Abstract
This research is centered on the optimization of coagulation–flocculation treatment of cosmetic wastewater. It analyzes blends of fishbone (BFB) and aluminum-based coagulant (ABC) to determine the efficacy of BFB as a potential coagulant–flocculants aid at optimum conditions using response surface methodology (RSM). The experiment was carried out employing the standard nephelometric procedure at 1000 rpm stirring rate. The central composite design (CCD) was used to examine the interactions of pH, dosage, and settling time to maximize the turbidity removal efficiency of the ABC- and BFB-driven coag–flocculation. The optimal pH, dosage, and settling time for ABC were obtained as 10, 0.1 g/L, and 2 min, while pH 6, 0.4 g/L, and settling time of 4 min were recorded for BFB following the established quadratic model of the RSM. The removal efficiency of ABC and BFB plots 80% and 88%, respectively; this corresponds to 262 NTU and 288 NTU of removal from the wastewater at optimal conditions. The kinetics result indicated that the rate constant (Kf) 3 × 10−3 (L/g min) of BFB surpassed 5 × 10−5 (L/g min) recorded for ABC following second-order coag–flocculation reaction, with correlation coefficients (R2) values of 0.999 and 0.9985, respectively. The research also applied cost–benefit analysis for the determination of the efficacy of BFB. The figure obtained shows that the benefit of using BFB will save $5.50 compared to ABC based on this work. At optimal conditions, BFB satisfied the environmental protection agency pH standard for industrial wastewater discharge, promising coagulant–flocculants aid for industrial wastewater purification purpose and the preservation of the environment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Growing demand and usage of cosmetic products in recent times have spurred remarkable growth in the cosmetics production industry. Consumers of cosmetics products have been spending high levels of disposable income on cosmetics than they had in the past. According to report, global cosmetic products market was valued around $532 billion in 2017 and is expected to reach approximately $863 billion by 2024 [1, 2]. The increasing consumption of cosmetic products for human beautification purposes has directly facilitated the increasing generation of wastewater associated with high turbidity and other contaminants from production sites [1, 3].
According to the environmental protection agency (EPA), turbidity in water should not exceed 40NTU [4]. The EPA guidelines also specified pH within (5.5–9) as standards for wastewater discharge [5, 6]; hence, cosmetic wastewater with turbidity contents greater than 250NTU is considered a point source pollutant. The level of contaminants present in wastewater poses serious industrial and environmental concerns. High turbidity promotes the growth of pathogens in water leading to waterborne disease outbreaks, which have caused significant cases of intestinal sickness [4, 7]. It also reduces significantly the aesthetic quality of water, having very harmful impact on the water bodies when not disposed properly. Industrial effects of turbidity are not left out of concerns, as it contributes to clog tanks and pipes corrosion, eventually damaging them [8]. Also, turbidity in effluents and their complex characteristics pose severe resistance to unit operations which undergoes in industrial wastewater treatment plants [4, 7]; hence, the importance of turbidity removal in wastewater treatment before discharge cannot be over emphasized.
There are different treatment processes applied to enhance the removal of contaminants from wastewater [9]. These processes includes coagulation and flocculation [10, 11], adsorption [8], and reverse oxidation [12] which was applied in recent times to remove bacterial from wastewater [8, 13, 14]. These are the most commonly sought-out treatment processes for the removal of turbidity from wastewater that contains significant amount of settle-able and suspended solids.
Handling high turbidity in wastewater is cost intensive, since the rising cost of purchasing conventional chemicals, energy required for treatment operations, and the adverse effect of inorganic chemicals on the environment have grown with the proportionate increase in pollution [15, 16]. However, the long-term application of some inorganic coagulants–flocculants has raised concerns associated with sludge, corrosion, and uncertainties regarding their environmental impacts [10, 15, 17]; to this end, organic materials are widely sorted as alternatives to synthesized chemicals previously used as coagulant–flocculants in water treatment processes. Also, most organic materials were applied as coagulants–flocculants aid for water treatments due to their ability to efficiently promote flocculation at optimum dosages and cost efficiency [10, 18].
Fisheries and aquaculture production is an important industry in a number of countries among the likes of Chile, India, Indonesia, Viet Nam, Bangladesh, Egypt, and Norway. China and Nigeria have consolidated their shares in regional or world production of fishery to varying degrees over the past two decades [19, 20]. In 2018, world production attained another all-time record as high as 114.5 million tons in live weight [20]. The sector is also expected to expand the most in Africa up to 48% driven by the additional culturing capacity put in place in recent years for tilapia and catfish, respectively [10, 11]. Asia will continue to dominate the aquaculture sector and will be responsible for more than 89% of the increase in production by 2030 [20]. These report reviews the possible availability of fishbone in surplus as raw material that can be harnessed and reused for any industrial and commercial purposes especially for industrial treatments of wastewater.
The works of references [21–23] reported that bones from fisheries and aquacultures possess high absorptive characteristics and were capable of inducing coagulation–flocculation exploited for the removal of contaminants from wastewater under varying operating conditions [9]. However, effective coagulation–flocculation for turbidity removal depends on a number of factors such as the compositions of the organic coagulant, the process variables, and equipment used. The conditions of experiment including the mixing rate, pH modifications, the concentration of the colloidal particles, acidity/alkalinity of the wastewater, and dosages of the organic coagulants are contributing factors toward achieving greater clarification efficiency [9, 10]. The challenges of using organic coagulants still remain that fundamental background knowledge about their pH modifications, clarification efficacy, and interactions with the substrates in the water medium is often scarce, or insufficient to infer the best chemical configuration for treating a specific effluent [9, 24, 25, 4]. These challenges have grown with the proportionate increase in their inability to satisfy the EPA pH standards for wastewater discharge.
