Abstract
Biomass is considering a source of organic carbon, which can replace fossil resources by using pyrolysis process, therefore an efficient biomass thermal modification technology has been target of so much research. The objective of this work is to study the potential energy of sugarcane bagasse and thermochemically modified bagasse for bioenergy potential for use in heat generation and energy. The thermal analysis was conducted by powder-shaped exposure of the three study samples (SB, AC-1, and AC-2) at three heating rates of (5, 7.5 and 10 °C min−1), it was possible to identify three stages of thermal degradation and study some thermochemical reactions, using two iso-conversional models, Kissinger–Akahira–Sunose (KAS) and Ozawa–Flynn–Wall (OFW) to calculate some kinetic parameters, such as activation energy (Ea) and pre-exponential factor (A). First step was about the devolatilization of volatile matter, moisture, and other substances. Degradation of hemicellulose, cellulose and lignin were shown in a second step. Characterization analyzes, such as SEM–EDX and textural parameters of the samples, show the presence of carbon in samples SB and AC-1. Due to SEM analyzes, morphological differences between the samples are showing as AC-1 and AC-2 samples present a rougher shape with pores, on the other hand, SB sample show a fibrous shape. In conclusion, sugarcane bagasse and thermochemically modified bagasse, show very promising results, for future studies, such as for bioenergy potential.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
1 Introduction
Thermochemically modified biomasses a carbonaceous material of porous structure that presents in its chemical composition some heteroatom, such as oxygen bound to carbon atoms. It is characterized by having a high surface area, claiming the ability to adsorb molecules and gases, in addition to the high functional groups on the surface, responsible for the high degree of reactivity [1, 2]. Various types of materials can be used as precursors of thermochemically modified biomass. These precursors are generally carbonaceous materials, carbon residues, biomass (lignocellulosic), biomass residue and polymers [3]. Biomass can be known as a group of organic material that can be converted into energy, with a great potential as a source of renewable energy. In this context, the biomass used was sugarcane bagasse, in which Brazil is the largest producer in the world [4].
According to data from the Ministry of Agriculture, Livestock and Supply of the Brazilian Government, the harvest 2018/2019 was about 643 million tons [5, 6], the bagasse generated from sugarcane milling is approximately 280 kg of bagasse per ton of sugarcane. These quantities show the importance of studying the bioenergy potential of sugarcane bagasse residues [7]. For lignocellulosic materials, such as sugar cane bagasse, there are some components: water (moisture) around 50%, cellulose ranging from 25 to 40%, hemicelluloses from 25 to 35%, and lignin with approximately 30% of the composition [5]. Precursor materials for production of thermochemically modified biomass, it is important to highlight the activating agent, if chemical impregnation is chosen, as is the case of this work. The activation step can understand the physical and chemical modification. Physical process modification can be performed in two consecutive stages: pyrolysis of the precursor material at high temperatures (above 300 °C) in inert atmosphere and thermal modification, at the same temperature or at temperatures higher than pyrolysis, in the presence of an oxidizing gas such as water vapor, carbon dioxide or both [8, 9]. These gases promote the oxidation of carbonaceous material inside the particles, promoting the clearance of existing pores, and consequently developing its porous structure [10]. On the other hand, in chemical modification, the material to be activated must be impregnated with chemical substances such as phosphoric acid, potassium hydroxide, zinc chloride, among others, before or after pyrolysis. Potassium hydroxide (KOH) was adopted because it is one of the chemical agents most used in this type of modification [11]. Traditionally, modified biomass and carbons are used in thermal and energy applications, through processes such as pyrolysis, which can be made in an inert atmosphere. Conversion of biomass into energy depends on some factors, such as the nature of biomass, pyrolysis factors, such as heating rate, pressure involved, resistance level, and inert atmosphere [12, 13].
According to Shen et al. [14], observed the thermal decomposition of biomass by thermogravimetry has been extensively used to investigate the combustion/pyrolysis properties of solid fuels, together with the thermogravimetric derivative, as they are complementary techniques. Combustion and biomass pyrolysis are part of direct routes to derive energy from lignocellulosic materials. There are tools to help to decide whether the pyrolysis can maintain self-energy balance, one of these tools are reaction heats with temperatures during biomass pyrolysis and combustion [14]. Ounas et al. [15] studied the thermal degradation of sugarcane bagasse and olive residue, dividing kinetic parameters by steps, in the main lignocellulosic components, such as hemicellulose and cellulose. They found an activation energy for hemicellulose in the range between 168–180 kJ mol−1 and 153–162 kJ mol−1; and for cellulose, this range ranged between 168–180 kJ mol−1 and 153–162 kJ mol−1, for sugarcane bagasse and olive residue, respectively. Rueda-Ordóñez and Tannous [16] showed the thermal decomposition of sugarcane straw, using iso-conversional models. Maia et al. [17] studied experimental investigation of the delignification process influence on thermochemical and kinetic properties of Capsicum waste, they found an activation energy for waste biomass in the range between 61–193 kJ mol−1 and 72–201 kJ mol−1 for Kissinger–Akahira–Sunose (KAS) and Ozawa–Flynn–Wall (OFW) methods, respectively. Three different heating rates of (5, 7.5 and 10 °C min−1) were used in this work for the kinetic study to obtain the activation energy and pre-exponential factor considering two isoconversional methods (KAS and OFW) [17].
