBW07309 (GSD-9)], and the results of each heavy metal recovery were shown in Table S1. All the reagents were guaranteed as analytical grades or higher.
2.4 Pollution assessment
The contamination factor (CF), enrichment factor (EF), the geo-accumulation index (Igeo), and the ecological risk index (Eir) were calculated in order to evaluate the heavy metals pollution degree and their ecological risk in surface sediments.
The CF was obtained as follows [22],
where Csi indicates the measured heavy metal concentrations (mg/kg); and Cni indicates the background values of heavy metal concentrations in Zhenjiang (mg/kg), which were equal to 26.03, 54.08, 252, 83.17, and 0.31 mg/kg for Pb, Cu, Zn, Cr, and Cd, respectively [23]. The classification criteria of CF are shown in Table S2.
The EF was obtained by heavy metal content to a reference metal, as follows [14]:
where Ssi indicates the measured content in sediment samples of a reference element for geochemical normalization purposes (mg/kg). Sref indicates the background value of reference element. In this present study, Mn element is used as a reference metal [24] and the background value (Sref) is 682 mg/kg for Mn [25]. The classification criteria of EF are shown in Table S3.
Igeo was calculated as follows [26]:
where k is the background matrix correlation factor, whose value was set at 1.5 [26]. The classification criteria of Igeo are shown in Table S4.
Eir [27] and the comprehensive potential ecological risk index (RI) [19] were calculated as follows:
where Tri indicates the toxic response coefficients, which were equal to 30 for Cd, 2 for Cr, 5 for Cu, 5 for Pb, and 1 for Zn. The classification criteria of Eir and RI are shown in Table S5.
3 Results and discussions
3.1 Spatial and seasonal variations of heavy metals in surface sediment
The statistical results of heavy metals concentration were as follows: 0.844–3.10 mg/kg for Cd; 24.8–117 mg/kg for Cu; 60.9–129 mg/kg for Cr; 29.6–218 mg/kg for Zn; and 11.8–54.7 mg/kg for Pb, in winter, respectively (Table 1). The highest value of mean concentration was measured for Cr, followed by Zn, Cu, Pb, and Cd in winter. The heavy metals concentrations were 0.431–3.31 mg/kg for Cd; 28.6–128 mg/kg for Cu; 34.4–95.3 mg/kg for Cr; 61.6–1311 mg/kg for Zn; and 12.3–52.1 mg/kg for Pb in summer, respectively (Table 1) and the order of mean concentration was, from highest to lowest, Zn ˃ Cu ˃ Cr ˃ Pb ˃ Cd in summer. This order differs from the results of Siddiqui and Pandey [28], who reported that the order, from highest to lowest, was Cr ˃ Zn ˃ Cu ˃ Pb ˃ Cd, and also from those of Ji et al. [29], who reported that in the sediments of Baiyangdian Lake, in China, the total metal concentrations followed the order Zn > Cr > Pb > Cu > As > Cd, from highest to lowest.
Compared to the local background values for Zhenjiang, the average concentrations in winter for Cd, Cu, Cr, Pb and Zn were 5.85, 1.02, 1.06, 1.05, and 0.345 folds in winter, and 3.71, 1.15, 0.632, 0.878, and 1.07 folds in summer, respectively. In winter, it was noticed that, apart from Zn, the average concentrations of all other heavy metals, i.e., Cd, Cu, Cr, and Pb, were above the local background values. In summer, the average concentrations of Cr and Pb were below the local background concentration levels. Compared to other lakes or rivers, the average concentrations of Cd, Cu, Pb, and Zn were found to be above the measured concentrations in the Yellow River [30]. Also, the average concentration of Cd in the Yangtze River was far lower than the one measured by the present study [31], which in turn was considerably lower than the one measured in the Pra River [32]. The average concentration of Cd in this study in winter were higher than the average concentrations in Baiyangdian Lake (1.22 mg/kg) [33] and Yanggze River (0.98 mg/kg) [34], whereas, lower than the average values in sediment of Taihu Lake (1.33 mg/kg) [35]. These comparisons indicate that the average concentrations of Cd, Cu, Cr, Pb, and Zn measured in the present study were within the concentration ranges in published data.
