Abstract
This study aims to characterise and discuss the potential use of sediment from the Arkelstorp bay located in the south of Sweden. Sediments from the Arkelstorp bay is collected and analysed on nutrient content, age, and potential contaminants. No organic environmental pollutants are found and the metal content are not elevated but still problematically high. For example, the amount of cadmium per phosphorus is 480 mg Cd kg−1 P. However, as the carbon 14-datings showed, the sediment is preindustrial. Therefore, the amount of registered cadmium comes naturally from the surrounding environment. Arkelstorp sediments present the potential to become a source of nutrition in agriculture. The results show that the material is a good source of nutrients, with a nitrogen content of 18 g kg−1 dry matter (DM), phosphorus 0.8 g kg−1 DM and potassium 2.4 g kg−1 DM respectively. However, the metal content is problematic to use the material without any pre-treatment. On the other hand, bioenergy production is expected to increase in the future, where this resource could potentially be helpful for the cultivation of bioenergy crops.
Highlights
-
Lake sediments were characterised in their nutrient content, and it was found that the material could be an interesting source as a replacement for mineral fertilizer.
-
Sediments contained no elevated amount of metals, but the cadmium content could limit the use of the material as fertiliser in agriculture.
-
Sediments were analysed for organic contaminants, and none were found.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
1 Introduction
Sweden is well known for its more than 100,000 lakes that cover about 9% of the total surface area of the Kingdom [1]. Centuries of human influence with agriculture and wastewater have left their mark on many of these lakes, which are struggling with eutrophication [2]. Furthermore, shallow lakes are quickly accumulating organic matter and, on the way, to be transformed from lakes to swamps instead. Nutrients in shallow lakes are also not steady bound to the sediments, but there is an exchange from the sediments to the water, and excess nutrients can thus migrate downstream [3]. Therefore, it is sometimes suggested to dredge these lakes to reduce their content of nutrients and create recreational water bodies that invite swimming and fishing.
Sediments are typically rich in beneficial compounds (like organic matter and nutrients) that could potentially be recovered to contribute to minimising the mining of resources [4]. However, dredged material is, according to European legislation 2008/98/EC, to be considered waste. Even though dredge material is regarded as a residue, the Waste Framework Directive dictates that disposal of dredged sediments should only be used as a last resort since the waste hierarchy dictates that the material should be recycled rather than landfilled [5]. As Renella [6] points out, other legislations also hinder the use of dredged sediments. In Sweden, the use of organic materials (such as sewage sludge or dredged sediments) as soil conditioners is regulated by the ratio between cadmium and phosphorous [7]. According to the regulation, ratios higher than100 mg Cd kg−1 P are not allowed on the Swedish market. The legislation, by setting a limit on the amount of cadmium per phosphorus in fertilisers, prevents the use of lake sediments in agriculture. This paper will show how the beneficial use of sediments would be better for the environment and society and stand as an example of the resources that are to be utilised in lakes.
1.1 Arkelstorp bay case
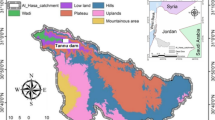
Arkelstorp bay has a long history of human activity around the beaches. The greatest impact on the bay was the lake lowering, which took place in 1878, where the water level was lowered by 2.1 m to create more agricultural land. Due to the lake lowering, the bay was separated from the lake, which now only has contact with Oppmannasjön through a more than one-kilometre-long canal. The lowering of the lake, together with the large nutrient load during the twentieth century, has created a shallow lake, which now risks being converted into wetlands (Fig. 1). Today the water level is around one meter and fluctuates by around 1 m during the year [8]. According to the Swedish guidelines, the bay is heavily eutrophicated [8]. The report confirms that the main reason for the eutrophication is the lowering of the lake that created a shallow bay divided from the main lake. This low amount of water combined with a high load of nutrients, mainly from sewage and agricultural runoff, causes algae blooms.
Immediately downstream of Arkelstorp bay, there are two lakes Ivösjön and Oppmannasjön pointed out by the European Union as a special area of conservation and part of the Natura 2000 network (SiteCode: SE0420319). Today, various projects are underway to reduce the nutrient load in this region. For example, in 2020, a project was completed and showed that an effective way to reduce the nutrient load downstream would be to dredge Arkelstorp bay [9]. By dredging 1–2 m, a greater water depth would be obtained. The bay ability to accumulate and retain phosphorus-rich sediment in the future would increase, lowering the nutrient load on the important biodiverse areas downstream. Furthermore, it would create an attractive residential area near the lake for the citizens in the Arkelstorp community. However, dredging an area of this size can be costly, especially if the sediment is contaminated. However, if the sediment could be used and seen as a resource, the cost would be significantly reduced. Characterisation of the chemical properties of sediments is the first step towards recycling/using these sediments as substrates for agriculture.
