Abstract
The concentration of CO2, one of the most important greenhouse gases (GHG), has reached to 409.8 ± 0.1 ppm in 2019. Although there are many carbon capture and storage (CCS) methods, they are very costly and their long term use raises concern about environmental safety. Alternatively, bio-sequestration of CO2 using microalgal cell factories has emerged as a promising way of recycling CO2 into biomass via photosynthesis. In the present study, Indigenous algal strain Pseudanabaena limnetica was cultivated in pneumatically agitated 60-L flat-panel photobioreactor system. The gas was released from Bio-CNG plant as by-product into Na2CO3-rich medium and cultivated in semicontinuous mode of operation. It was observed that when CO2 was sparged in seawater-based 0.02 M Na2CO3 solution, maximum CO2 was dissolved in the system and was used for algal cultivation. Control system produced 0.64 ± 0.035 g/L of biomass at the end of 15 days, whereas CO2 sparged Na2CO3 medium produced 0.81 ± 0.046 g/L of biomass. When CO2 from Bio-CNG station was fed, it resulted in biomass production of 1.62 ± 0.070 g/L at the end of 18 days compared to 1.46 ± 0.066 g/L of biomass produced in control system which was not fed with gas released from Bio-CNG plant as by-product. Thus, feeding CO2 directly into Na2CO3 medium and operating the system semicontinuously would be efficient for scrubbing CO2 from commercial Bio-CNG plant. This study proves that feeding CO2 gas from Bio-CNG plant into Na2CO3-rich alkaline system can be used to feed algae for enhanced biomass production.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Global warming is being caused due to emission and increase in three greenhouse gases (GHGs), namely CO2, methane and N2O. Atmospheric CO2 concentration in 2018 was 407.3 ppm which increased by 2.5 ± 0.1 ppm in 2019 [1]. The Kyoto Protocol and the Paris Agreement (2015) have asked the participating countries to curb climate change impact by setting up policies out of which crucial are CO2 emissions by reducing fossil fuel usage and increasing carbon capture and sequestration [2, 3].
Most of chemical or physical means of capture of CO2 from smoke stack emission involve three major steps which are carbon capture, separation and storage which enormously increase the cost of project. [4]. Microalgae possess inbuilt mechanisms to capture CO2 from the atmosphere even in smallest concentrations. These mechanisms are referred to as carbon capture mechanisms (CCM) [5]. Also, microalgae can withstand toxic waste gasses such as CO2 and NOx, SOx coming from industrial and sewage wastewater sources. It is a known fact that microalgal cultures show enhanced growth when they are provided with CO2-enriched air. Hence, microalgae are used as biological scrubbers which utilize CO2 from the industrial gas released as by-product resulting into enhanced biomass production. Biomass can be utilized for applications such as food, animal feed, fuel and other value-added by products which could be incorporated into cosmetics and nutraceutical industry [4].
Microalgae captures CO2 in three ways like conversion of bicarbonates into CO2 which diffuses into cell directly, assimilation of CO2 directly via plasma membrane and direct uptake of bicarbonates though cell membrane transporters [6]. One of the challenges in providing CO2 into algal systems is its efficient transport to algal cells. The most common way of feeding CO2 is through fine-pore spargers but the barrier is the solubility of CO2 in aqueous medium. Sparging excess CO2 does not mean that it will not cause CO2 limitation because most of the sparged CO2 escapes into the atmosphere. This conventional way of CO2 feeding does not become economical, especially when large-scale algal cultivation is a target [7]. Alternative way of capturing CO2 present in gas released from thermal power plant or Bio-CNG plant is to absorb CO2 using solvents such as NaOH, K2CO3, etc., and this CO2-loaded solvent is then pumped directly into raceway ponds or photobioreactors and used for algal cultivation [8].
Present communication deals with the use of two different solvents NaOH and Na2CO3 for absorption of CO2. The CO2 released from CNG station was sparged in 0.01 M NaOH solution. After feeding CO2, the NaOH was converted to NaHCO3 and then Na2CO3. The conversion of NaOH to NaHCO3 and Na2CO3 was checked using pH double indicators. The amount of Na2CO3 solution formed was also calculated. This CO2-enriched Na2CO3 solution was used as carbon source while preparing the culture medium for growth of our own algal strain Pseudanabaena limnetica (Lemm.) komàrek isolated from salt pans of eastern suburban regions of Mumbai capable of sustaining elevated concentration of Na2CO3 [9]. It will serve dual purpose, sequestration of CO2 from Bio-CNG station and enhancement of algal biomass which can be utilized for several commercial applications.