Experimental characterization analyzes such as scanning electron microscopy (SEM) and textural parameters of porosity were performed to give full support and scientific knowledge. For these reasons, the objective of this work was to investigate the thermal behavior and to study the kinetic parameters of sugarcane bagasse, and thermochemically modified biomass for bioenergy potential.
The focus of this work was to prepare biochar using a process of thermochemical modification of biomass residues that were doped with KOH. The sugarcane bagasse chemically doped was taken to a muffle with controlled temperature and inert atmosphere so that a pyrolysis process could be carried out. Two biochar were prepared, AC-1 and AC-2, these two products presented important morphological characteristics and kinetic parameters, when their application in heat generation and bioenergy is aimed.
2 Materials and methods
2.1 Sample preparation
Sugarcane bagasse waste were washed with water after this the samples were dried in an oven (Solab-SL-100/42) at 105 °C until constant weight [18]. After reaching constant weight, the samples were ground in a knife mill (MA048-Marconi) and Solotest sieves (NBR # 48-300 μm).
2.2 Biochar production
In an analytical balance, about 0.6 g of dry biomass (SB) were weighed [19]. The samples were arranged in porcelain crucibles with partially open lid in inert atmosphere, to exit the most volatile and pyrophoric materials in a ceramic furnace (muffle). Pyrolysis temperature was 350 °C at a heating rate of 10 °C min−1. Because higher temperatures, ash formation was observed. After the pyrolysis process, the biochar was cooled in a desiccator and packed in previously sanitized and closed bags and later processed as thermochemically modified bagasse. Figure 1 showed the route of preparation of the biochar samples.
2.3 Production of thermochemically modified bagasse
Methodology used in the steps of chemical impregnation and action under temperature were based on the studies by Correia et al. [20], however, adaptations were made for the biomass under study in this work. A biochar sample (AC-1) was produced, which was chemically modified by KOH impregnation (85%—Synth) in aqueous solution with ratio of 2 wt% [21, 22]. Chemical agent KOH was chosen due to its high employability in the production thermochemically modified bagasse [23, 24].
Thermochemically modified bagasse initially went through the impregnation process with KOH, then was stirred (Micropr, Q225M) for 30 min, then was vacuum filtered (Tecnal), to be drying at 105 °C. After this process, the doped and dry thermochemically modified bagasse was taken to a muffle furnace for heat treatment at 450 °C, adopting the same procedures and conditions of the pyrolysis previously performed. After pyrolysis test, the sample was washed in distilled water at 60–70 °C to clean the area surface, and pore clearance, and then be cooled to room temperature.
Subsequently, the pH of the sample was neutralized using a solution of HCl 0.5 M. Finally, the sample was vacuum filtered and carried to the oven for drying at 105 °C. After all the procedures, the samples were collected and stored in properly sealed plastic bags. Another thermochemically modified bagasse sample was produced (AC-2) by impregnating KOH (2 wt%) directly into biomass SB, and pyrolysis was taken at 350 °C and then washed and its pH corrected, finally, to drying at 105 °C for 24 h.
Figure 2 show the procedures performed, totaling three study samples (SB, AC-1, AC-2).
2.4 TG analyses
Study samples were characterized in thermogravimetric (TG) and thermogravimetric differential (DTG) terms in a thermogravimetric analyzer (simultaneous TGA–DSC, TA Instruments SDT Q600), with a heating rates of 5, 7.5 and 10 °C min−1, and temperature from 25 to 700 °C, under nitrogen atmosphere (100 mL min−1). For each analysis, about 1.5 mg of each sample was put in platinum crucibles. TA Instruments software provided the TG and DTG curves. For this study, only the rate of 5 °C min−1 was discussed, because it was the reason with the highest degree of detail.