1.2 Recycling off lake sediments
Anthropogenic and natural weathering activities lead to the accumulation of significant contents of organic and inorganic compounds (like metals and nutrients) in limnic environments. Especially in sediments, as they are the final sink of these compounds [10]. Metals threaten aquatic ecosystems with their biogeochemical persistence in nature. Nutrients also present a hazard to marine environments. Industrial and agriculture activities are the common sources of metals and nutrients through mining, smelting, combustion of fuels, and drainages from farms and landfills [11]. This could lead to the accumulation of significant amounts of different elements in water resources, such as lakes. Metals (like Pb, Hg, Cd, and others) are well known for their toxic effects on the environment and human health. When the elements are stored in substantial levels in living cells, they lead to negative impacts like carcinogenic effects [12]. In contrast, nutrients are responsible for the eutrophication of small and big lakes and water bodies in different parts of the world [13].
On the other hand, nutrients are vital for the growing of plants. Mineral fertiliser is available in many forms on the market; however, the main components are nitrogen (N), phosphate (P) and potassium (K). These minerals are also essential for living [14]. For instance, adults daily need about 4000 mg of K to process important body functions, like regulation of fluid balance [15]. There are several reasons to discuss different methods and techniques to recycle these elements. Nitrogen, as an example, is harvested from atmospheric nitrogen gas, which is an energy-demanding process. The main source of phosphate and potassium is from mining activity [14]. Thus, phosphorus is considered one of the critical elements within the European Union and the whole world [16]. The known supply of phosphorus minerals is expected to be exhausted within 50–100 years [17]. In comparison, potassium is more accessible within the next 600 years [18]. One more reason to discuss the availability of nutrients is that mineral deposits are highly aggregated globally, and different geopolitics can cause shortages. It is well known that more than 75% of the world phosphorus reserves are found in Morocco [19], which has the ability to control the prices from a long-range perspective. For example, in 2008, the world phosphate prices spiked to high records to more than 800% during days [20]. These elements are natural-finite resources, and therefore, recycling and reusing need to be considered. Sweden alone used 215,200 tons of N, 32,400 tons of K, 16,600 tons of P during the agricultural season of 2019/20 [21].
Alternative to the use of sediments as fertilisers, different other uses exist depending on the physical and chemical composition of the material. For example, sediments can be used for landscape reconstruction [6], road construction and filling material in the building sector [22]; Also building materials such as bricks can under certain conditions be created by sediment [22]
Dredging of sediments is a potential solution to remediate water bodies and reach beneficial water levels [23]. Specifying the properties of sediments through characterisation studies is essential to understand the potential recycling routes and risks associated with future use. The goal should be to employ the material as far as possible for beneficial purposes. If sediments are not contaminated, the simplest method of using nutrients should be employing the material as a soil conditioner in agricultural fields. This manuscript will first show a characterisation of lake sediments on both nutrient content and potential contamination parameters. Then, it discusses the potential usability of the sediment, intending to use its nutrient content.
2 Materials and methods
2.1 Site description
Arkeltstorp bay (56.16075° N 14.28572° E) is a semi-enclosed bay located at Kristianstad, southern Sweden. The bay is connected to Oppmanna lake on the north side by a narrow channel created in the nineteenth century by lowering the lake (Fig. 1). The total surface area is 80 hectares. Three smaller urban communities (Immeln, Arkelstorp and Oppmanna) are located near the bay, with approximately 1000 people living around the drainage area. The main activities around the bay are agriculture and forestry. Agriculture is diverse, but the area is famous for growing different fruits and berries (such as apples, pears, strawberries, and raspberries). Thus, pesticides were expected to be found in the lake.