The present study investigated the efficacy of reuse waste materials, blends of fishbone (tilapia, salmon, and catfish) as coagulant–flocculants aid for the removal of high turbidity from cosmetic wastewater. The objectives of the research included the determination of the optimum operating conditions for the coag–flocculation treatment of the wastewater using RSM, and investigating the coag–flocculation kinetics model for the blends of fishbone and alum-driven coag–flocculation, in order to compare their rate constants which was applied as a criterion to determine the effectiveness of BFB at high mixing rate. The study also included the cost–benefits analysis of the process, to examine the efficacy of BFB over ABC at optimal conditions, and to satisfy the EPA pH standards for wastewater discharge.
2 Materials and methods
2.1 Materials
The wastewater sample was collected from Emos Best Industries Nigeria Limited. The organic coagulant samples used for this work were obtained from reused waste materials consisting of dried fishbones which were received as a gift from local fish traders in the riverine area of the Niger Delta, the aim of ensuring the availability of the organic coagulant source.
2.2 Preparation of the coagulant
The organic coagulant consisted of fishbone samples from tilapia, salmon, and catfish weighing 5 kg, was sun-dried for about 3 weeks, crushed, and ground to increase its surface area. The crushed samples were mixed thoroughly and screened through 20 mesh sizes to obtain flour of finer particles which were collected in a container ready for use. The blended fishbone coagulant was characterized at Pymok Research Laboratory Enugu, Nigeria.
The proximate analysis on the prepared organic coagulant sample was done at Spring Board Research Centre, Awka, Nigeria. The characterization and proximate analysis of the blended fishbone samples were carried out to ascertain the chemical constituents, compositions, and concentrations of the organic structure following the standard procedure in accordance with reference [15]. The results of the characterization and proximate analysis on blended fishbone (BFB) coagulant are presented in Table 1.
The conventional coagulant used in this work consisted of 2 kg of aluminum-based salt (Alum) of industrial grade has a chemical formula: \({\text{Al}}_{2} \left( {{\text{SO}}_{4} } \right)_{3} \cdot 18{\text{H}}_{2} {\text{O}}\), molecular weight (666.42 g/mol), made up of 51–59% \({\text{Al}}_{2} \left( {{\text{SO}}_{4} } \right)_{3}\), with pH range (2.5–5.5) was bought from Kincel Excel International Nigeria Limited. The aluminum-based coagulant (ABC) was ground to fine powdered form to increase the surface area of the coagulant. It was then stored in a separate container ready for use.
2.3 Procedure
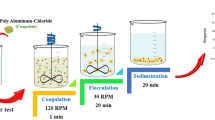
The wastewater sampling was carried out at Halden Nigeria Limited, Port Harcourt, Nigeria. In the course of the experiment, the preserved sample of the cosmetics wastewater was characterized in accordance with the standard method of examination of wastewater as stated in reference [15]. The standard nephelometric test was conducted on the wastewater samples. In the course of the treatment process, the initial turbidity (NTU) of the wastewater sample was measured and recorded. The pH of the solution was adjusted using 0.1 M HCL or 0.1 M NaOH just before the measured doses of the coagulants ABC and BFB ranging from 0.1 to 0.5 g/L were, respectively, added. The mixing speed of 1000 rpm was maintained for 10 min after which it was reduced to 250 rpm for 20 min using a magnetic stirrer, while the settling time following the coag–flocculation treatment was varied from 2 min through 30 min.
In the course of each experimental run, a pipette was used to draw samples for testing, and the corresponding turbidity (NTU) of each samples drawn was measured using the turbidity meter. The corresponding experimental results were used to calculate the percentage turbidity removal efficiency which served as the response parameter following the process optimization.
2.4 Experimental conditions
The pH was varied to 2, 4, 6, 8, and 10, respectively, using a Delta-320 pH meter and dosage range between 0.1 and 0.5 g/L. The settling time was varied from 2 min through 30 min, respectively, at room temperature and atmospheric pressure condition. The percentage turbidity removal efficiency was calculated by Eq. 1.
where N0 is the initial turbidity (NTU) content of wastewater sample before treatment, and Nt is the turbidity content of the wastewater after treatment with blended fishbone and alum-based coagulant, respectively. The concentrations of the turbidity particles removed from the wastewater were evaluated by a multiplying factor of 2.35 [10, 11] as shown in Eq. 2, where Ci represents the concentrations (g/L) of the colloidal particles causing turbidity in the wastewater sample.
2.5 Experimental designs
The central composite design (CCD) of the response surface methodology (RSM) was used for the optimization study. The CCD is an experimental design used for building a quadratic model for responses without the need to use a complete three-level factorial experimental design and provides information about interactions among experimental variables within the range studied, leading to better knowledge of the process. The CCD design was randomized but not replicated at each experimental condition [8]. The relationship between the coded values and the actual values is obtained by Eqs. 3 and 4.
where xt is the coded value, Xk is the actual value of variable k, Xk is the midpoint value of variable k, and R is the range of variable k. The three levels of factors included the optimum values obtained from the RSM experimental runs and two other points that were within the experimented region but not values outside the experimental design matrix used for the central composite design.
The most common empirical models fit to the experimental data take either a linear, cubic, or a quadratic form, with the coefficients of the model used to establish the design model equations showing the relationship and interactions of the factors and their significance with the response.
3 Results and discussion
3.1 Characteristics of the blends of fishbone
The characterization result of BFB showed that the organic material contains varying proportions of calcium, iron, zinc, sodium, potassium, and magnesium presented in terms of their concentrations.