The concentration levels and spatial patterns of distribution of Cd, Cu, Cr, Pb, and Zn at the 21 samples sites were dissimilar. For example, in all 21 sites, Cd accumulation was above the local background value. With regards to Cu, 47.6% and 52.4% of sediment accumulation values were above the local background value in both winter and summer. Moreover, 47.6% and 4.76% of Cr accumulation values in sediments were above the local background value in winter and summer, respectively. For Pb, 52.4% and 19.1% of sediment accumulation values were above the local background value in winter and summer, respectively. In winter, the Zn concentration was below the local background value in all 21 sites, while in summer it was above that level on sites S6, S12, S13, S16, and S18. In general, the maximum concentration levels in both winter and summer were recorded for Cd at S13, for Cu at S18, for Cr at S16, for Pb at S6 (in summer) and S18 (in winter), and for Zn at S18. The minimum concentration levels in winter and summer were measured for Cd at S15 and S14, for Cu at S14 and S8, for Cr at S15 and S19, for Pb at S12 and S14, and for Zn at S14 and S11, respectively. Previous studies confirmed significant spatial changes in element concentration. For instance, Duncan et al. [32] confirmed that the concentration levels of heavy metals in the dry season were higher than in the wet season, which could be attributed to the dilution factor in the wet season. The spatial differences in the concentrations of the five heavy elements investigated, obtained in this study, may be due to downstream movements, and fluvial suspended sediments containing heavy metals combined with local exogenous pollution inputs, related to cruise ships, boats, and other human activities. Maanan et al. [36] reported that the heavy metals discharged into natural waterbodies can be easily absorbed by silt and ultimately deposit into sediments. Wang et al. [12] found higher heavy metals concentrations on the surface than in core sediments. In our previous studies [21], significant spatial changes in Cd, Cu, Cr, Pb, and Zn concentrations in the overlying water were found to occur in a same water area. In the current study, the maximum concentration was found mainly in both the main stream and the tributaries of Guyun River, which flow through urban areas and, as such, are greatly affected by human activities.
Apart from spatial variation, the concentrations of the five elements investigated displayed a wide seasonal variability (Table 1). Moreover, seasonal changes also followed inconsistent patterns. For example, the average concentrations of Pb, Cd, and Cr were higher in winter than in summer, whereas the winter contamination levels for Cu and Zn were lower than in summer for the entire sediment. The results of the analysis of variance (ANOVA) indicate significant differences in concentrations between winter and summer for Cr, Zn, and Cd (P < 0.01).
3.2 Contamination factor of heavy metals in surface sediments
The contamination factor (CF) was used for evaluating contamination levels and determine the anthropogenic contribution of heavy metal in sediment [14, 15]. In this study, the CF values for Cd, Cu, Cr, Pb, and Zn are shown in Fig. 1 and Table 2. The range values of CF in winter and summer were, respectively, 2.72–10.0 and 1.39–10. 7 for Cd; 0.459–2.17 and 0.529–2.37 for Cu; 0.732–1.55 and 0.413–1.15 for Cr; 0.452–2.10 and 0.472–2.00 for Pb; and 0.118–0.866 and 0.245–5.20 for Zn. The average CF values for Cd, Cu, Cr, Pb, and Zn were, respectively, 5.85, 1.02, 1.06, 1.05, and 0.345 in winter, and 3.71, 1.15, 0.632, 0.878, and 1.07 in summer. The order of mean CF value was recorded for Cd, followed by Cr, Pb, Cu, and Zn in winter and Cd, Cu, Zn, Pb, and Cr in summer, from highest to lowest.
According to the CF classification’s criterion [27, 37], a total of 7 sites in winter and 3 sites in summer had a recorded CF value for Cd above 6, indicating that 33.3% of the sites in winter, and 14.3% of the sites in summer, were found to have a very high contamination degree. In addition, 52.4% of the sampling sites in winter and 38.1% in summer were classified as having a considerable contamination degree, and 14.3% in winter and 47.6% in summer as having a moderate contamination degree. For Zn, the CF values were lower than 1 for all winter samples, indicating a low contamination degree. However, in summer a total of 2 sites (9.52%) were found to have a considerable contamination degree; 3 sites (14.3%) had a moderate contamination degree; and the CF values of the remaining 16 sites (76.2%) were lower than 1, indicating a low contamination degree. Pb is commonly regarded as a marker element of traffic, as it originates from leaded gasoline [37]. In this study, the winter CF values for Pb were between 1 and 3 in 11 sites (52.4%), which therefore were classified as having a moderate contamination degree; and 10 sites (47.6%) were considered as having a low contamination degree. A dissimilar situation was revealed in summer for Pb, where 17 sites (81.0%) were evaluated as having a low contamination degree, and 4 sites (19.1%) were classified as having a moderate contamination degree, in line with the results of Said et al. [37]. Furthermore, the contamination degree for Cr in winter was evaluated as moderate in 47.6% of sites, and low in 52.4% of sites. In summer, 95.2% of the sites had a low Cr contamination degree, and only 4.76% of sites had a moderate Cr contamination degree. An identical situation was observed for Cu in sediments. A total of 47.6% of sites in winter and 52.4% of sites in summer were found as having a moderate Cu contamination degree, while 52.4% of sites in winter and 47.6% of sites in summer had a low contamination degree.