2.2 Sample collection and processing
Lake sediment samples were collected from three different areas in the bay during February 2019. Sampling was planned to be done from the ice to facilitate sampling and collection of the sediment cores. Three samples in a transect from north to south were planned. Unfortunately, the winter did not turn out as expected, and the ice had poor strength, after which the sampling sites had to be adjusted (Fig. 1). Samples were taken from the frozen lake during the winter. As a first step, the water depth from the ice to the lake bottom was defined. The difference between the water phase and sediment phase (lake bottom) is diffuse at this lake. Therefore, the water depth was measured using a plastic disc with a diameter of 12 cm. The disc was loaded with a weight of 325 g. The transit where this disc stayed on the sediment surface was defined as the lake bottom. A Russian core manual sampler was employed to extract sediment samples. One core was taken from each sampling point. Using a plastic spatula, the 2 m cores were sliced on four different depths listed as the top (0.1–0.6 m), top-medium (0.6–1.1 m), medium-bottom (1.1–1.6 m) and bottom (1.6–2.1 m). The first 10 cm were not sampled since the material was too liquid, and the core sampler did not allow it collection. The samples froze fast due to the winter conditions, and they were stored in a freezer until further analyses. The sediment depth was additionally measured at each point using a manual sampler. The probe was introduced until reaching the bottom of the sediment column. At sites 2 and 3, the sediment depth was deeper than the expected values. Therefore, the probe (including its extension) could not reach the lake bottom.
2.3 Analyses
The samples were analysed at the commercial laboratory SYNLAB, Sweden. The analyses included solid and organic content and metals (As, Pb, Cd, Cu, Ni, Zn, Fe and Hg), organic compounds (DDT, PAH, PCB, HCH, Aldrin and glyphosate) and nutrient (total N, P and K) contents. Additionally, carbon 14 was analysed for the bottom depth to determine the age of the sediments.
Dry matter was calculated following the standard [24] by measuring the difference in weight before and after heating a specific weight of each sample at 105 ± 5 °C until reaching a constant weight. In contrast, the loss on ignition was found according to the standard [25] by measuring the difference in weight before and after heating a specific weight from each sample at 500 °C. Trace-element content was analysed according to [26] and [27] by inductively coupled plasma atomic emission spectroscopy (ICP-AES). Organic compounds were measured by gas-chromatography–mass-spectrometry (GC–MS). Total nitrogen was analysed according to the Kjeldahl procedure [28], and phosphorus and potassium according to [27].
Carbon dating samples were sent to Lund University, radiocarbon dating laboratory. Since there was a possibility that waves and boat activities disturb the top sediment layers, only samples from 1.6–2.1 m down were dated. The dating was carried out on plant mater with a terrestrial origin, as limnic mater could be contaminated with carbon from dissolved limestone. The material was collected by sieving a part of the collected lake sediment. From the sieved material, birch seeds and leaf fragments were collected and dried. The sample at point 3 was divided into two samples for different dating, allowing to do a control of the method.
2.4 Contamination risk factor
The level of metal pollution was calculated using the contamination factor. Equation 1 was employed, where Cs represents the metal content on the samples and Cb the natural background levels of the element. Results could be interpreted as Cf < 1 no pollution; 1 < Cf < 3 moderate pollution; 3 < Cf < 6 considerable contamination; and Cf > 6 very high pollution [29]. Background levels were adopted from the Swedish Environmental Protection Agency (SEPA), using the content provided for pristine-natural conditions for the national territory [30].
2.5 Statistics
The analysis of variance (ANOVA) was employed to calculate the statistical significance (p < 0.05) of the organic and dry content and the metal and nutrient content of the samples for the different sampling points and heights. The conditions of normality and homogeneity were determined prior to the analysis. When the requirements were violated, the Wilcoxon and Kruskal test was also run to verify the results from the ANOVA test. The Tukey test was used to find the difference between groups when a significant impact was detected. Additionally, the correlation between the variables was calculated using the Pearson correlation test. The statistical analyses were calculated using the software R version 3.6.1.
3 Results and discussion
3.1 Physical characteristics
The water and sediment depths for the different sampling points are shown in Fig. 1. The water depth denotes low values, with the shallowest point presenting a depth of 60 cm in the first sampling station. The additional points present a water depth of about 1 m. The sediment column has high depths of 6 m for point 1 and higher than 7 m for points 2 and 3. The water and sediment depths show a potential to dredge the bay to improve and increase the water level, allowing leisure activities such as bathing, canoeing, or boating.
Table 1 presents the results of the physical characteristics as an average for each layer. Sediments are typically constituted by water and require dewatering before further management [31]. In Arkeltstorp bay, most of the sediment consists of water (dry matter: 9.97 ± 2.19%), and the mean content is significantly constant for each of the sampling points (ANOVA, F2,9 = 0.22, P = 0.81), suggesting homogeneity through the lake. Additionally, the dry content significantly increases further down in the sediment column (ANOVA, F3,8 = 26.33, P = 0.00017). A Tukey-test (α = 0.05) shows that the top and medium-top layers have a considerably higher mean solid content than the medium-bottom and bottom sections.