2.5 Kinetic models
Isoconversional methodology in non-isothermal experiments is recommended from International Confederation for Thermal Analysis and Calorimetry—ICTAC kinetics committee and it was applied to this work to calculate the activation energy (Ea) and the pre-exponential factor (A) [25]. Activation energy was calculated using non-isothermal TG/DTG curves at three different heating rates of 5, 7.5 and 10 °C min−1, calculated according to the Ozawa–Flynn–Wall (OFW) and Kissinger–Akahira–Sunose (KAS) models.
2.5.1 Ozawa–Flynn–Wall
For the calculation of thermodynamic parameters, the OFW model was used, in which it is one of the most claimed models for this calculation, besides being one of the most accepted in the literature [25, 26]. Equation (1) was applied, based on the TG and DTG curves of the samples [25, 27].
β is the heating rate (°C min−1), Cα is a function of the degree of conversion α, R is the constant of gases (8.31 J K−1 mol−1) and T and the absolute temperature (K) that designates the heating rate and the mass conversion is calculated plotting a graph of ln(β) versus 1/T, with known Cα. Three different values for heating rate (β) were considered and the same values of degree of conversion (α) and absolute temperatures (T).
2.5.2 Kissinger–Akahira–Sunose
Mathematical model KAS was adopted because it comprises a model that is considered one of the best iso-conversional methods [25, 28]. It was applied considering Eq. 2 [25, 29].
Ea and A are respectively, activation energy (KJ mol−1) and exponential pre factor (s−1) at a certain degree of conversion (α). An ln (β/T2) versus 1/T graph allows the calculation of the activation energy by the slope of the straight line (−Ea/R).
2.6 Thermodynamic parameters
Thermodynamic parameter studied was the pre-exponential factor (A), which was calculated according to Eq. 3 [27, 29].
Tm is the temperature of the maximum peak of DTG (K).
2.7 Textural parameters
Textural parameters (BET surface area, micropore volume, pore volume and pore diameter) were obtained by a porosimeter (Micromeritics—ASAP 2020), calculated from N2 adsorption, interfaced to a microcomputer. Surface areas and micropore volumes were calculated from the Brunauer–Emmett–Teller (BET) and t-plot method, respectively. The total pore volumes were calculated from the adsorbed amount of N2 at P/P0 and average pore size was calculated with the BET method.
A sample of approximately 0.25 g of the study material was used. Weighted in analytical balance, which was heated to 300 °C and vacuum degassed to lose moisture and other contaminants, and then analyzes were made.
2.8 Scanning electron microscopy (SEM) and energy dispersive X-ray spectroscopy (EDX) analysis.
The samples were analyzed using a scanning electron microscope and energy dispersive spectroscopy (SEM, JEOL-JSM-6010LA). The powder samples were metallized (Denton Vacuum V Metallizer) for electrical conduction. In addition, they were placed on a carbon tape and observed under a potential difference in range of 0 to 5 keV and increases of 500 and 1000×. For EDX analysis, the In-Touch Scope equipment was used, coupled to the same scanning electron microscope used.
3 Results and discussion
3.1 Thermal analysis
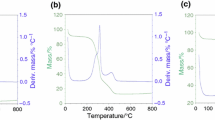
Thermal decomposition behavior was studied using a thermogravimetric technique at the heating rates of 5, 7.5 and 10 °C min−1 in an inert atmosphere (N2, flow 100 mL min−1) within the temperature range of ambient (30 °C) to 700 °C. Different heating ratios were used to demonstrate the different behaviors that can be observed in the TG and DTG curves, only the thermogravimetric curves of the samples at 5 °C min−1 were discussed, because this rate is the one that presented a higher degree of detail for thermal analysis. However, for kinetic studies, the others heating rates were used. Figure 3 illustrates the TG-DTG curves of the samples of SB, AC-1 and AC-2 in an inert atmosphere at 5 °C min−1.
When analyzing the TG-DTG curves of SB, it was observed that it presented features of the burning process of lignocellulosic components such as hemicellulose, cellulose and lignin. It was also observed the decrease in mass with the increase in temperature, and curves consistent with those of dry sugarcane bagasse, according to published studies [15, 30].
Percentage of residual mass in samples SB, AC-1 and AC-2 was 26.82%; 66.26% and 65.63%, respectively. According to Morais et al. [25], higher mass loss (lower residual mass), higher the amount of volatile material present, and the smaller the amount of ash formed, this can be attributed to the presence of aromatic rings, making the biomass more stable. High residual mass value for samples AC-1 and AC-2 can be explained by the pyrolysis process to which the thermochemically modified bagasse were previously submitted (AC-1) and later (AC-2), besides the fact that the samples are impregnated by the inorganic KOH.