The spatiotemporal variation of heavy metals contamination degree is the result of the diverse inputs of heavy metal concentrations in the sediments. The higher the release of heavy metal contaminants, the higher the contamination degree in the sediments [38]. In this study, the highest CF level was recorded at site S13 for Cd, at S18 for Cu and Zn, at S16 for Cr, and at S6 (in winter) and S18 (in summer) for Pb. Moreover, the location of the CFs peak values was found as consistent with the points where the maximum concentrations occurred. The highest CF value was obtained for Cd, indicating that the contamination caused by Cd to sediments was higher compared to other elements.
3.3 Enrichment factor of heavy metals in surface sediments
The enrichment factor (EF) is a common parameter to determine the anthropogenic contribution of heavy metal levels in sediment [14, 15]. In this study, the values of the enrichment factor (EF) for Cd, Cu, Cr, Pb, and Zn are shown in Fig. 2 and Table 2. The range values of EF in winter and summer were, respectively, 2.79–15.6 and 1.44–18.3 for Cd; 0.201–2.93 and 0.505–4.13 for Cu; 0.358–2.19 and 0.506–1.98 for Cr; 0.254–2.11 and 0.492–3.48 for Pb; and 0.052–1.17 and 0.263–9.06 for Zn. The average EF values for Cd, Cu, Cr, Pb, and Zn were, respectively, 6.24, 1.13, 1.11, 1.12, and 0.397 in winter, and 4.92, 1.52, 0.802, 1.15, and 1.57 in summer. The order of mean EF value was recorded for Cd, followed by Cu, Pb, Cr, and Zn in winter and Cd, Zn, Cu, Pb, and Cr in summer, from highest to lowest. The results of the analysis of variance (ANOVA) indicate significant differences in EF between winter and summer for Cr (P < 0.01) and Zn (P < 0.05).
According to the EF classification’s criterion [14], only 1 site in winter had a recorded EF value for Pb, Cr and Cu above 2 and below 5, indicating that 4.76% of the sites in winter were found to have a moderate enrichment for Pb, Cr and Cu in winter. All of sites in winter for Cu were found to be in minimal enrichment (EF < 2) in winter. 33.3% and 66.7% of the sites in moderate enrichment (2 ≤ EF < 5) and significant enrichment (5 ≤ CF < 20) for Cd in winter. In summer, a total of 2 sites (9.52%) were found to have a moderate enrichment (2 ≤ EF < 5) and the EF values of the remaining 19 sites (90.5%) were lower than 2, indicating a minimal enrichment for Pb; All of sites in winter for Cr were found to be in minimal enrichment (EF < 2) in summer. 81.0% and 19.0% of the sites in minimal enrichment (EF < 2) and moderate enrichment (2 ≤ EF < 5) for Cu and Zn in summer. A total of 76.2% of sites and 23.8% of sites in summer were found as having a moderate enrichment (2 ≤ EF < 5) and significant enrichment (5 ≤ CF < 20) for Cd in summer, respectively.
3.4 Geo-accumulation index of heavy metals in sediments
The Geo-accumulation index (Igeo) is a pollution assessment tool that predicts the anthropogenic inputs of heavy metals [10, 39]. In this study, the Igeo values for winter sediment samples ranged from − 1.97 to 0.485 for Pb; from 0.840 to 2.61 for Cd; from − 1.71 to 0.532 for Cu; from − 3.67 to − 0.792 for Zn; and from − 1.04 to 0.023 for Cr (Fig. 3 and Table 2). In summer, the Igeo values ranged from − 1.67 to 0.409 for Pb; from − 0.130 to 2.82 for Cd; from − 2.61 to 0.657 for Cu; from − 2.63 to 1.79 for Zn; and from − 1.86 to − 0.420 for Cr. The contamination caused by Cd was more than for other heavy metals in both winter and summer.
According to the Igeo classification’s criterion [22], in winter a total of 20 sites were classified in Class 0 and 1 site in Class 1 (Fig. 4), indicating that 95.2% of the research area was unpolluted, and 4.76% was from unpolluted to moderately polluted for Pb, Cu, and Cr. The sediments analyzed were unpolluted by Zn in winter, as all the corresponding Igeo values were below 0. For Cd in winter, 3 sites were classified in Class 1, 13 sites in Class 2, and 5 sites in Class 3, indicating that 14.3% of sampling sites were from unpolluted to moderately polluted, 62.0% of sites were moderately polluted, and 23.8% of sites were from moderately to heavily polluted. Similar Igeo and pollution levels were obtained for summer sediments. Ghrefat and Yusuf [40] reported strong to extreme Cd contamination levels in the Wadi Al-Arab Dam. Varol and Şen [41] found that the sediments of the upper Tigris River, Turkey, were unpolluted to moderately polluted class for Cr. In the present study, Igeo values were found as closely related to reference background concentrations, whereby the higher the reference background of a metal, the lower the Igeo value. Therefore, differences in background values lead to dramatic changes in Igeo assessment results for a same element. For example, the lower Igeo value for Pb in this study was mainly attributed to the higher reference background value in this study compared to that for Sheyang Lake [42].