The organic content is an important parameter in sediments since it relates to the availability of metals and nutrients [31]. In this study, the loss on ignition suggests that 30.7 ± 3.6% of the dry matter is organic, this is significantly higher than other reported findings [5]. The parameter has a similar mean content among the sampling points (ANOVA, F2,9 = 0.074, P = 0.93). However, the mean organic content has a significant difference by layers (ANOVA, F3,8 = 10.78, P = 0.0035). The top and top-medium are significantly more organic than the lower layers (Tukey test, α = 0.05), potentially related to the degradation of the material in lower layers.
3.2 Carbon 14 results
Since the lake is heavily eutrophicated and affected by human activities, an important question is if the nutrient and metal compositions on the sediments come from natural origins or human activity. There was a suspicion that large amounts of the elements came from the dairy, fruit-must, starch and wood industries that historically have been found in the area. As shown in Table 2, the dating varies between 152 AD and 967 BC, meaning that the samples are about 2400 years old ± 550 years old. This big spread was expected as the samples were a composite sample created from half a meter of the sediment column. However, it tells us that the samples are significantly older than the industrial age (around 1850). The results suggest that, in the bottom layers, the content of nutrients and metals comes from natural sources since the human discharges at that time were unlikely. This rough measurement of sediment age indicates a sedimentation rate of 0.8 mm year−1 calculated as mean depth divided by mean age. which is in line with sedimentation rates used in other studies [32, 33]. The rate of sedimentation can vary greatly throughout history [34], but the purpose was only to show that the sediments were not caused by modern human activity. As shown with point 3, there was a low difference in the age estimation between the different sub-samples, indicating a reasonable accuracy with the method.
3.3 Nutritional results
The total content of nitrogen, phosphorous and potassium are on average 18,083 ± 3343, 832 ± 133 and 2425 ± 386 mg kg−1 dry matter (DM), respectively, on average a ratio 22:1:3 (N:P:K). Table 1 shows the different mean content by layers. The nitrogen level is high compared to other sediments. Burdige [35] reported continental high organic sediments with a maximum total nitrogen content of 12,000 mg kg−1 DM. In addition, compared to commercial organic and chemical fertilisers, Arkeltstorp sediments present medium–high contents of nitrogen and low content of phosphorous and potassium. Regarding organic soil conditioners, compost is a widely employed product, and its nutrient composition depends on the material employed for its production. Typical values (in mg kg−1 DM) for N, P and K are 3000–15,000, 1000–10,000 and 3000–10,000, respectively [36]. Additionally, the commonly used 20-20-20 fertiliser has a composition of 20,000 mg kg−1 of fertiliser for N, P and K, highlighting that the sediments require higher levels of phosphorous and potassium to reach the quality from marketable products. Potentially, dredged sediments could be mixed with compost or other organic substrates to improve the nutrient distribution and increase the content of micro and macro nutrients [37].
The nutrient content are homogeneous and show no significant variation between the sampling points (N: ANOVA, F2,9 = 0.10, P = 0.91; P: ANOVA, F2,9 = 0.25, P = 0.78; K: ANOVA, F2,9 = 1.09, P = 0.38), suggesting that elements of all points has the same source. Oppositely, the mean content of nutrients is significantly different in layers of the sediment column (N: ANOVA, F3,8 = 12,7, P = 0.0021; P: ANOVA, F3,8 = 4.92, P = 0.032; K: ANOVA, F3,8 = 4.80, P = 0.034). According to a Tukey test (α = 0.05), for nitrogen and phosphorous, the top has a significantly higher content than the medium-bottom and bottom sections. Additionally, the top-medium presents a considerably higher level of nitrogen and potassium compared to the bottom depth. The higher content of nutrients in top layers could be explained by a higher discharge in the present times potentially related to the farms around the study site.
Regarded the potential recovery amount of nutrients, the study counted with only 3 sites to determine the sediment depth. It is therefore not possible to exactly estimate the mean sediment depth in the bay. However, every probing shows a depth of 6 m or higher. As mentioned in the introduction, 1–2 m would be enough to improve water quality. Since the bay is roughly 80 ha, if one meter is dredged, the total wet volume of sediment to remove would be around 800,000 m3. The dry weight is on average 10%; hence, the dry volume is around 80,000 tons of dry material. Accounting for the nutrient sources, the dredge masses then has 72 tons of phosphorus and 1440 tons of nitrogen in total.