2 Material and methods
2.1 Strain selected
Microalgal strain selected was Pseudanabaena limnetica (Lemm.) komàrek, which is an indigenous halophilic strain isolated from salt pans of Mumbai suburban region.
2.2 Culture medium
BG-11 medium used for growth of cyanobacterial strains was prepared as mentioned by Rippka et al. 1979 [10]. Modified seawater BG11 medium (MSWBG11) was formulated from the BG11 medium. MSWBG11 medium was modified BG11 seawater-based medium containing ten times higher carbonate (Na2CO3) compared to normal BG11 composition [11].
2.3 Design and culture conditions
To conduct the experiments for CO2 sequestration, the operationally optimized 60-L photobioreactor was utilized. The photobioreactor was made up of glass having dimensions of 20 cm × 50 cm × 70 cm (breadth × length × height). Construction and operational standardization were carried out in our previous study by Magar and Deodhar 2018 [11]. The culture was supplied with light intensity of 185–222 µmol m−2 s−1for 12 h every day using white fluorescent light. The agitation was provided using spargers at the rate of 4LPM using acrylic rotameters (Napro Scientific, Pune). Perforated horizontal tubes were placed at the base of the photobioreactor through which air was sparged into the system [11].
2.4 CO2 feeding
The CO2 supply was fed from the commercially available 100% compressed CO2 cylinders (Super Industrial Gases, Thane). The flow rate of CO2 was controlled using a commercial pressure regulator. Further for accurate flow rate, rotameter of 0.001–10 LPM capacity was procured from Napro Scientific, Pune. The gaseous CO2 was fed into the medium at a flow rate of 0.001 LPM through the borosilicate sintered glass sparger (Deepali enterprises, Mumbai). For experiment purpose, flow rate of ≤ 0.001LPM was maintained using the same rotameter and sparger system.
2.5 pH, Temperature, dissolved carbon dioxide (DCO2) detection and biomass estimation
The detection and monitoring of pH, temperature and DCO2 were carried out using electronic sensors, which were immersed in the working liquid system of the photobioreactor. Each of the sensors, i.e. pH and DCO2, along with inbuilt temperature sensor in each of them was obtained from Mettler-Toledo India Pvt. Ltd. The dry weight of the biomass (DWB) was estimated gravimetrically.
2.6 Experimental setup for dissolution of CO2 in different solvent systems
Experiment was performed by adding two different salts like NaOH and Na2CO3 having concentration of 0.01 M and 0.02 M, respectively, in tap water as well as seawater. Both alkaline solutions were separately prepared, each one having total volume of 5L. CO2 gas was sparged at the rate of 0.001 LPM in the system using sintered glass sparger as mentioned above. Sampling from each of the sets was done every 10 min, and molar concentration of NaOH, Na2CO3 and NaHCO3 was estimated and calculated by volumetric titration method using double indicators, namely phenolphthalein and methyl orange [15]. Change in pH was also measured every 10 min using pH sensor. Solution capable of capturing maximum CO2 was further used to study its effect on growth of algae.
2.7 Effect of CO2-sparged alkaline solutions on the growth of algae
In control experiment, the microalgae P. limnetica were grown in 60-L photobioreactor in MSWBG11 medium without any artificial CO2 supplementation. The initial inoculum was added in the reactor which was aerated using spargers at rate of 4 LPM, and the algal growth was continued for 15 days. Culture was exposed to white fluorescent light of 10,000–12,000 lx intensity for 12 h every day. Dissolved CO2 (henceforth mentioned as DCO2) concentration was noted and recorded continuously using data logger system. The associated pH change was also noted with pH sensor. The growth of dried algal biomass (g/L) was weighed after every 3-day interval in order to get measureable change in biomass weight [11].
In Test 1 and test 2, the culture of P. limnetica was inoculated in a MSWBG11 medium in the 60-L-capacity flat-panel photobioreactor. For test 1 and test 2, the carbonate content in MSWBG11 medium was replaced by CO2-enriched NaOH and Na2CO3 solutions, respectively. The culture was exposed to the white fluorescent light of 10,000–12,000 lx intensity for 12 h every day. The effect of CO2 sparged NaOH /Na2CO3 solution on algal growth was interpreted in terms of biomass produced which was measured at the intervals of 3 days. The DCO2 and pH change was also reported at every hour.