3.5 Potential heavy metals ecological risks assessment
The ecological risk values (Eir) in the winter season were in the range 81.6–300 for Cd; 2.30–10.8 for Cu; 1.46–3.10 for Cr; 2.26–10.5 for Pb; and 0.118–0.866 for Zn, with average values of 176, 5.11, 2.12, 5.25, and 0.345 for Cd, Cu, Cr, Pb, and Zn, respectively. In summer, the Eir values were in the range 41.7–320 for Cd, 2.65–11.8 for Cu, 0.827–2.29 for Cr, 2.36–10.0 for Pb, and 0.245–5.20 for Zn, and the average Eir values were 111 for Cd, 5.75 for Cu, 1.26 for Cr, 4.39 for Pb, and 1.07 for Zn (Fig. 5 and Table 2). The ranking of the elements according to their mean values of Eir was, from highest to lowest, Cd ˃ Pb ˃ Cu ˃ Cr ˃ Zn in winter and Cd ˃ Cu ˃ Pb ˃ Cr ˃ Zn in summer.
The average Eir values for Cd indicate a situation of high ecological risk in winter and considerable ecological risk in summer. In fact, 42.9% of sites were exposed to considerable ecological risk by Cd in both winter and summer. More in detail, in summer 42.9% of sites were exposed to a moderate ecological risk, 9.52% to high risk, and 4.76% to very high risk, whereas in winter 57.1% of sites were exposed to high ecological risk. The Eir values of Cu, Cr, Pb, and Zn were all lower than 40 in both winter and summer, indicating that these heavy metals posed a low ecological risk to the sediments. The results indicate also that Cd posed a considerable or high ecological risk, whereas the other heavy metals posed a low ecological risk. These results are consistent with previous research. For example, Zhang et al. [16] found that Cd is more toxic than other heavy metals in the surface sediments of the tributaries of the Han River. Ke et al. [43] revealed that Cd was the major contributor to the ecological risk level in Liaohe River. Cheng et al. [44] surveyed a total of 150 freshwater lakes in China, and found that they were at a moderate risk at national scale for Hg and Cd contamination. Tian et al. [45] reported that the total Cd emissions by coal burning reached 262 t in 2008. Ultimately, such huge emissions flow into freshwater systems and accumulate in lake sediments, resulting in serious environmental pollution [44].
The potential ecological risk index (RI) measures the total ecological risk posed by the contamination of various heavy metals to an ecosystem [43]. In this study, the RI value of the heavy metals investigated ranged from 91.4 to 314 in winter and 48.4–342 in summer, with average values of 188 and 124 in winter and summer, respectively. According to the RI classification criterion shown in Table S5, the recorded RI value in winter was lower than 150 in 7 sites (Figs. 6 and 7), indicating that 33.3% of the sediments were at a low potential ecological risk in winter; in 12 sites, it was higher than 150 and lower than 300, indicating that 57.1% of the sediments were classified at a moderate potential ecological risk; and in 2 sites it was higher than 300 and lower than 600, indicating that 9.5% of the sediments had a considerable ecological risk. In summer (Figs. 6 and Fig. 7), the recorded RI value was lower than 150 in 17 sites, indicating that 81.0% of the sediments were at a low potential ecological risk; in 3 site it were higher than 150 and lower than 300, indicating that 14.3% of the sediments were at a moderate potential ecological risk; in 1 site it was higher than 300 and lower than 600, indicating that 4.8% off the sediments had a considerable ecological risk.
The average RI contributions for the five heavy metals examined were computed; the ranking of the elements was, from highest to lowest, Cd ˃ Pb ˃ Cu ˃ Cr ˃ Zn in winter and Cd ˃ Cu ˃ Pb ˃ Cr ˃ Zn in summer (Fig. 8). Therefore, Cd was the major contributor to the potential ecological risk in both winter and summer. Previous research conducted in Liaohe River revealed a similar result, with the sequence Cd ˃ As ˃ Cu ˃ Pb ˃ Cr ˃ Zn and an Eir value for Cd of 750 in the surface sediment, demonstrating a very high Cd pollution level [43]. Similar work by Yi et al. [46] for the Yangtze River revealed that Cd levels posed a relatively high ecological risk, whereas the levels of Pb, Cu, Cr, and Zn posed a low ecological risk. In addition, Ji et al. [29] reported that Cd entailed a clearly higher potential ecological risk to the sediments of Baiyangdian Lake. Zhang et al. [16] also reported that Cd is more toxic than any other heavy metal in the tributaries of the Han River. Therefore, the issue of how to reduce Cd emissions and their associated ecological risk is important for local heavy metals pollution control.