3.4 Pesticides and organic pollutants
In total, 58 different pollutants were analysed, including DDT, PAH, PCB, HCH, Aldrin and glyphosate. As expected, organic contaminants were not traceable in the samples due to the lack of industrial discharges. Only monobutyltin and monofenyltin were present above detection limits. However, both substances were only slightly above the detection limit, not presenting any harmful risk. The content of the compounds for the different sampling points is found in the additional material section. The lack of organic compounds is also related to the age of the sediments. According to the results, the sedimentation rate is only approximately 1 mm per year. Thus, the latest hundred years are found in the top 10 cm layer, which was not sampled because of the lack of a proper sampling procedure. Due to the human activity around the area, it is possible that this first layer could contain traces of organic pollutants of PAH, glyphosate and others. However, the liquid state, which prevents sampling of this top 10 cm, indicates an extremely low amount of dry matter. Thus, any concentrations found in this layer would not represent as much in mass, likely not affecting beneficial uses.
3.5 Trace elements
Metals are present, even according to the obtained sedimentation rate, the sampled sediments are older than 100 years. The content of metals follow the order Fe < Zn < Cu < Cr < Ni < Pb < As < Cd < Hg, presenting a mean content (in mg kg−1 DM) of 30,000 ± 4285 for Fe, 145 ± 28 for Zn, 30 ± 4 for Cu, 27 ± 3 for Cr, 21 ± 2 for Ni, 18 ± 11 for Pb, 6.3 ± 0.6 for As, 0.65 ± 0.28 for Cd and 0.48 ± 0.42 for Hg. Table 3 illustrates the mean content of metals/metalloids in the different sediment depths. Regarding the distribution of metals in the lake, on the one hand, the content of elements is significantly homogeneous between the sampling points (As: ANOVA, F2,9 = 0.27, P = 0.77; Pb: ANOVA, F2,9 = 0.21, P = 0.88; Cd: ANOVA, F2,9 = 0.12, P = 0.89; Cu: ANOVA, F2,9 = 1.58, P = 0.26; Cr: ANOVA, F2,9 = 0.91, P = 0.44; Ni: ANOVA, F2,9 = 4.33, P = 0.055; Zn: ANOVA, F2,9 = 0.37, P = 0.71; Fe: ANOVA, F2,9 = 1.83, P = 0.21; Hg: ANOVA, F2,9 = 2.73, P = 0.12). The constant distribution suggests a same source of origin. Person-correlation coefficients for metals and loss of ignitions are shown in Table 1. Positive trends among metals also express a similar potential origin. The highest correlations (R > 0.85) are Pb–Cd, Pb–Cu, Cu–Cd, Zn–Pb, Zn–Cd, and Zn–Cu. A medium correlation is shown (0.86 < R < 0.6) for Cr–Pb, Cr–Cd, Cr–Cu, Ni–Cr, Ni–Cu, Zn–Cr, Fe–Ni, Fe–As and Fe–Cr.
On the other hand, arsenic, lead, cadmium, copper and zinc show a significant difference in the mean content between layers (As: ANOVA, F3,8 = 9.01, P = 0.006; Pb: ANOVA, F3,8 = 21.42, P = 0.00035; Cd: ANOVA, F3,8 = 25.69, P = 0.00019; Cu: ANOVA, F3,8 = 5.911, P = 0.020; Zn: ANOVA, F3,8 = 17.8, P = 0.00067). The top denotes a considerably higher content than the top-medium, medium-bottom and bottom depths for Cd, Pb and Zn. The same trend applies to Cu, but the top-medium layer and the top have a similar mean content (Tukey test, α = 0.05). The higher presence of metals in the top section expresses that the discharge of elements was intensified during more-recent times, possibly related to the human activities around the lake. However, the concentration is still below the guidelines found in Table 4.
Oppositely to the other elements, the arsenic content in the bottom and medium-bottom layers is significantly higher than the top and top-medium depths (Tukey test, α = 0.05). The trend could be explained by the distribution of iron in the sediment column (where high contents are present in the bottom layers). Arsenic is highly bound to iron [38, 39], explaining the high content of both elements in the bottom layers. Results are confirmed with the medium–high correlation between the elements (R = 0.7). Moreover, arsenic and iron could be present in higher contents in the bottom layers due to a higher weathering rate in older times.