2.8 Semicontinuous mode of operation for maximum biomass yield
Algal culture P. limnetica was cultivated in three different 60-L flat-panel photobioreactors set A, set B and set C. The culture conditions were same as described above in Sect. 2.7. Algal culture was initially allowed to grow for 10 days till log phase was achieved and then semicontinuous mode of operation was adopted. From three sets, set A, set B and set C, three different volumes of culture media (1 L, 2 L and 3 L) were removed every day and it was replaced by the same amount of fresh MSWBG11 medium. The experiment was continued for 60 days. Everyday increase in the algal dried biomass (g/L) was measured gravimetrically and noted.
2.9 Effect of CO2 from Bio-CNG plant on growth of Algae P. limnetica in semicontinuous mode of operation
Experiment was conducted in the premises of Primove Engineering Pvt. Ltd., Pune. Composition of Bio-CNG coming from plant contains 50% CH4, 49% CO2 and 1% other gases. CO2 gas coming from Bio-CNG plant had 99% purity with less than 1% of other trace gases such as H2S, N2, etc. CO2 gas was directly sparged in MSWBG11 medium. This CO2-sparged MSWBG11 medium was used for replacing the medium in a semicontinuous mode of operation. Experiment was conducted in 60-L photobioreactor that was inoculated with 0.3 g/L of culture, and operating conditions were the same as mentioned above. DCO2 concentration (mg/L), % CO2 consumption, pH and dried biomass yield (g/L) were analysed and calculated daily.
2.9.1 Statistical analysis
All the data were statistically analysed using one-way ANOVA and Tukey’s test (α = 0.05 and 0.01) wherever applicable.
3 Results and discussions
Emission of CO2 is the major contributor to global climate change. About one-third of global CO2 fixation occurs due to algae. This is because algal cells have special cell organelles pyrenoids to accelerate the process of CO2 assimilation. In algal cell, HCO3¯ from medium passes through plasma membrane to chloroplasts and then to thylakoids where it is catalysed to CO2. There it is fixed by RuBisCO enzyme to produce many sugars by Calvin’s cycle. Amount of HCO3¯ thus enhances the rate of photosynthesis, biomass formation and growth rate of algae. When CO2 gas is directly bubbled into microalgal medium, most of it is wasted due to short resident time and low utilization efficiency of raceway pond. There should be a mechanism to improve efficiency of converting CO2 to HCO3¯. CO2 bicarbonate absorbers are generally adapted to efficiently convert CO2 to NaHCO3 which dissolves easily in culture medium and promotes algal growth [12].
Numbers of promising materials are used to capture CO2. Best ones are aqueous amines. But the major disadvantages in its use are amine evaporation, corrosion of equipment and requirement of heat energy to regenerate the solvent [13]. As alternative alkaline sorbents, K2CO3, Na2CO3 have received a wide attention. Recently, hydrated Na2CO3 was used to absorb CO2 [14]. Also NaOH was used to capture CO2 from coal-fired power plant [15]. CO2 reacts with aqueous NaOH to form Na2CO3 and NaHCO3. In bench-scale reactor, the operational conditions were standardized in such a way that 97% NaHCO3 was produced which has several industrial applications as baking soda.
In the present study, CO2 released from Bio-CNG plant was sparged in NaOH or Na2CO3 solutions and resulting mixture was used for preparation of culture medium for growing algae.
The algal strain Pseudanabaena limnetica (Lemm.) komàrek indigenous algal strain isolated from salt pan capable of withstanding Na2CO3-enriched alkaline seawater medium. The normal concentration of Na2CO3 in the BG11 medium is 0.02 g/L, whereas the modified seawater BG 11 medium (MSWBG11) contains ten times greater amount of Na2CO3 (0.2 g/L). The 60-litre flat-panel photobioreactor was used for cultivation. At the end of 18 days, the algae produces 30% enhanced biomass than normal BG11 medium [11].