The result of the analysis confirmed that the structure of the organic material is predominantly made of calcium and iron in concentrations of 0.04044 g/L and 0.02676 g/L, respectively, while zinc, sodium, and potassium were recorded in the amounts of 0.009 g/L, 0.001 g/L, and 0.002 g/L. The copper and manganese contents in the structure of BFB were found in smaller volumes in concentrations lesser than 0.001 g/L. These results suggested that BFB is likely to be a natural metal-based organic coagulant. The blending of the different species of bones increased the alignments of the metal ions in reasonable proportions in the structure, thereby increasing the potential of BFB to induce coagulation when the positive-charged metallic ions are precipitated from the surface and diffuse into the water medium [9, 26, 27, 28].
However, the result of the proximate analysis carried out on the blends of fishbone shows that the powdered form of the organic coagulant contains low percentage of moisture recorded at 2.5% in value. Thus, we suggested that the powdered form of BFB is possibly hydrophilic [29]. Also, 47% ash content was reported for the organic coagulant. The blends of fishbone also contained 5% of carbohydrates, while the fiber contents were reported to be 37%. Depending on the functional groups present in the structure of the organic material, we reasoned that the fiber contents may act as an integral part of the organic coagulant to producing better absorptive surfaces that can facilitate the absorption of contaminants in the water medium.
The protein and fat contents presented in BFB following the analysis were reported to be 4.3% and 5%, respectively. The percentage of proteins in the BFB structure are indication of the presence of amino groups, with polymeric characteristics [18, 30], that are capable of promoting aggregation of colloidal particles during coagulation [16, 28, 31]. The result of the analysis for blended fishbone is shown in Table 2.
3.2 Characteristics of the wastewater
The result of the wastewater characteristics shows that total suspended solid (TSS) and total dissolve solid (TDS) contents of the water were recorded as 401 mg/L and 232 mg/L, respectively. The biological oxygen demand (BOD) and chemical oxygen demand (COD) of the wastewater were reported to be less than 4 mg/L. The wastewater sample collected from the industrial facility has a low acidity of 6.02 mg/L. It has a high alkalinity of 134 mg/L. The turbidity of the water was recorded as 350 NTU, at pH of 4.2. We can infer from the result in Table 2 that the wastewater is highly contaminated going by its characteristic, and it does not satisfy the EPA acceptable standards for wastewater discharge. Consequently, measures must be put in place to ensure the water is treated to satisfy the EPA standard for industrial wastewater discharge.
3.3 Coagulation–flocculation process optimization and analysis
The optimization of the coagulation–flocculation treatment of the cosmetics wastewater using blends fishbone (BFB) and alum-based coagulant (ABC) was carried out using the response surface methodology (RSM). The central composite design (CCD) was selected with zero block and used to build the quadratic model for 20 experimental runs to accommodate more interactions of the experimental factors A = dosage (g/L), B = pH, and C = settling time ( min) as independent factors used for coag–flocculation optimization. The quality of the quadratic model was expressed by the coefficient of determination of R2 value, and its statistical significance and the model adequacy were checked by the Fisher’s F value in the same program using the trial version of the Design Expert Software version 12.0.
Model terms were evaluated by the p value (probability) with 95% confidence level. Three-dimensional surface plots and their respective contour plots were obtained for the BFB-driven and ABC-driven coag–flocculation treatment of cosmetic wastewater based on the effects of the three factors: dosage, pH, and settling time at two levels. The quadratic model was selected with p values less than 0.0500 indicated model terms are significant with the interactions of the three factors for BFB-driven and alum-driven coag–flocculation.
The coefficients estimated represent the expected changes in the response per unit change in the significant factor values when all other remaining factors are held constant. The intercept in an orthogonal design is the overall average response of all the runs. The coefficient of determination R2 (0.9706) obtained shows that the degree of fitness confirms there is a very high correlation between the experimental responses and the predicted values of the factors. The correlation between the actual versus the predicted values of the response is presented in Fig. 1 of ESM. An Adjusted R2 (0.9441) obtained with the analysis of variance is in reasonable agreement with the coefficient of correlation R2 value of 0.9706. The values of R2 are close to unity and confirm the validity of the quadratic model used for the experimental design.
The analysis of variance (ANOVA) report for the experimental run of the BFB-driven coag–flocculation treatment is presented in Table 3 of ESM. A p value less than 0.0500 with the model F value of 36.68 indicated model terms are significant. In this case, A, B, C, AB, B2, and C2 are significant model terms. The predicted R2 = 0.7932 is in reasonable agreement with the adjusted R2 = 0.9441, i.e., the difference is less than 0.2 with a standard deviation of 4.46. The model equation relating the response %turbidity removal efficiency (Y) and the interactions of the independent process variables: dosage (A), pH (B), and settling time (C), for the BFB-driven coag–flocculation is represented by Eq. (5):
The dosage of BFB was found to have the most significant influence on the response followed by the pH of the solution as observed from the respective coefficients of the model (Eq. 5). The interactions of the factors with their effects of the model terms on the response are ranked in the order:
However, the quadratic model terms for the ABC-driven coag–flocculation were evaluated at 95% confidence level. The quadratic model’s F value of 31.17 and P value of 0.075 obtained following the central composite design indicated that the model terms A, C, A2, B2, and C2 are significant. A lack of fit values of 4.05 obtained with the model was greater than 0.1000 which indicated that the lack of fit is not significant. The interactions of the model terms with their significant effects on the response are in the order:
The quadratic model developed for ABC follows that the dosage (g/L) has the most significant effects on the turbidity removal efficiency, followed by the pH of the solution. The coefficient of determination R2 (0.9706) obtained for the model with the degree of fitness confirms there is a very high correlation between the experimental responses and the predicted values of the factors. The correlation between the actual versus the predicted values of the response for ABC-driven coagulation is presented in Fig. 4 of ESM.