In Sweden, when sediments are extracted from water bodies, the maximum permissible metal contents are determined according to the potential use on what the material will be employed. The guideline includes thresholds for sensitive and less-sensitive land uses. Table 4 illustrates the limits, and it is shown that less-sensitive thresholds are never exceeded. Sensitive limits are overpassed by cadmium in the top and mercury in all depths. The contents are near the threshold values. Therefore, before using the sediments in agriculture, it is recommended to further collect more samples around the lake and analyse the metals to guarantee compliance with the Swedish legislation. Potential methods to reduce the content of metals in dredged sediments are phytoremediation, bio-leaching and chemical washing [40]. In addition, new emerging remediation techniques are being developed based on different types of combinations of physical, chemical and biological methods [41]. Additionally, in Sweden, the use of organic materials (such as sewage sludge or dredged sediments) as a soil conditioner is also regulated by the ratio between cadmium and phosphorous [8]. According to this regulation, ratios higher than 100 mg Cd kg−1 P are banned on the Swedish market. Therefore, denoting a material with low benefit to be used as soil improver since it could spread high volumes of cadmium. In sediments from the study, the cadmium per phosphorus is high (480 mg Cd kg−1 P), raising issues to use the material as a fertiliser. The reduction of cadmium in the sediments could improve the conditions by reducing the ratio and providing less of the metal per kg of phosphorous. Potential clean-up technologies to remove cadmium from the sediments are phytoremediation and chemical leaching processes [42]. However, the use of the techniques can highly increase the cost of treatment of sediments, reducing the feasibility of currently implementing the use of the material in a full-scale application. An additional way to improve the cadmium phosphorus ratio could be by mixing the dredged material with a more phosphorus-rich substrate. If the material cannot be employed as fertiliser in agricultural soils, other beneficial uses could be carried out with the material. The sediments could potentially be employed as landfill coverage or as a source of nutrients for crops with less sensitive uses, such as the production of bioenergy plants.
The contamination-factor calculations are shown in Table 5. Mercury expresses medium–high contamination in all layers; similarly, cadmium and lead present medium contamination in the top. Additionally, chromium, copper, nickel, zinc and the three lower layers of cadmium and lead show low pollution. Arsenic presents no pollution. The general low pollution (compared to general natural-metal content in lakes in Sweden) shows that, in the sampled sediments from Arkeltstorp bay, the elements originate from natural origins rather than anthropogenic sources. Elements with a degree of contamination suggest that the enrichment of the metal is due to a source of contamination, potentially related to activities around the lake, such as farming.
4 Conclusions
Arkelstorp bay is a shallow water body heavily affected by human influence. Especially the lowering of the water table made the bay less efficient to cope with the nutrient load and is heavily affected by eutrophication. Dredging and increasing the water depth was proposed. Therefore, sediments were characterised to define their potential usage.
Regarding the quality, Arkelstorp sediments were surprisingly homogeneous, and no real difference was found between different sampling sites. The sediments were highly organic (about 31%) and showed high content of total nitrogen. Oppositely, potassium and phosphorous were found in lesser content, proposing that if the sediments are employed as soil fertiliser, the material could be mixed with other organic substrates (like compost) to improve the content and distribution of nutrients.
The organic contaminants found in the sediments were lesser than expected. In fact, concerned organic contaminants were not present. However, as the carbon dating showed, these sediments might be too old to contain any contaminants. Surprisingly, as there were no organic pollutants that could be connected to the modern industrial age, there was still a significant amount of metals, like cadmium and lead. As the carbon dating’s show a significant age, the only reasonable conclusion is that the elements originate from the surrounding bedrock.
The use of sediment presents an issue with the cadmium phosphorus ratio in fertilisers. The Swedish legislation prevents the use of fertilisers when the cadmium to phosphorus ratio is higher than 100 mg Cd kg−1 P. However, the European Union Waste Ordinance points out that the use of dredged material is the priority step. One possible solution is to mix the sediments with other P-rich materials to circumvent the legislation. Another possibility could be pre-treating the material with phytoremediation or chemical extraction techniques. If the material could not be employed as a soil conditioner, it could be used in other uses, such as landfill coverage, or why not as a source of nutrition to produce bioenergy.