TG-DTG curves allowed the identification of three steps of biomasses degradation process. The first stage (Step 1) is related to devolatilization, loss of moisture, volatile matter occurring at temperatures up to 130 °C [31]. The second stage (Step 2) comprised the thermal degradation of the lignocellulosic components, being much more evident in the SB sample, because it is the sample without any previous heat treatment, compared to samples AC-1 and AC-2. In this phase, there are two mass losses, the first between 130 and 380 °C, which probably corresponded to the decomposition of hemicellulose, and most of the volatile organics, elucidating an intense peak in the DTG at 338 °C, representing the loss of 70% of the entire mass, characterizing the high reactivity of the sample and the second between 390 and 550 °C, which may correspond to cellulose decomposition, small amount of lignin, hemicellulose, carbohydrates, and biodegradable matter [15, 25]. This same process for samples AC-1 and AC-2 were evidenced in the range of 130–550 °C, probably attributed to decomposition of organics, hemicellulose, and cellulose. Hameed et al. [32] observed for sugarcane bagasse two reaction zones of thermal degradation (Zone I: 200–400 °C and Zone II: 400–600 °C). Other studies were described. Naqvi et al. [33] obtained similar behavior for decomposition of sugarcane bagasse biomass, active pyrolysis started after 200 °C, and concluded at 560 °C.
Kumar et al. [31] observed similar behavior for banana trunk biomass. Kumar et al. [34] obtained similar behavior for decomposition of rice husk biomass samples. In addition, acai pyrolysis was studied by Santos et al. [35] and they found the same thermal behavior. This temperature variation in the curves can be explained by the thermal variation of the lignocellulosic components present. For example, hemicellulose and cellulose decomposes at temperatures in the range 200–450 °C, while lignin decomposes at temperatures in the range of 450–1000 °C [3, 36]. The third stage (Step 3) is responsible for ash formation, carbonates, less volatile and inorganic matter at temperatures above 550 °C.
3.2 Kinetic study
Through thermogravimetric analysis, it is possible to calculate kinetic parameters, such as activation energy, Ea, and pre-exponential factor, A. For this, the iso-conversional methods OFW and KAS were applied in the three study samples in 5, 7.5 and 10 °C min−1. For the calculation of the activation energy, the degrees of conversions (α) chosen in range of 70–95%, because in this range there was the higher loss of mass of the material except that for the sample that underwent pyrolysis process (AC-1 and AC-2), there was a great reduction in mass loss.
Figure 4 showed the conversion degree curves (α) vs. temperature (K) at the heating rates of 5, 7.5 and 10 °C min−1 in inert atmosphere. Through these results, the degree of conversion and mass loss can be used to better understand the thermal behavior of the study biomass [4]. Activation energy can show the limit at which thermal reactions start, so its calculation showed the energy threshold that was overcome before the molecules reacted and formed new compounds. The values found by the OFW and KAS models for activation energy and exponential factor in inert atmosphere can be observed in Tables 1 and 2. In comparative terms of Ea, Fig. 5 shows a comparison of the OFW and KAS models in inert atmosphere.
Figures 6 and 7 shows the calculations of kinetic parameters of activation energy and pre-exponential factor according to OFW and KAS models in inert atmosphere. The kinetic results of the samples of activated carbons were presented, because the SB sample presented R2 with low adjustment, distant values of 1. According to Tables 1 and 2 that the activation energy values for the AC-1 sample can vary from 7.16 to 73.82 kJ mol−1 for the OFW model and from 4.90 to 68.41 kJ mol−1 using the KAS model. Finally, the activation energy of the AC-2 sample can vary from 16.31 to 75.88 kJ mol−1 for the OFW model and from 8.79 to 77.55 kJ mol−1 for the KAS model, this variation may indicate changes in reactivity as results of extensive reactions and the complex nature of biomass [25]. In comparative, Fig. 5 showed that it was important to use more than one iso-conversional model to calculate the activation energy and pre-exponential factor. The OFW and KAS models showed a certain similarity for all samples. The activation energy comprises the barrier necessary to initiate thermochemical reactions, in this case, thermochemically modified bagasse sample AC-1 presented the lowest Ea value (4.90 kJ mol−1), followed by the other sample of thermochemically modified bagasse AC-2 (8.79 kJ mol−1) according to KAS, this means that they need a little energy to initiate chemical reactions, this can be explained by the fact that these samples are more reactive, because they were samples of chemically activated carbons with KOH. The Ea values found for the AC-1 and AC-2 samples were below the mean when compared to the biomass of sugarcane bagasse [15, 25]. Overall, low activation