3.6 Multivariate statistical analysis
Previous research confirmed that the content of heavy metals in sediment might be influenced by the two primary sources, namely natural and anthropogenic sources [16]. Natural sources determine the local background value of heavy metals, which were impact by various geochemical factors, including the original contents in rocks and parent materials, various processes of weathering, depositional and adsorption; Anthropogenic sources were impact by a variety of human activities linked to human population density, urban sewage and garbage, as well as the application of fertilizers and pesticides, etc.[47], As a result, generally bring additional heavy metal enrichment in sediment [16]. Multivariate statistical analysis, including Pearson correlation analysis, principal component analysis (PCA) and cluster analysis (CA), has been widely used to identification the sources of heavy metals [14] in order to timely and effectively controlling the input and enrichment of heavy metals to sediment [16].
In fact, correlation among heavy metals concentrations may reflect a same origin and sources [48], while no correlation may imply multiple sources of release [49]. In the present study, a significant positive correlation was found between Pb and Cu (P ˂ 0.05), Pb and Zn (P ˂ 0.01), and Cu and Zn (P ˂ 0.01) in winter (Table 3). Furthermore, in summer there was a positive significant correlation between Cu and Cd (P ˂ 0.01), Pb and Cd (P ˂ 0.01), and Zn and Cd (P ˂ 0.01), and Zn and Pb (P ˂ 0.01). No significant correlation was observed between Cr and all the other heavy metals in the surface sediments (P > 0.05), except with Cu in winter (P < 0.05). The strong positive relationship between Pb, Zn, and Cu indicates that these heavy metals may have a similar source in the research area.
Further, PCA was employed to determine the similarities in the distribution behavior of heavy metals and their relationships [49]. The PCA results were presented in Fig. 10. Two prominent components with eigenvalues greater than 1 were detected, that explained the 74.2% and 89.3% of the total variance in winter and summer, respectively (Fig. 9), indicating that these two principal components could reflect the distribution of the heavy metals measured from the sampled sediments. The values of the first principal component (PC1) were 2.58 and 3.45, representing 51.5% and 68.9% of the total variance, respectively; these were heavily weighted with winter loadings of 0.962 for Cu, 0.620 for Cr, 0.664 for Pb, and 0.903 for Zn, and with summer loadings of 0.907 for Cd, 0.893 for Cu, 0.849 for Pb, and 0.960 Zn. Previous studies reported that factor loadings ˃0.6, 0.4–0.6, and 0.3–0.4 were classified as strongly, moderately, and weakly associated with elements in the same class, respectively [50]. According to this standard, PC1 was strongly dominated by Cu, Pb, Cr, and Zn in winter, and by Cu, Pb, Cd, and Zn in summer. In addition, PC2 was heavily weighted by Cd with loadings of 0.89 for winter sediments, and was heavily weighted with Cr with loadings of 0.892 in summer. These results indicate that the sources of Cd and Cr in the collected sediments were more complex. Dai et al. [51] reported that different elements may have a similar distribution pattern and sources when they are clustered into one component. In this study, Cu, Pb, Cr, and Zn were clustered in PC1 for winter, indicating that these heavy metals may have a common source in the study region. These results are consistent with the correlation results. In addition, Audry et al. [52] and Qiao et al. [53] reported that Cu and Pb were related to the rapid industrial development and fossil fuels burning. Zheng et al. [54] reported that Cd was associated with the industrial wastes, including electroplating, battery, paint, metallurgy industries, etc., and agricultural wastes, including phosphate fertilizers, pesticides, etc. Therefore, the above-mentioned heavy metals may have originated from anthropogenic sources. The positive loadings of PC2 by Cd in winter and by Cr in summer, measured in this study, indicate that Cd and Cr have a mixed source, which may include both anthropogenic and lithogenic sources.
Cluster analysis is used to determine similarities between observed individuals and the smaller the distance value, the closer these clusters [14]. In this present study, cluster analysis results demonstrated Cd was more distinct from other element in winter and Cr was more distinct from other element in summer (Fig. 10). Obviously, the cluster analysis results were in good agreement with the correlation coefficients and PCA results.