References
Larson M (2012) Sweden’s Great Lakes. In: Herschy Reginald W, Fairbridge Rhodes W, Bengtsson L (eds) Encyclopedia of Lakes and Reservoirs. Springer, Dordrecht, pp 761–764
Ulén B, Bechmann M, Fölster J, Jarvie HP, Tunney H (2007) Agriculture as a phosphorus source for eutrophication in the north-west European countries, Norway, Sweden, United Kingdom and Ireland: a review. Soil Use Manag 23:5–15. https://doi.org/10.1111/j.1475-2743.2007.00115.x
Müller S, Mitrovic SM, Baldwin DS (2016) Oxygen and dissolved organic carbon control release of N, P and Fe from the sediments of a shallow, polymictic lake. J Soils Sediments 16:1109–1120. https://doi.org/10.1007/s11368-015-1298-9
Ferrans L, Jani Y, Gao G, Hogland W (2019) Characterization of dredged sediments: a first guide to define potentially valuable compounds-the case of Malmfjärden Bay, Sweden. Adv Geosci 49:137–147. https://doi.org/10.5194/adgeo-49-137-2019
Sheehan C, Harrington J (2012) Management of dredge material in the Republic of Ireland—a review. Waste Manag 32:1031–1044. https://doi.org/10.1016/j.wasman.2011.11.014
Renella G (2021) Recycling and reuse of sediments in agriculture: Where Is the Problem? Sustainability 13:1648. https://doi.org/10.3390/su13041648
Swedish Ministry of the Environment, Förordning (1998:944) om förbud m.m. i vissa fall i samband med hantering, införsel och utförsel av kemiska produkter in 1998:944, Miljödepartimentet, Editor. 1999
Djerf, H. (2021) Teknisk slutrapport: en vik i Sjöriket Skåne. Available from: https://researchportal.hkr.se/files/40950738/FULLTEXT01.pdf
Djerf, H. (2020) Åtgärdsförslag för att förbättra vattenkvaliteten i Arkelstorpsviken. 2020; Available from: https://researchportal.hkr.se/files/40952824/FULLTEXT01.pdf
Chen R, Chena H, Songa L, Yaob Z, Mengc F, Teng Y (2019) Characterization and source apportionment of heavy metals in the sediments of Lake Tai (China) and its surrounding soils. Sci Total Environ. https://doi.org/10.1016/j.scitotenv.2019.133819
Rajeshkumar S, Liua Y, Zhanga X, Ravikumarb B, Baia G, Li X (2018) Studies on seasonal pollution of heavy metals in water, sediment, fish and oyster from the Meiliang Bay of Taihu Lake in China. Chemosphere 191:626–638. https://doi.org/10.1016/j.chemosphere.2017.10.078
Gonçalves RA et al (2018) Decadal and spatial variation of Hg concentrations in sediments of a multi-stressor impacted estuary. Mar Pollut Bull 135:1158–1163. https://doi.org/10.1016/j.marpolbul.2018.08.053
Smith VH, Schindler DW (2009) Eutrophication science: where do we go from here? Trends Ecol Evol 24:201–207. https://doi.org/10.1016/j.tree.2008.11.009
Timilsena YP, Adhikari R, Casey P, Muster T, Gill H, Adhikari B (2015) Enhanced efficiency fertilisers: a review of formulation and nutrient release patterns. J Sci Food Agric 95:1131–1142. https://doi.org/10.1002/jsfa.6812
Strohm D, Ellinger S, Leschik-Bonnet E, Maretzke F, Hesekerc H (2017) Revised reference values for potassium intake. Ann Nutr Metab 71:118–124. https://doi.org/10.1159/000479705
Azam HM et al (2019) Phosphorous in the environment: characteristics with distribution and effects, removal mechanisms, treatment technologies, and factors affecting recovery as minerals in natural and engineered systems. Environ Sci Pollut Res Int 26:20183–20207. https://doi.org/10.1007/s11356-019-04732-y
Cordell D, Drangert J-O, White S (2009) The story of phosphorus: global food security and food for thought. Glob Environ Chang 19:292–305. https://doi.org/10.1016/j.gloenvcha.2008.10.009
Roberts T, Stewart W (2002) Inorganic phosphorus and potassium production and reserves. Better Crops 86:6–7
Cooper J et al (2011) The future distribution and production of global phosphate rock reserves. Resour Conserv Recycl 57:78–86. https://doi.org/10.1016/j.resconrec.2011.09.009
Cordell D, White S (2011) Peak phosphorus: clarifying the key issues of a vigorous debate about long-term phosphorus security. Sustainability 3(10):2027–2049. https://doi.org/10.3390/su3102027
Statistics Sweden (SCB), Försäljning av mineralgödsel för jord- och trädgårdsbruk under 2019/20. 2021. p. 20
Jani Y, Mutafela R, Ferrans L, Ling G, Burlakovs J, Hogland W (2019) Phytoremediation as a promising method for the treatment of contaminated sediments. Iran (Iranica) J Energy Environ 10(1):58–64