energies (60–120 kJ mol−1) may indicate that high values of activation energy are required to decompose lignocellulosic biomass [25]. Ounas et al. [15] investigated pyrolysis in sugarcane bagasse using the OFW method and found the range of 168–180 kJ mol−1 for activation energy related to hemicellulose degradation, and 321–240 kJ mol−1 for cellulose degradation. In general, activation energies in the range of 80–200 kJ mol−1 may indicate the decomposition of hemicellulose, 195–236 kJ mol−1 represents the thermal degradation range of cellulose, and 18–65 kJ mol−1 for lignin [25]. Morais et al. [25] found Ea values for sugarcane bagasse biomass that can vary from 11 to 88 kJ mol−1 following the OFW method and 3–78 kJ mol−1 for the KAS model. Jayaraman et al. [37] studied pyrolysis and gasification for energy recovery of sugarcane bagasse and found activation energy values in the range 164–240 kJ mol−1 using OFW and 163–243 kJ mol−1 adopting the KAS model. Hameed et al. [32] studied pyrolysis of sugarcane bagasse and the highest Ea value found was 89.27 kJ mol−1. Pre-exponential factor (AC-1) ranged from 4.02 × 10–1 to 1.02 × 106 s−1 for OFW and 6.56 × 101 to 2.16 × 102 s−1 for KAS were found. The AC-2 sample presented pre-exponential factor can vary from 1.96 × 100 to 5.22 × 104 s−1 for OFW and 1.13 × 102 to 4.09 × 102 s−1 for KAS. These low values of the pre-exponential factor (≤ 109 s−1), may indicate that the reactions occurred on the surface of the samples, not suffering alterations and interferences of the surface area, difficult to rupture, indicating a complex reaction, as can be seen in most of the samples studied. On the other hand, values ranging from 104 s−1 to 1018 s−1, as seen in the OFW model for AC-1 and AC-2, may indicate first-order reactions. According to the values found for the pre-exponential factor, Hameed et al. [32] obtained 4.03 102 s−1 for sugarcane bagasse pre-exponential factor with Jander equation. Therefore, the results of the methods revealed that the activation energy depends on the degree of conversion, which were obtained through two iso-conversion models OFW and KAS, by which they provided different values of activation energy. For this reason, the comparison of the results by two methods was of great help in the validation of the data.
3.3 Textural parameters
Table 3 shows the results for the textural parameters of SB, AC-1 and AC-2.
Specific surface area (BET) values can vary from 4.58 to 11.83 m2 g−1, and the total pore volume can vary from 0.04 to 0.08 cm3 g−1. Micropore volume for this case was not very representative, being below 0.01 cm3 g−1. Finally, the pore diameter can vary from 25 to 57 nm. It was noted that before chemical activation, the total pore volume in the SB sample was low (0.04 cm3 g−1). After the modification performed with KOH, there was an increase in the total pore volume in the samples AC-1 (0.07 cm3 g−1) and AC-2 (0.08 cm3 g−1), a fact that allow to the intensified creation of pores, as they were evidenced in the SEM analyzes [37]. There was also an increase in the BET surface area in the thermochemically modified bagasse samples, this increase is directly related to the temperature increase to which the samples were submitted, since the sample AC-1 was under the temperature of 450 °C while the AC-2 was under 350 °C. In general, a high surface area and a high volume of micropores are basic requirements for the adsorption process, in combination with the attractive van der Waals forces and the affinity of functional groups with the substance of interest [38, 39]. David and Kopac [39] studied CO2 adsorption on activated carbons of a nutshell mixture, finding a value of specific surface area BET and pore volume < 1 m2 g−1 and < 0.01 cm3 g−1, respectively. In addition, Shafeeyan et al. [40], studied CO2 adsorption on activated carbon based on palm bark and found a BET specific surface area value and pore volume of 723 m2 g−1 and 0.37 cm3 g−1, respectively. Alhassan et al. [41] investigated the CO2 adsorption on activated carbon from sugarcane bagasse, obtaining a BET specific surface area value and a pore volume of 563.8 m2 g−1, and 0.825 cm3 g−1, respectively. The pore diameter (Pd) allowed the classification of the pores of the studied samples. According to IUPAC, the AC-1 sample (Pd = 25.48 nm) is classified as mesoporous (2 < Pd < 50 nm). Therefore, the textural parameters, served to characterize the samples in terms of physical analysis of porosity, providing knowledge for future studies of processes such as adsorption gas capture, and other processes. Figure 8 illustrates the variation of the Pd with the BET surface area, where there is a non-linear relationship between the BET surface area and the pore diameter (Pd), showing once again the complex nature of the samples, as seen, the Pd decreased in relation to the SB for the AC-1 sample and increased from AC-1 to AC-2. The reverse process was seen for the BET surface area, the BET surface area increased from SB to AC-1 and regressed from AC-1 this non-linearity can be considered normal given the complexity of biomass [39].