However, the model fit statistics shows that the predicted R2 (0.8211) and the adjusted R2 (0.9346) have a difference less than 0.2 and are in reasonable agreement with the correlation coefficient R2 value of 0.9656. These statistical values are close to unity and plot a linear correlation between the actual value and the predicted values of the response as shown in Fig. 2 of ESM. An adequacy precision value of 22.716 which measures the signal-to-noise ratio indicated a ratio greater than 4; hence, there is an adequate signal ratio with the quadratic model established for ABC-driven coagulation–flocculation optimization process.
The final quadratic model equation in terms of experimental factors (pH, dosage, and settling time) that were used to make predictions about the percentage turbidity removal efficiency (Y) at given levels of each factor for the ABC-driven coag–flocculation is given by Eq. 6.
The optimal results for the ABC- and BFB-driven coagulation–flocculation treatment of the cosmetic wastewater were determined based on the interpretation of the solution to the final model Eqs. 5 and 6, respectively, from Design Expert Software version 12.0. The optimization goal for factors: dosage, pH, and setting time, was maintained within the range of experimental data with the objective of maximizing the response %turbidity removal efficiency.
The ramp plots presented in Figs. 3 and 4 of ESM were obtained at a desirability of 1.0. The plots also show predicted values of the output responses which corresponds to the optimal points located within the range of experimental design boundary. These points are represented by the depth, data components, and the intensity of the colors gradients of the surface and contour plots. They confirm the optimal results for the BFB- and ABC-driven coagulation–flocculation treatment processes.
3.4 Optimization results for ABC and BFB coag–flocculation
The optimal pH, dosage, and settling time were recorded as 6, 0.4 g/L, and 4 min, respectively, for BFB, while an optimum pH 10, 0.1 g/L dosage, and settling time of 2 min were recorded for ABC, respectively, as presented in ramp plots in Figs. 3, 4 of ESM. It can be deduced from the results that at optimum operation, the turbidity in the wastewater was reduced from an initial 350 NTU to a value of 42 NTU with BFB, while ABC reduced the turbidity from an initial 350 NTU to a residual value of 70 NTU. These values correspond to 88% of turbidity being removed with BFB, while the ABC-driven coag–flocculation plots 80% turbidity removal efficiency, respectively.
The CCD following the response surface methodology (RSM) used to develop the 3D surfaces shown in Figs. 1, 2 and 2D contours presented in Figs. 3, 4 confirms the precise location of the optimal points for the ABC- and BFB-driven coag–flocculation process following the interpretations of the quadratic model Eqs. 5 and 6. However, other areas of good performances are well represented by the orientation and elevations of the surfaces and contours of Figs. 3, 4, 5 and 6, respectively.
The respective surface plots in Figs. 5, 6 are drawn for a better comprehension of the coag–flocculation optimization process. The data, depths, and positions of the 3D surfaces serve as a graphical technique showing precisely the functional relationship between the variables and the response parameter (removal efficiency). It explains the interactions of the variables pH and settling time [13].
However, the plots (Figs. 5, 6) show that two out of the three factors were interdependent, such that there were significant interactive effects between pH and settling time on the removal efficiency compliance to BFB and ABC coag–flocculation. The curvatures of the 3D surfaces posted the best performance are achieved at an elevation from pH 6 through pH 10 for the BFB- and ABC-driven coag–flocculation. The pH elevation of the medium is an indication of the strong alignment of the coagulants in the solution. The higher range of pH attained by the solution was to ensure the complete destabilization of the particles causing turbidity in the wastewater medium.
Figure 6 plots the best performance which was achieved at the pH elevation of the wastewater from 8 to 10 for ABC. At the pH range (8–10), the turbidity removal efficiency increased appreciably from 63% through 78%. The increase in pH of the medium is an indication of an elevation toward a predominantly alkaline solution. An optimum pH 10 was recorded for the ABC coag-flocculation treatment. The optimal pH result indicated that ABC does not satisfy the EPA standard for industrial wastewater discharge. However, the trough in the 3D surface in Fig. 5 shows contour patterns whose curvatures follow that the areas of best performance of BFB were achieved as pH increases from 6 through 10, which corresponds to 75% through 96% turbidity removal efficiency. Higher degree of removal efficiency was largely dependent on the dosage output of BFB; for the process to be economically viable, an optimal dosage of 0.4 g/L was recorded. The optimum pH of 6 was recorded for BFB. The result obtained confirms that the optimal pH falls within the range of the EPA acceptable standards for wastewater discharge. Hence, we concluded that the finish water is stable and will neither be corrosive. The optimal pH was determined to help reduce coagulant wastage and cost of services [4, 27].
The surface plots in Figs. 5 and 6 also posted the effect of settling time on the coag–flocculation process. It posted the areas of good performance for the ABC- and BFB-driven coag–flocculation occurred at settling time lesser than 5 min. This result is in agreement with the optimum settling time of 2 min and 4 min reported in Figs. 3, 4 of ESM for the ABC and BFB, respectively. The result obtained for the settling time is an indication of a short coag–flocculation period. Hence, we can conclude that the theory of fast coagulation holds for both the BFB- and ABC-driven coag–flocculation, respectively.
However, the quadratic model developed with the CCD for the optimization process shows that the coagulant dosage has significant main effect on the turbidity removal efficiency. The optimization results presented in Figs. 3 and 4 of ESM show that the optimum dosages were 0.1 g/L for ABC and 0.4 g/L for BFB, respectively. The optimal values of the dosages were considered to be minimal since these values of dosages are less than 0.5 g/L [27]. An optimal dosage value lesser than 0.5 g/L is an indication that minimal residues were produced during the coag–flocculation period, thus resulting in smaller volumes of residual turbidity. The optimal dosage of the BFB-driven coag–flocculation maintains that there will be little or no scaling in the finish water.