4 Conclusion
In this study, the concentrations of the five heavy metals investigated (namely, Cd, Cu, Cr, Pb, and Zn) displayed a wide spatial and seasonal variability in the surface sediments of the freshwater ecosystem of Zhenjiang. The average concentration of Cd, Cu, Cr, and Pb in winter was above the local background concentration levels, whereas in summer the average concentration of Cr and Pb was below. The average concentrations of Cd, Cu, Cr, Pb, and Zn were within the concentration ranges in published data. The highest CF value was recorded for Cd in both seasons, followed by Cr, Pb, Cu, and Zn in winter, and Cd, Cu, Zn, Pb and Cr in summer. These EF results reveal that the enrichment caused by Cd in surface sediments was more severe than for the other heavy metals. Looking at the Eir classification’s criterion, 42.9% of sties were exposed to considerable ecological risk for Cd in both winter and summer, respectively; in summer, 42.9%, 9.52% and 4.76% of sites were exposed to a moderate ecological risk, a high risk and a very high risk, respectively, whereas 57.1% of sites were exposed to a high ecological risk in winter. The other four heavy metals analyzed (i.e., Cu, Cr, Pb, and Zn) posed a low ecological risk to the sediments in the research area in both winter and summer. The multivariate statistical analysis results indicate that in the study region, Cd and Cr have a mixed source of pollution, which might include both anthropogenic and lithogenic sources, whereas other elements may have a common source. However, the above results were solely based on surface sediment samples, the interactions of heavy metals among sediment, overlying water, aquatic plants and aquatic animals in freshwater ecosystem of Zhenjiang City, China, should be conducted in the future. In addition, vertical sediment samples need to be further collected in order to comprehensive record heavy metals contamination level over time by human activities.
Data availability
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.
References
Liang P, Bartzas G (2021) Editorial overview: Heavy metals and metalloids: a serious threat to environment and human health. Curr Opin Environ Sci Health 23:100287
Zhao YQ, Yang Y, Dai RK, Leszek S, Wang XY, Xiao LZ (2021) Adsorption and migration of heavy metals between sediments and overlying water in the Xinhe River in central China. Water Sci Technol 84(5):1257
Hassimi H, Taleb A, Bouezmarni M, Karzazi O, Taleb M, Kherbeche A, Debbaut V (2019) The effect of the physicochemical conditions variations on the behavior of heavy metals trapped in polluted fluvial system sediments: the case of OuedSebou, Morocco. Appl Water Sci 9:17
Agah H, Leermakers M, Elskens M, Fatemi SMR, Baeyens W (2009) Accumulation of trace metals in the muscles and liver tissues of five fish species from the Persian Gulf. Environ Monit Assess 157(1–4):499–514
Sobhanardakani S (2017) Potential health risk assessment of heavy metals via consumption of caviar of Persian sturgeon. Mar Pollut Bull 123(1–2):34–38
Li JL, Zuo QT, Feng F, Jia HT (2022) Occurrence and ecological risk assessment of heavy metals from Wuliangsuhai Lake, Yellow River Basin, China. Water 14(8):1264
Wang QQ, Duan WY, Yao XM, Guo XN, Liu DY, Gao WM, Zhang JD (2022) Distribution and ecological risk assessment of heavy metals in the sediments of Changli ecological monitoring area, northwest of Bohai Bay, China. Environ Pollut Bioavailab 34(1):180–189
Paramasivam K, Ramasamy V, Suresh G (2015) Impact of sediment characteristics on the heavy metal concentration and their ecological risk level of surface sediments of Vaigai river, Tamilnadu, India. Spectrochim Acta A 137:397–407
Filgueiras AV, Lavilla I, Bendicho C (2004) Evaluation of distribution, mobility and binding behaviour of heavy metals in surficial sediments of Louro River (Galicia, Spain) using chemometric analysis: a case study. Sci Total Environ 330:115–129
Maina CW, Sang JK, Raude JM, Mutua BM (2019) Geochronological and spatial distribution of heavy metal contamination in sediment from Lake Naivasha, Kenya. J Radiat Res Appl Sci 12(1):37–54
Sobhanardakania S, Taghavi L (2016) Nickel, lead and zinc contamination in the surface sediments of Agh gel wetland. Iran Arch Hyg Sci 5(3):172–178
Wang SH, Wang WW, Chen JY, Li Z, Zhang B, Jiang X (2019) Geochemical baseline establishment and pollution source determination of heavy metals in lake sediments: a case study in Lihu Lake, China. Sci Total Environ 657:978–986