3.4 SEM–EDX analyzes
Images obtained from SEM analyze revealed two morphological stages: fibrous structure and rough structure. The fibrous surface is formed by strips in parallel covered with a residual material while the rough morphology is more fragile having a fragmented structure containing deformities through which small pores connect neighboring cells with the surface of the walls [25]. The SEM images are shown in Fig. 9 with a 500 and 1000× magnification for the samples of SB (a, b), AC-1 (c, d) and AC-2 (e, f), respectively. AC-1 and AC-2, due to being subjected to thermal degradation presented rough structures forming numerous heterogeneous pores demonstrating that there is more uniformity before the pyrolysis process [25, 39]. A possible explanation is that during thermal conversion a large amount of volatile material flows through the solid material for a short period that changes the particles on the surface shrinking and making them rough [25]. AC-1 sample showed a porous structure with a disorganized pattern which is related to the surface area. The same sample still retains parts of the fibrous morphology of fresh cane bagasse (SB) [42]. Fibers present in AC-1, presented a more transversal shape. Lignin was partially removed by the pyrolysis process showing a further morphological difference to the natural sample (B). Thermal degradation process provided the formation of a porous structure indicating that the pyrolysis removed external fibers which increased the surface area facilitating the cellulose degradation. In addition, the morphology of thermochemically modified bagasse AC-2 showed a reduction in the fibrous structure, however with numerous pores formed [25]. Through EDX analysis it was possible to identify some elements present on the surface of each sample (Fig. 10). According to Fig. 10, it was possible to identify the following elements and their percentages in the SB sample: carbon (81%) and oxygen (19%). After the activation process for the sample AC-1 the presence of carbon (70.86%), oxygen (22.65%) and potassium (6.49%) were verified. Finally, for the directly pyrolyzed sample AC-2 the presence of carbon (30.64%) potassium (present in the activating agent KOH) (56.70%) was seen maintaining the proportion for oxygen (12.66%). A rigorous increase in potassium was observed in AC-2, and a decrease in carbon, most possibly explained by the fact that this sample was impregnated directly in the biomass in nature passing through only a pyrolysis process.
4 Conclusions
To study the potential energy of sugarcane bagasse and thermochemically modified bagasse, the thermal behavior of the samples showed the presence of three steps by which there was variation in the temperature range involved in each one. First step involved the devolatilization of more volatile substances and loss of moisture. Second stage showed the thermal degradation of lignocellulosic components such as hemicellulose, cellulose and parts of lignin. The final stage (third stage) of thermal degradation showed the presence of ash and materials that were not decomposed. All applied models OFW and KAS for calculating kinetic parameters are in accordance with literature data, with a variation in the activation energy range and pre-exponential factor observed during thermal degradation indicating that the pyrolysis process occurred in several stages. Through the textural parameters, it was possible to characterize the samples in relation to the pore size, specific BET surface area and pore volume. SEM analyzes, on the other hand, showed morphological differences between the samples with the SB sample having a fibrous, tubular shape and the AC-1 and AC-2 samples, a rougher shape with pores not evenly spread but maintaining part of its fibrous structure. EDX analyzes allowed to verify the presence of carbon for all samples, for samples AC-1 and AC-2, it was possible to identify the presence of potassium most likely coming from the activating agent. For future studies, some prospective was related such as studies of energy balance, ignition and burnout temperatures and applied studies, such as adsorption analysis for CO2 capture. Therefore, according to the results in this search, sugarcane bagasse and thermochemically modified bagasse showed very promising results it has considerable potential that can lead to the development of new studies for bioenergy potential or adsorption process.
References
Gorgulho HF, Mesquita JP, Gonçalves F, Pereira MFR, Figueiredo JL (2008) Characterization of the surface chemistry of carbon materials by potentiometric titrations and temperature-programmed desorption. Carbon 46:1544–1555
Li H, An N, Liu G, Li J, Liu N, Jia M, Zhang W, Yuan X (2016) Adsorption behaviors of methyl orange dye on nitrogen-doped mesoporous carbon materials. J Colloid Interface Sci 466:343–351
Yagmur E, Tunc MS, Banford A, Aktas Z (2013) Preparation of activated carbon from autohydrolysed mixed southern hardwood. J Anal Appl Pyrolysis 104:470–478
Jayaraman K, Gokalp I, Petrus S, Belandria V, Bostyn S (2018) Energy recovery analysis from sugar cane bagasse pyrolysis and gasification using thermogravimetry, mass spectrometry and kinetic models. J Anal Appl Pyrolysis 132:225–236
Morais LC, Maia AAD, Yamaji FM, Viana SRF, Resende P (2020) Energy analysis of sugarcane bagasse after enzymatic catalysis process. Biomass Conv Bioref. https://doi.org/10.1007/s13399-020-01097-y
National Supply Company (CONAB). Harvest of sugarcane 2018/2019. Ministry of Agriculture, Livestock and Supply, Brazil. Available in: http://www.conab.gov.br. Accessed 06 Nov 2020. (in Portuguese).