The darker red regions of the underlying contours in Figs. 7 and 8 indicated areas of local maxima corresponding higher quality corresponding to 92% turbidity removal efficiency with a dosage of 0.5 g/L of BFB. The pH elevation represented in the index contour of Fig. 9 of ESM terminated at a threshold pH 10, and the result was associated with 78% removal efficiency indicating that ABC requires supplementary alkalinity to further drive the coag–flocculation reaction to completion. Hence, we can conclude from the optimization results that the effectiveness of ABC was not depended on dosage.
3.5 Coag–flocculation kinetics of turbidity removal for BFB and ABC coag–flocculation
The kinetics parameters which include the rate constants and the order of reaction were developed following the analogies of the kinetic model Eqs. 7–12. In real practice, empirical evidence shows that the nth order of coag–flocculation reactions lies between zero, first-order, pseudo-first-order, or second-order rate laws, such that \(\left( {0 \le n \le 2} \right)\) [13, 14]. The model was developed based on the kinetics dependency of the BFB- and ABC-driven coag–flocculation characteristics on the concentration of particles causing turbidity \((C_{i}\)) with time (t). Thus, the rate equation for the n-th order coag–flocculation can be expressed as:
where n is the order of the reaction at time (t) and \(K_{n}\) is the nth-order coag–flocculation rate constant of the reaction. Based on Eq. 7, the rate for coag–flocculation performed for a first-order (n = 1) at given reaction time (t) is given thus:
Integrating both sides of Eq. 8
where \(\left( { - r_{A } } \right)\) is the rate of coag–flocculation reaction, \(K_{{\text{f}}}\) is the rate constant for the first-order coag–flocculation expressed in \(\left( {{\text{L/g}}\;{\text{min}}} \right)\),\(C_{i}\) are concentrations of particles after treatment expressed in g/L, C0: is the initial concentration of the particles before treatment (g/L), and \(n\) is the order of the coag–flocculation reactions.
The rate constant \((K_{{\text{f}}} )\) for a first-order kinetics can be obtained by plotting values of (In Ci) against time (t), where the slope gives the rate constant (Kf). In most cases, a second-order kinetics rate laws hold for the coag–flocculation treatments of wastewater following the use of organic materials as coagulants [14].
The second-order (n = 2) model derivation follows from Eqs. 11 through 12, such that:
Integrating both sides of Eq. 11, we arrive at:
where \(K_{{2{ }}}\) is the constant for the rate of second-order (n = 2) coag–flocculation, the initial concentration of the suspended and dissolved particles in the wastewater medium before represented by \(C_{0 }\) treatment, and \(C_{i }\) represents concentrations after coag–flocculation treatment. Expressing the rate constant in terms of other parameter in Eq. 11, and using \(K_{f }\) as the reference for K2, we arrive at Eq. 12, where t is the time ( min) and \(K_{{\text{f }}} \left( {{\text{L/g}}\;{\text{min}}} \right){ }\) is the rate constant for the second-order coag–flocculation treatment of the wastewater at optimum conditions. The values of the rate constants of BFB and ABC, respectively, were obtained from the evaluation of kinetics trace of the plot in Figs. 11, 12, 13 and 14 of ESM, which follows from the graphical evaluations of the concentration \((C_{i } )\) against time (t) at optimum conditions. The values for the linear correlation coefficients (R2) were employed to evaluate the level of fits of the experimental data on Eq. 12. From the values of the coag–flocculation rate constants obtained, we can evaluate the clarification efficacy of using BFB to ABC by comparing their rate constants at optimum conditions.
However, Tables 5, 6, 7, and 8 of ESM present the values of the rate-related parameters obtained from the analysis of Eq. 12 that strongly influenced the ability of ABC and BFB to remove turbidity from the cosmetic wastewater. Such parameters \(\left( {K_{{\text{f}}} } \right)\) have direct bearing on design, fabrication, and practical implementation of this study. The linear plot (Figs. 11, 12, 13, 14 of ESM) shows that BFB and ABC, respectively, obeyed the second-order coag–flocculation rate law (n = 2).
The result presented in Table 5 of ESM shows that the rate constant \(\left( {K_{{\text{f}}} } \right)\) value of 1.3 × 10−3 L/g min was obtained at a dosage of 0.5 g/L, while the rate constant value of 7.7 × 10−4 L/g min was obtained at 0.4 g/L dosage for the BFB-driven coag–flocculation kinetics at optimum pH. The lowest rate constant (Kf) value of 1 × 10−4 L/g min was obtained at a dosage of 0.3 g/L at pH 6 and settling time of 4 min, respectively. From the trend of values obtained for the coag–flocculation kinetics, it was observed that at optimum pH 6, the Kf values increased appreciably with the increase in dosage of BFB added to the water medium. Higher values of rate constant were obtained with an increase in dosages of BFB as the pH of the solution increased from 6 through 10. The kinetics result confirms that pH and dosage are the most important factors that influenced the clarification efficiency of BFB. Higher rate constant was obtained as pH increases from 6 to 10, and dosage from 0.3 to 0.5 g/L. The pH 10 does not satisfy the EPA standard for wastewater discharge, and 0.5 g/L dosage outputs for BFB are an indication of coagulant wastage. Thus, we concluded that at pH 10, the finish water may cause scaling, with the same potential to blind filters leading to increasing operational cost. The optimum dosage 0.4 g/L of BFB yielded high Kf value \({\text{of}}\; 7.7\; \times \;10^{ - 4} \left( {{\text{L/g}}\;\min } \right)\) at the optimal pH 6. Hence, we conclude from the kinetics result that the efficacy of the blends of fishbone satisfied the EPA standard for industrial wastewater discharge. Thus, the kinetics result is in obvious agreement with the optimization result for BFB obtained from the RSM.