Radenovic D et al (2019) Long-term application of stabilization/solidification technique on highly contaminated sediments with environment risk assessment. Sci Total Environ 684:186–195. https://doi.org/10.1016/j.scitotenv.2019.05.351
EN 12880–1:2000, Characterization of sludges—determination of dry residue and water content
EN 12879: 2000, Characterization of sludges—determination of the loss of ignition of dry mass
EN 16173:2012, Sludge, treated biowaste and soil. Digestion of nitric acid soluble fractions of elements
ISO 11885:2007, Water quality—determination of selected elements by inductively coupled plasma optical emission spectrometry (ICP-OES)
EN 16169:2012, Sludge, treated biowaste and soil—determination of Kjeldahl nitrogen
Håkanson L (1980) An ecological risk index for aquatic pollution control.a sedimentological approach. Water Res 14:975–1001. https://doi.org/10.1016/0043-1354(80)90143-8
SEPA, (2009) Repport 5976. Riktvärden för förorenad mark Swedish Environmental protection Agency Available from: https://www.naturvardsverket.se/globalassets/media/publikationer-pdf/5900/978-91-620-5976-7.pdf
Ali IBH, Zaoubeir L, Mounir B, Imen S (2014) Characterization of Tunisian marine sediments in Rades and Gabes harbors. Int J Sedim Res 29:391–401. https://doi.org/10.1016/S1001-6279(14)60053-6
Bindler R, Rydberg J, Renberg I (2011) Establishing natural sediment reference conditions for metals and the legacy of long-range and local pollution on lakes in Europe. J Paleolimnol 45:519–531. https://doi.org/10.1007/s10933-010-9425-5
Johansson K, Andersson A, Andersson T (1995) Regional accumulation pattern of heavy metals in lake sediments and forest soils in Sweden. Sci Total Environ 160:373–380. https://doi.org/10.1016/0048-9697(95)04370-G
Routh J, Meyers P, Hjorth T, Baskaran M, Hallberg R (2007) Sedimentary geochemical record of recent environmental changes around Lake Middle Marviken, Sweden. J Paleolimnol 37:529–545. https://doi.org/10.1007/s10933-006-9032-7
Burdige DJ (2021) Geochemistry of marine sediments. Princeton University Press, USA
Roman P, Martinez M.M, Pantoja A, (2013) Fundamentos teoricos del compostaje, in Manual de compostaje del agricultor, FAO, Editor. 2013, FAO: Chile. pp 21–44
Mattei P et al (2017) Evaluation of dredged sediment co-composted with green waste as plant growing media assessed by eco-toxicological tests, plant growth and microbial community structure. J Hazard Mater 333:144–153. https://doi.org/10.1016/j.jhazmat.2017.03.026
Mhanna R et al (2021) Concomitant behavior of arsenic and selenium from the karst infillings materials of the fractured carbonate dogger aquifer (hydrogeological experimental site, Poitiers, France). Chemosphere. https://doi.org/10.1016/j.chemosphere.2021.129935
Polettini A et al (2006) A kinetic study of chelant-assisted remediation of contaminated dredged sediment. J Hazard Mater 137:1458–1465. https://doi.org/10.1016/j.jhazmat.2006.04.022
Shih Y-J, Syu S-J, Chen C-W, Chen C-F, Dong C-D (2019) Assessment of ex-situ chemical washing of heavy metals from estuarine sediments around an industrial harbor in Southern Taiwan. J Soils Sediments 19:3108–3122. https://doi.org/10.1007/s11368-019-02321-7
Zhang M, Wang X, Yang L, Chu Y (2019) Research on progress in combined remediation technologies of heavy metal polluted sediment. Int J Environ Res Public Health 16:5098
Peng J-F, Song Y-H, Xiao-yu P-Y, leiQiu C-G (2009) The remediation of heavy metals contaminated sediment. J Hazard Mater 161:633–640. https://doi.org/10.1016/j.jhazmat.2008.04.061
SEPA. Report 5976, Riktvärden för förorenad mark 2009; Available from: https://www.naturvardsverket.se/globalassets/media/publikationer-pdf/5900/978-91-620-5976-7.pdf
Funding
Open access funding provided by Kristianstad University. The project has been funded by Leader Sydöstra Skåne and Havs- och Fiskerifonden.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Data collections were performed by Henric Djerf, and analyses were performed by Henric Djerf and Laura Ferrans. The first draft of the manuscript was written by Henric Djerf, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Djerf, H., Ferrans, L. Defining potential valuables through the characterisation of lake sediments: case study in Arkelstorp bay, Sweden. SN Appl. Sci. 4, 106 (2022). https://doi.org/10.1007/s42452-022-04988-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-022-04988-z