Lin KN, Lim YC, Chen CW, Chen CF, Kao CM, Dong CD (2022) Spatiotemporal variation and ecological risk assessment of heavy metals in industrialized urban river sediments: Fengshan river in southern Taiwan as a case study. Appl Sci 12(3):1013
Ustaoğlu F, Tepe Y (2019) Water quality and sediment contamination assessment of Pazarsuyu Stream, Turkey using multivariate statistical methods and pollution indicators. Int Soil Water Conserv Res 7(1):47–56
Ustaoğlu F, Tepe Y, Aydin H (2020) Heavy metals in sediments of two nearby streams from Southeastern Black Sea coast: contamination and ecological risk assessment. Environ Forensics 21(2):145–156
Zhang JH, Li XC, Guo LQ, Deng ZM, Wang DW, Liu LS (2021) Assessment of heavy metal pollution and water quality characteristics of the reservoir control reaches in the middle Han River, China. Sci Total Environ 799:149472
Rajeshkumar S, Liu Y, Zhang X, Ravikumar B, Bai G, Li X (2018) Studies on seasonal pollution of heavy metals in water, sediment, fish and oyster from the Meiliang Bay of Taihu Lake in China. Chemosphere 191:626–638
Lorena SM, Buddhi W, Godwin AA, Prasanna E, Ashantha G (2021) Water-sediment interactions and mobility of heavy metals in aquatic environments. Water Res 202:117386
Li Z, Liu JJ, Chen HL, Li QQ, Yu C, Huang X, Guo HC (2020) Water environment in the Tibetan Plateau: heavy metal distribution analysis of surface sediments in the Yarlung Tsangpo River Basin. Environ Geochem Health 42:2451–2469
Bi B, Liu X, Guo X, Lu S (2018) Occurrence and risk assessment of heavy metals in water, sediment, and fish from Dongting Lake, China. Environ Sci Pollut Res 25(34):34076–34090
Dadzie AA, Yuan LB, Xing SY, Liu X, Zhou XH (2020) Survey and assessment of metals distribution in the overlying water of the representative lake and rivers in Zhenjiang, China. SN Appl Sci 2:776
Islam SM, Ahmed MK, Raknuzzaman M, Habibullah-Al-Mamun M, Islam MK (2015) Heavy metal pollution in surface water and sediment: a preliminary assessment of an urban river in a developing country. Ecol Indic 48:282–291
Wu CD, Qu J, Li MJ, Zhang B (2009) Distribution features and evaluation on potential ecological risk of heavy metals in sediment of Neijiang River of Zhenjiang city. Environ Monit China 25(5):93–97 (In Chinese)
Liu LH (2022) Pollution characteristics and risk assessment of heavy metals in sediments in the coastal areas of southwest Fujian. Mar Environ Sci 41(2):200–207 (In Chinese)
Yan MC, Chi QH, Gu TX, Wang CS (1995) Average element content of various sediments in China. Geophys Geochem Explor 19(6):468–472
Mueller G (1979) Heavy-metals in sediment of the rhinechanges since 1971. Umsch Wiss Tech 79(24):778–783
Hakanson L (1980) An ecological risk index for aquatic pollution control. A sedimentological approach. Water Res 14(8):975–1001
Siddiqui E, Pandey J (2019) Assessment of heavy metal pollution in water and surface sediment and evaluation of ecological risks associated with sediment contamination in the Ganga River: a basin-scale study. Environ Sci Pollut Res 26(11):10926–10940
Ji Z, Zhang Y, Zhang H, Huang C, Pei Y (2019) Fraction spatial distributions and ecological risk assessment of heavy metals in the sediments of Baiyangdian Lake. Ecotoxicol Environ Safe 174:417–428
Yan N, Liu W, Xie H, Gao L, Han Y, Wang M, Li H (2016) Distribution and assessment of heavy metals in the surface sediment of Yellow River, China. J Environ Sci 39:45–51
Zhang W, Feng H, Chang J, Qu J, Xie H, Yu L (2009) Heavy metal contamination in surface sediments of Yangtze River intertidal zone: an assessment from different indexes. Environ Pollut 157(5):1533–1543
Duncan AE, de Vries N, Nyarko KB (2018) Assessment of heavy metal pollution in the sediments of the River Pra and its tributaries. Water Air Soil Pollut 229(8):272
Zhang C, Shan BQ, Zhao Y, Song ZX, Tang WZ (2018) Spatial distribution, fractionation, toxicity and risk assessment of surface sediments from the Baiyangdian Lake in northern China. Ecol Ind 90:633–642
Wang Y, Yang ZF,·Shen ZY, Tang ZW, Niu JF, Ga F (2011) Assessment of heavy metals in sediments from a typical catchment of the Yangtze River, China. Environ Monit Assess172: 407–417
Yu T, Zhang Y, Meng W, Hu XN (2012) Characterization of heavy metals in water and sediments in Taihu Lake, China. Environ Monit Assess 184:4367–4382
Maanan M, Saddik M, Maanan M, Chaibi M, Assobhei O, Zourarah B (2015) Environmental and ecological risk assessment of heavy metals in sediments of Nador Lagoon, Morocco. Ecol Ind 48:616–626