The kinetics results obtained for the ABC-driven coag–flocculation plot the highest Kf value of \(3.4\; \times \;10^{ - 4} \;{\text{L/g}}\;\min\) at 0.1 g/L dosage. \({\text{The}}\;{\text{lowest }}\left( {k_{{\text{f}}} } \right)\;{\text{value}}\;{\text{of}}\;{ } 5\; \times \;10^{ - 5} \;{\text{L/g}}\;{\text{min}}\) was obtained at 0.3 g/L dosage of ABC at the optimum pH 10 and settling time of 2 min. The Kf values decreased from \(5\; \times \;10^{ - 5} \;{\text{L/g}}\;{\text{min}}\) to \(3.4\; \times \;10^{ - 4} \;{\text{L/g}}\;{\text{min}}\) with an increase in dosage of ABC added to the water medium. The highest rate constant Kf value of ABC was obtained at optimum dosage 0.1 g/L, pH 10, and settling time of 2 min. Thus, the kinetics result obtained for ABC-driven coag–flocculation confirms the optimization results obtained with the RSM.
Figure 7 is used to compare the rate constants of both coagulants at their optimum pH and dosage. The plot shows that the rate constant Kf of the BFB-driven coag–flocculation surpassed rate value of ABC at their optimum conditions. Thus, we concluded that the organic coagulant aligned well with the solution at the optimum pH 6 and was more effective than aluminum sulfate coagulant used for the treatment of cosmetics wastewater at their optimum conditions. We also concluded that since the pH terminates at 10, an increase in dosage of BFB from 0.4 to 0.5 g/L is an indication that the alkalinity of the solution was well preserved. As such, BFB can function as a coagulant aid that can be used to raise alkalinity of any wastewater whose alkalinity is reported to be greater than 100 mg/L, and acidity lower than 20 mg/L. Also, the inability of ABC to drive the stability below pH 10 might be due to excess negative charges of the particles of the water medium. Consequently, ABC requires further acidity to necessitate the pH depression below 10.
Considering the viability of ABC in terms of the dosage required to induce coag–flocculation, hosting the efficacy of 0.4 g/L dosage of BFB over 0.1 g/L ABC at optimal conditions will depend on the cost–benefit analysis of both coagulants. This includes the cost of maintaining a pH between 6 and 8 throughout the coag–flocculation treatment process in order to satisfy the EPA standards for industrial wastewater discharge.
3.6 Cost–benefit analysis of the present study
The present proposal was to test and implement blends of fishbone (BFB) as a natural coagulant–flocculants aid or as a substitute or to be used as hybrids with other conventional chemicals for the coag–flocculation treatments of wastewater. Although the pH output for BFB satisfied the EPA standard for industrial wastewater discharge at an optimum dosage of 0.4 g/L, the small amount of dosage loading of 0.1 g/L reported for ABC is considered to be very viable economically. A maximum value of 96% threshold turbidity removal was recorded with BFB at a pH of 10, setting time of 5 min, and a dosage of 0.5 g/L. From the results obtained from Figs. 2 and 4, the threshold removal efficiency of 80% was reported with ABC under the same conditions of pH and settling time. The wastewater treatment and respective cost requirements are more or less similar for both coagulants cosmetic to be used in wastewater treatment plants. However, the major difference will be concerned for the industrial production outputs of the coagulants.
The cost–benefit analysis was evaluated by Eq. 13 to determine the cost for energy consumption based on instrumentation requirements for the BFB and ABC coag–flocculation process. The energy consumption Ec equation is given by:
where PD is the power consumed by the device in (KW), a is the load factor, t is the time in (Hr), and CC is the cost per consumption. The values of a = 1 are used when the equipment was used at full-load module, and a = 0.5 for half-load mode.
The raw material cost prices for this study were obtained from the local vendors. It should be noted here that the variability of the coagulants and operational cost depends on the quantity of the final results of the analysis. The organic coagulant was predominantly made from varieties of fishbone taking into consideration the availability of the raw materials needed for the commercial production. The coagulant comprises of fishbone of tilapia which is relatively common, and catfish is reported in abundant supply in the West Africa and Asian region, while salmon can be found in sufficient supply across parts of the globe. The cost remediation is calculated and translated to the US Dollar (USD).
The present cost analysis took into account the market prices, as well as prices provided by vendors across the world. The raw materials took into account multiple market prices for other coagulants used specifically for turbidity removal from cosmetics wastewater. The electricity costs per KWH (kilowatt hour) used for this study are based on the average price in Nigeria for the year 2020. An estimated 0.107$/KWH for businesses included all components of electricity bills such as the cost for power, distribution, and taxes calculated as an average annual level of electricity consumption. This price was retrieved from the Benin Electrical Distribution Company (BEDC) [32]. The total electricity cost for this project was calculated and estimated to 2.48 USD/kg of coagulant. The raw material cost for alum bought from a chemical vendor, Kincel Excel International Nigeria Limited, was 1.75 USD per kilogram.
Labor cost consisted of compensations for researchers participating in the project, with the addition of taxes and benefits. For the purpose of this study, the personnel requirements and synthesis process comprised of one postgraduate researcher student, and one research supervisor working for 1 workday (i.e., 3 h). The average wages of the personnel were assessed based on the cost of contract service cost information, which maintains a rich database with employee wages per company depending on the position.
Table 9 of ESM is drawn to calculate the recipe cost, gathering all appropriate information presented with relative structures. Energy cost was calculated as 2.48 USD. Therefore, the total cost of production was estimated 140.98 USD per kilogram for this proposal including the cost of transportation to the site of remediation.
Total cost: This is the sum of the direct and indirect cost.
Opportunity Cost: This is the difference in the return of the forgone option and the chosen. Mathematically, it can be seen as:
where F0 = Return on best forgone option; in this case ,we are considering ABC = 146.48 USD and R0 = Return on chosen option blends of fishbone (BFB) = 140.98 USD.
Hence, opportunity cost (OC) = \(146.48 - 140.98\) = 5.5 USD.