Alcarde AR (2020) Embrapa Sugarcane. Brasília. Available in: http://www.agencia.cnptia.embrapa.br/gestor/canadeacucar/arvore/CONTAG01_108_22122006154841.html. Accessed 06 Nov 2020.
Al Bahri M, Calvo L, Gilarranz MA, Rodriguez JJ (2014) Diuron multilayer adsorption on activated carbon from CO2 activation of grape seeds. Chem Eng Comm 203:103–113
El-Hendawy ANA, Alexander AJ, Andrews RJ, Forrest G (2008) Effects of activation schemes on porous, surface and thermal properties of activated carbons prepared from cotton stalks. J Anal Appl Pyrolysis 82:272–285
Li G, Tian F, Zhang Y, Ding J, Fu Y, Wang Y, Zhang G (2015) Bamboo/lignite-based activated carbons produced by steam activation with and without ammonia for SO2 adsorption. Carbon 85:448
Cao Y, Wang K, Wang X, Gu Z, Ambrico T, Gibbons W, Talukder A (2017) Preparation of active carbons from corn stalk for butanol vapor adsorption. J Energy Chem 26:35–41
Alhumade H, da Silva HJCG, Ahmad MS, Çakman G, Yıldız A, Ceylan S, Elkamel SA (2019) Investigation of pyrolysis kinetics and thermal behavior of Invasive Reed Canary (Phalaris arundinacea) for bioenergy potential. J Anal Appl Pyrolysis 140:385–392
Slopiecka K, Bartocci P, Fantozzi F (2012) Thermogravimetric analysis and kinetic study of poplar wood pyrolysis. Appl Energy 97:491–497
Shen J, Igathinathane C, Yu M, Pothula AK (2015) Biomass pyrolysis and combustion integral and differential reaction heats with temperatures using thermogravimetric analysis/differential scanning calorimetry. Bioresource Technol 185:89–98
Ounas A, Aboulkas A, El Harfi K, Bacaoui A, Yaacoubi A (2011) Pyrolysis of olive residue and sugar cane bagasse: non-isothermal thermogravimetric kinetic analysis. Bioresour Technol 102:11234–11238
Rueda-Ordóñez YJ, Tannous K (2015) Isoconversional kinetic study of the thermal decomposition of sugarcane straw for thermal conversion processes. Bioresour Technol 196:136–144
Maia AAD, Enriquez YAM, Morais LC (2020) Experimental investigation of the delignification process influence on thermochemical and kinetic properties of biomass. J Therm Anal. https://doi.org/10.1007/s10973-020-10061-x
Morais JPS, Freitas Rosa M, Marconcini JM (2010) Procedures for lignocellulosic analysis. In: Embrapa cotton (Ed.), 1. ed. Campina Grande: [s.n.]. 54.