Said I, Salman SA, Elnazer AA (2019) Multivariate statistics and contamination factor to identify trace elements pollution in soil around Gerga City, Egypt. Bull Natl Res Centre 43(1):43
Ullah R, Muhammad S, Jadoon IA (2019) Potentially harmful elements contamination in water and sediment: evaluation for risk assessment and provenance in the northern Sulaiman fold belt, Baluchistan, Pakistan. Microchem J 147:1155–1162
El-Kady AA, Wade TL, Sweet ST, Klein AG (2019) Spatial distribution and ecological risk assessment of trace metals in surface sediments of Lake Qaroun, Egypt. Environ Monit Assess 191:413
Ghrefat H, Yusuf N (2006) Assessing Mn, Fe, Cu, Zn, and Cd pollution in bottom sediments of Wadi Al-Arab Dam, Jordan. Chemosphere 65(11):2114–2121
Varol M, Şen B (2012) Assessment of nutrient and heavy metal contamination in surface water and sediments of the upper Tigris River, Turkey. CATENA 92:1–10
Zhao YF, Xu M, Liu Q, Wang ZF, Zhao L, Chen Y (2018) Study of heavy metal pollution, ecological risk and source apportionment in the surface water and sediments of the Jiangsu coastal region, China: a case study of the Sheyang Estuary. Mar Pollut Bull 137:601–609
Ke X, Gui S, Huang H, Zhang H, Wang C, Guo W (2017) Ecological risk assessment and source identification for heavy metals in surface sediment from the Liaohe River protected area, China. Chemosphere 175:473–481
Cheng HX, Li M, Zhao CD, Yang K, Li K, Peng M, Yang ZF, Liu F, Liu YG, Bai RJ, Cui YJ, Huang ZF, Li LH, Liao QL, Luo JL, Jia SJ, Pang XG, Yang J, Yin GS (2015) Concentrations of toxic metals and ecological risk assessment for sediments of major freshwater lakes in China. J Geochem Explor 157:15–26
Tian H, Cheng K, Wang Y, Zhao D, Lu L, Jia W, Hao J (2012) Temporal and spatial variation characteristics of atmospheric emissions of Cd, Cr, and Pb from coal in China. Atmos Environ 50:157–163
Yi YJ, Sun J, Tang CH, Zhang SH (2016) Ecological risk assessment of heavy metals in sediment in the upper reach of the Yangtze River. Environ Sci Pollut R 23(11):11002–11013
Jiang YF, Ye YC, Guo X (2019) Spatiotemporal variation of soil heavy metals in farmland influenced by human activities in the Poyang Lake region, China. CATENA 176:279–288
Hadlich HL, Venturini N, Martins CC, Hatje V, Tinelli P, de Oliveira Gomes LE, Bernardino AF (2018) Multiple biogeochemical indicators of environmental quality in tropical estuaries reveal contrasting conservation opportunities. Ecol Indic 95:21–31
Christophoridis C, Evgenakis E, Bourliva A, Papadopoulou L, Fytianos K (2020) Concentration, fractionation, and ecological risk assessment of heavy metals and phosphorus in surface sediments from lakes in N.Greece. Environ Geochem Health 42:2747–2769
Hadzi GY, Ayoko GA, Essumang DK, Osae SKD (2019) Contamination impact and human health risk assessment of heavy metals in surface soils from selected major mining areas in Ghana. Environ Geochem Health 41(6):2821–2843
Dai LJ, Wang LQ, Li LF, Liang T, Zhang YY, Ma CX, Xing BS (2018) Multivariate geostatistical analysis and source identification of heavy metals in the sediment of Poyang Lake in China. Sci Total Environ 621:1433–1444
Audry S, Schafer J, Blanc G, Jouanneau JM (2004) Fifty-year sedimentary record of heavy metal pollution (Cd, Zn, Cu, Pb) in the Lot River reservoirs (France). Environ Pollut 132:413–426
Qiao Y, Yang Y, Gu J, Zhao J (2013) Distribution and geochemical speciation of heavy metals in sediments from coastal area suffered rapid urbanization, a case study of Shantou Bay, China. Mar Pollut Bull 68:140–146
Zheng JP, Jiao XM, Fang NJ et al (2017) Source and risk assessment of heavy metals in sediments in Jiangsu coastal areas. China Environ Sci 37(4):1514–1522
Acknowledgements
This study was, in part, supported by the Grants from the National Natural Science Foundation of China (No. 31971472), by the Jiangsu province water conservancy science and technology project (No. 2016050), and by the Jiangsu provincial environmental monitoring and research fund project (No. 1816).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors confirm that there is no conflict of interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Liu, X., Dadzie, A.A., Yuan, L. et al. Analysis and potential ecological risk assessment of heavy metals in surface sediments of the freshwater ecosystem in Zhenjiang City, China. SN Appl. Sci. 4, 258 (2022). https://doi.org/10.1007/s42452-022-05127-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-022-05127-4