In this case, the benefit of using BFB will save 5.5 USD based on this work. The diagrammatical representation of the cost benefits remediation is presented in Fig. 8. The result of the cost comparison of blends of fishbone (BFB) to alum (ABC) plots that it is slightly higher to operate the coagulation–flocculation treatment of the cosmetic wastewater at optimum conditions using ABC for turbidity removal than BFB.
3.6.1 Cost of potential risks
There is little or no cost associated with potential risk as the BFB is biodegradable and environmentally friendly. The regulatory risk is very minimal because it does not pose any hazardous threat to lives. In this cost benefit analysis, we are comparing the cost of using blended fish bone (BFB) as an alternative to alum which is a widely used coagulant. Aside the decrease in water pH which increases the alkalinity of the water and the possible introduction of dissolved toxic aluminum compounds, the intangible cost of no environmental impact of BFB is a major factor that cannot be quantified. This is a major cost benefit over a period of time in terms of return on investment.
3.7 Comparison of the result of the present study with other research work
To summarize the efficacy of BFB, we compared the results from this study with the results of other authors referenced in this work. Table 3 gives a summary of the comparison.
From Table 3, an optimal pH equal to 6 recorded for BFB is in wide contrast with the optimum pH results of fishbone used by the other researcher, and thus, we concluded that the blends of fishbone yielded better performance than unblended fish bones used by the other researchers in terms of the pH satisfying the EPA standard for wastewater discharge. Also, when compared with the work of the other authors, the pH result shows that fishbones are viable sources for wastewater treatments. This report goes a long way to justify the efficacy and potentials of BFB for satisfying the World Health Organization (WHO) pH guidelines (6–8) for wastewater discharge [5, 10].
The table further emphasizes the difference in stirring rate, and removal efficiencies of fishbone as it varied between references [23] and the present work, and those of references [22, 33], respectively. The results show a wide disparity in their removal efficiency with the variations in stirring speeds. We reasoned that the results varied due to the differences in the compositions of the water medium and their characteristics. The comparisons of the results from the works of references [22, 33] depict that the stirring speeds have little or no direct bearing on their removal efficiencies; rather the coag–flocculation shear characteristics between the particles and the specialty index of the coagulant alignment with the wastewater are the determinant factor that greatly influenced the removal efficiency [9, 22, 23, 24, 27, 34].
The difference in the properties of the water medium will determine the quantity of coagulants applied at the optimum conditions. The optimal dosage of 0.4 g/L achieved for the present work makes BFB a viable coagulant when compared with the referenced results. It has also proven that BFB posted higher removal efficiency corresponding to 96% when compared to unblended fish bones used by the other researchers when used in an industrial environment. The cost–benefit analysis compared with the results for alum as applied to the present work further elaborates the efficacy and importance of BFB coagulant in the treatment of cosmetic wastewater systems.
4 Conclusion
The conclusions drawn from the research findings on the coagulation–flocculation treatment of cosmetic wastewater include that blends of fishbone (BFB) were proven to be more efficient than aluminum-based coagulant (ABC) in terms of pH. The output of the blends of fishbone satisfied the pH standard of the EPA for industrial wastewater discharge, while alum does not. The best performance of fishbone was found to occur at an optimum dosage with minimal potential to blind filters, short coag–flocculation time, scaling was reduced and energy conserved. It effectively reduces high turbidity in cosmetics wastewater to small residual volumes at short periods of settling time. The organic material was confirmed as a viable coagulant–flocculants aid showing strong capacity to function best with alum. We concluded further from the cost remediation analysis that it is economically viable to treat wastewater with low acidity and high alkalinity using blends of fishbone at high stirring rate. The reused material was proven to save cost compared to alum for water treatment processes [35, 36].
References
Sample PDF documentation on “Global Cosmetics Products Market Top Country Data” (2020). http://www.360marketupdates.comsample/12884489, http:www.wfmj.com.
Google documents New York. Published by Zion market research New York, (2018). http://www.zionmarketresearch.com/sample/cosmetic-products-market.
Sample PDF document “Cosmetic and skin care products Global Industry perspective, Comprehensive analysis and Forecast (2018): 2017–2024”Report code: ZMR-893, pp 1–160. http://www.zionmarketresearch.com/repor/cosmetic-products-market.
Recommended Standards for Wastewater Facilities: Policies for Design, Review and Plans for Wastewater Collections and Treatments Facilities (2014): Health Research, Inc, Health Education Service Division. http://www.healthresearch.org/store/ten-state-standards.