Viotto RS, Maia AAD, Yamaji FM, de Morais LC (2017) Thermogravimetric investigation of spent shiitake substrate to solid biofuel. Can J Chem Eng 96:845–854
Correia LB, Fiuza RA Jr, Andrade RC, Andrade HMC (2018) CO2 capture on activated carbons derived from mango fruit (Mangifera indica L.) seed shells. J Therm Anal Calorim 131:579–586
Elmouwahidi A, García BE, Cadenas FP, Hódar JM, Marín FC (2017) Activated carbons from KOH and H3PO4-activation of olive residues and its application as supercapacitorelectrodes. Electrochim Acta 229:219–228
Deng S, Wei H, Chen T, Wang B, Huang J, Yu G (2014) Superior CO2 adsorption on pine nut shell-derived activated carbons and the effective micropores at different temperatures. Chem Eng J 253:46–54
Labus K, Gryglewicz S, Machnikowski J (2014) Granular KOH-activated carbons from coal-based cokes and their CO2 adsorption capacity. Fuel 118:9–15
Król M, Gryglewicz G, Machnikowski J (2010) KOH activation of pitch-derived carbonaceous materials: effect of carbonization degree. Fuel Process Technol 92:158–165
Morais LC, Maia AAD, Guandique MEG, Rosa AH (2017) Pyrolysis and combustion of sugarcane bagasse. J Therm Anal Calorim 129(3):1813–1822
Damartzis T, Vamvuka D, Sfakiotakis S, Zabaniotou A (2011) Thermal degradation studies and kinetic modeling of cardoon (Cynara cardunculus) pyrolysis using thermogravimetric analysis (TGA). Bioresour Technol 102:6230–6238
Kim YS, Kim YS, Kim SH (2010) Investigation of thermodynamic parameters in the thermal decomposition of plastic waste-waste lube oil compounds. Environ Sci Technol 44:5313–5317
Xu Y, Chen B (2013) Investigation of thermodynamic parameters in the pyrolysis conversion of biomass and manure to biochars using thermogravimetric analysis. Bioresour Technol 146:485–493
Leroy V, Cancellieri D, Leoni E, Rossi J (2009) Kinetic study of forest fuels by TGA: model-free kinetic approach for the prediction of phenomena. Thermochim Acta 497:1–6
Teixeira Cardoso AR, Conrado NM, Krause MC, Bjerk TR, Krause LC, Caramão EB (2019) Chemical characterization of the bio-oil obtained by catalytic pyrolysis of sugarcane bagasse (industrial waste) from the species Erianthus Arundinaceus. J Environ Chem Eng 7:102970
Kumar M, Shukla SK, Upadhyay SN, Mishra PK (2020) Analysis of thermal degradation of banana (Musa balbisiana) trunk biomass waste using iso-conversional models. Bioresour Technol 310:123393
Hameed Z, Aman Z, Naqvi SR, Tariq R, Ali I, Makki AA (2018) Kinetic and thermodynamic analyses of sugar cane bagasse and sewage sludge co-pyrolysis process. Energy Fuels 32(9):9551–9558
Naqvi SR, Ali I, Nasir S, Ali Ammar Taqvi S, Atabani AE, Chen WH (2020) Assessment of agro-industrial residues for bioenergy potential by investigating thermo-kinetic behavior in a slow pyrolysis process. Fuel 278:118259
Kumar M, Mishra PK, Upadhyay SN (2020) Thermal degradation of rice husk: effect of pre-treatment on kinetic and thermodynamic parameters. Fuel 268:117164
Santos VO, Queiroz LS, Araujo RO, Ribeiro FCP, Guimarães MN, da Costa CEF, de Souza LKC (2020) Pyrolysis of acai seed biomass: kinetics and thermodynamic parameters using thermogravimetric analysis. Bioresour Technol Rep 12:100553
Maia AAD, de Morais LC (2016) Kinetic parameters of red pepper waste as biomass to solid biofuel. Bioresour Technol 204:157–163
Liu SH, Huang YY (2018) Valorization of coffee grounds to biochar-derived adsorbents for CO2 adsorption. J Clean Prod 175:354–360
Huang G, Liu Y, Wu X, Cai J (2019) Activated carbons prepared by the KOH activation of a hydrochar from garlic peel and their CO2 adsorption performance. Carbon 153:804–806
David E, Kopac J (2014) Activated carbons derived from residual biomass pyrolysis and their CO2 adsorption capacity. J Anal Appl Pyrol 110:322–332
Shafeeyan MS, Daud WMAW, Houshmand A, Arami-Niya A (2011) Ammonia modification of activated carbon to enhance carbon dioxide adsorption: effect of pre-oxidation. Appl Surf Sci 257:3936–3942
Alhassan M, Andrew I, Auta M, Umaru M, Garba MU, Isah AG, Alhassan B (2018) Comparative studies of CO2 capture using acid and base modified activated carbon from sugarcane bagasse. Biofuels 9:719–728
Pérez NP, Pedroso DT, Machin EB, Antunes JS, Tuna CE, Silveira JL (2019) Geometrical characteristics of sugarcane bagasse for being used as fuel in fluidized bed technologies. Renew Energ 143:1210–1224
Acknowledgements
The authors would like to gratefully acknowledge the São Paulo Research Foundation (FAPESP) (process number: 2018/03138-7) for the financial support, São Paulo State University (UNESP Sorocaba-ICTS) for the opportunity to develop the project, the Laboratory of Activated Carbon (LCA) of Federal University of Paraiba (UFPB) for performing textural analyses.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Peres, C.B., Rosa, A.H. & de Morais, L.C. Investigation of pyrolysis kinetics parameters and thermal behavior of thermochemically modified bagasse for bioenergy potential. SN Appl. Sci. 3, 337 (2021). https://doi.org/10.1007/s42452-021-04345-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-021-04345-6