EPA guidelines for water quality-based decisions (1991) The TMDL Process Doc. (1991); No. EPA 440/4-91-001
Environmental Protection Agency (EPA) (1999) Quality-Based Decisions. The TDML Process Doc. No EPA 440/4–91.001: TDML Proc. Doc., 1999
Malik QH (2018) Performance of alum and assorted coagulants in turbidity removal of muddy water. J Appl Water Sci 40:1–10
Usefi S, Asadi-Ghalhari M (2019) Optimization of turbidity removal by coagulation and flocculation process from synthetic stone cutting wastewater. Int J Energy Water Resour 3(11):33–41. https://doi.org/10.1007/s42108-019-00010-2
Enerst E, Onyeka O, David N, Blessing O (2017) Effect of PH, dosage, temperature and mixing speed on the efficiency of water melon seed in removing the turbidity and colour of Atabong River, Akwa-Ibom State, Nigeria. Int J Adv Eng Manag Sci (IJAEMS) 2454–1311
WHO, UNEP, GEMS (1989) Global freshwater quality. Alden Press, Oxford
Tran NVN, Yu QJ, Nguyen TP, Wang S-L (2020) Coagulation of chitin production wastewater from shrimps scraps with by-product from chitosan and chemical coagulant polymers. MDPI Academic Open Access Publishing. 126072020 (2020): 20
Khan AH, Khan NA, Ahmed S, Dhingra A, Singh CP, Khan SU, Mohammadi AA, Changani F, Yousefi M, Alam S, Vambol S, Vambol V, Khursheed A, Ali I (2020) Application of advanced oxidation processes followed by different treatment technologies for hospital wastewater treatment. J Clean Prod 269:122–411
Menkiti MC, Nnaji PC, Onukwuli OD (2009) Coag–flocculation kinetics and functional parameters response of periwinkle shell coagulant (PSC) to PH variation in organic rich coal effluent. J Natl Sci 7(6):1–18
Nnaji PC, Okoye CC, Umeuzuegbu JU (2020) Efficiency of Luffa cylindrica and Muccuna Sloaneai seeds in dye removal: a new approach. World Sci News Int J 2392–2192:84–201. https://doi.org/10.1007/s13201-018-0662-5
Greenberg LS, Eaton AD Standard methods for the examination of water and wastewater, 20th ed. APHA, USA
Farajnezhad H, Gharbani P (2012) Coagulation treatment of wastewater in petroleum industry using poly aluminum chloride and ferric chloride. IJRRAS 13(1)
Iwuozor KO (2019) Prospects and challenges of using coagulation and flocculation method in treatment of effluents. Adv J Chem 2(2):105–127. https://doi.org/10.29088/SAMI/AJCA.2019.2.105127
Lourenco A, Arnold JL, Gamela JAF, Cayre OJ, Rasterio MG (2018) Anionic poly-electrolytes synthesized” an aromatic-free-oils process for application as flocculants in dairy-industry effluent treatment. J Ind Eng Chem Res 57(49):16884–16896. https://doi.org/10.1021/acs.iecr.8b03546
Subasinghe R (2017) FAO fisheries and aquaculture circular FIAA/C1140 (En): world aquaculture production 2015. A brief overview: (2017). Yearbook of Fisheries Statistics. http://www.fao.org
United Nations Food and Agriculture: Food and Agriculture Organization of the United Nation, 2002. . No 1774. http://www.fao.org
Rahman NS, Yhaya MF, Azahari B, Ismail WR (2018) Ultilization of natural cellulose fibres in wastewater treatment. Springer, New York
Ebrahimi A, Arami M, Bahrami H, Pajootan E (2013) Fish bone as a low-cost adsorbent for dye removal from wastewater: response surface methodology and classical method. J Environ Model Assess. https://doi.org/10.1007/s10666-013-9369-z
Mu Y, Saffarzadeh A, Shimaoka T (2016) Feasibility of using natural fishbone Apatite for removal of Pb from municipal solid waste inceneration (MSWI) fly ash. J Procedia Environ Sci J 31:345–350. https://doi.org/10.1016/j.provenv.2016.02.046
Kan CC, Huang C-P (2002) Coagulation of high turbidity water: the effects of rapid mixing. J Water Supply Res Technol 51:77–85
Othmani B, Rasteiro MG, Khadhraoui M (2020) Towards green technology: a review on some efficient model plant-based coagulants/flocculants for fresh-water and wastewater remediation. J Clean Water Technol Environ Policy 22:1025–1040
Muruganandam L, Saravana Kumar MP, Jena A, Gulla S, Godhwani B (2017) Treatment of wastewater by coagulation and flocculation using biomaterials. In: IOP conference series: materials science and engineering, vol 263, p 032006. https://doi.org/10.1088/1757-899X/263/3/032006
Greville AS (1997) How to select a chemical coagulant and flocculants. In: Alberta waste & wastewater operators association 22nd annual seminar
Pivokonsky M, Naceradska J, Brabenec T, Novotna K (2015) The impact of interactions between algea organic matter and himic substances on coagulation. PubMed Water Res 84:278–284
Tetteh EK, Rathilal S (2019) Application of organic coagulants in water and wastewater treatment. Inter-open 4. https://doi.org/10.5772/Inter-open.84556
Feng Y, Chu Z, Man L, Hu Y, Zhang C, Yuan T, Yang Z (2019) Fishbone-like polymer from green cationic polymerization of methyl eleostearate as bio-based nontoxic PVC plasticizer. ACS Sustain Chem Eng 7(23):18976–18984
Vivian C, Mohan Dhas S, Ashwin G (2019) Evaluation of bio-based fibers for treatment of wastewater from textile industry. Int J Innov Technol Explor Eng 8(8):2278–3075
New tariff rate and customers classification-BEDC ELECTRICITY-TARRIF www.bedcpower.com/new-electricitytariff-rate-2, February (2016): internet material
Okey-Onyesolu CF, Onukwuli OD, Ejimofor MI, Okoye CC (2020) Kinetics and mechanistic analysis of particles decontamination from abattoir wastewater (ABW) using novel Fish Bone Chito-protein (FBC). Elsevier Publication Ltd, New York. https://doi.org/10.1016/j.heliyon.2020.e04468
Bayar S, Yildiz YS, Erdem A, Irdemez S (2011) Effect of stirring speed and current density on removal efficiency of poultry slaughter house wastewater by electrocoagulant method. Desalination 280:103–107
Abidemi BL, Adekunle O, Temitope A, Joshua O, Rachael A (2019) Treatment technology of wastewater from cosmetic industry a review. Int J Chem Bio Mol Sci 4(4):69–80
Cui H, Having X, Yu Z, Chen P, Cao X (2020) Application Progress of enhanced coagulation in water treatment. RSC Adv 34
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ovuoraye, P.E., Ugonabo, V.I. & Nwokocha, G.F. Optimization studies on turbidity removal from cosmetics wastewater using aluminum sulfate and blends of fishbone. SN Appl. Sci. 3, 488 (2021). https://doi.org/10.1007/s42452-021-04458-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-021-04458-y