3.1 Use of different alkaline sorbents for feeding CO2
When gaseous CO2 is fed into the NaOH solution, CO2 is first dissolved in water to form aqueous CO2 (Eq. 1). Subsequently, aqueous CO2 reacts with OHˉ to generate HCO3¯and CO32¯ (Eqs. 2 and 3):
When gaseous CO2 is fed into the NaOH solution, CO2 reacts with aqueous NaOH and forms sodium carbonate (Na2CO3) and later forms sodium bicarbonate (NaHCO3) in turn. These CO2 absorption reactions are expressed by Eqs. (4) and (5)
The reaction rates and pH for above reactions 4 and 5 are different [16]. The mole fraction of each carbonate species could be expressed as function of pH which is described in Fig. 1 which is called Bjerrum plot. As seen in Fig. 1, carbonates are prominently produced at alkaline pH 14 to 10 [15]. As more CO2 is sparged, pH becomes less alkaline and bicarbonates are maximally produced at pH 8. At lower pH 4–6, HCO3¯ is produced.
3.1.1 Dissolution of CO2 in tap water- and seawater-based 0.01 M NaOH solution
In the present study, 0.01 M NaOH solution was used for sparging of CO2 gas. CO2 gas was purged through fine-pore sintered glass sparger at flow rates of 0.005 – 0.01 LPM. Initially, when CO2 was sparged in the NaOH solution, the pH of solvent is very alkaline (pH 11) At this pH, most of the fed CO2 reacted with NaOH to form Na2CO3 which was analysed by acid base titration method using double indicators (phenolphthalein and methyl orange). Every 10-min interval, sample was harvested and titrated against 0.2 M HCl. Change in pH was also measured. Phenolphthalein end point from purple to orange is an indicator of consumption of all NaOH and Na2CO3. And only bicarbonate is present in the solution that is further determined by methyl orange indicator. Methyl orange end point gives the measure of Na2CO3 that is converted to NaHCO3 after titrating with HCl. The amount of OH¯, CO3¯ and HCO3¯ formed during the course of CO2 sparging was calculated as described in the literature [17].
In this experiment, capacity of tap water and seawater to capture CO2 was studied. When NaOH was added to pure water, it dissociates to form Na+ and OHˉ almost completely making the solution highly alkaline. NaOH, Na2CO3 and NaHCO3 species are generated as explained in Sect. 3.1.1. At zero time, the amount of NaOH is highest and it is responsible for highest phenolphthalein reading. The amount of NaOH is 0.054 ± 0.004 mol/L. The pH of solution is 11.72 ± 0.012. As more and more CO2 was sparged over time, amount of NaOH gradually started decreasing and Na2CO3 was formed. When the reaction mixture was titrated with HCl, the Na2CO3 was converted to NaHCO3. The amount of NaOH or Na2CO3 decreased from 0.054 ± 0.004 to 0.003 ± 0.002 mol/L. The amount of NaHCO3 increased from 0.00 to 0.062 ± 0.002 mol/L. During CO2 sparging, pH of reaction mixture decreased from 11.72 ± 0.012 to 9.06 ± 0.038. At this pH, NaOH is converted to NaHCO3 that added to additional increase in methyl orange reading (Table 1).
When 0.01 M NaOH solution was prepared, seawater was used as the medium for dissolution of CO2 (Table 2). It was observed that initial pH of the solution was only 10.05 ± 0.017, which might be because of inherent buffering nature of seawater which restricts drastic changes in pH. CO2 was sparged in the solution, and changes in NaOH, Na2CO3 and NaHCO3 were analysed. pH decreased from 10.05 ± 0.017 to 8.24 ± 0.006 in just 110 min. Concentration of NaOH decreased from 0.026 ± 0.000 to 0.001 ± 0.000 mol/L, and at the same time, amount of NaHCO3 increased from 0.010 ± 0.001 to 0.034 ± 0.006 mol/L which was approximately three times lower than that of compared with NaHCO3 concentration measured in tap water.
3.1.2 Dissolution of CO2 in tap water- and sea water-based 0.02 M Na2CO3 solution
Similarly, 0.02 M Na2CO3 solution in tap water and seawater was also used to study the dissolution of CO2 and generation of NaHCO3 in the system. Initially, when Na2CO3 was added in water, it dissociates to form NaOH and H2CO3. Thus, total available Na2CO3 in the system reduces. When CO2 sparging was initiated, NaOH previously formed is converted to Na2CO3. And all Na2CO3 is converted to NaHCO3 which was detected by methyl orange indicator. NaOH and Na2CO3 concentrations were measured as already mentioned in the above experiment. It was observed that when tap water was used, the initial pH of the system was 11.31 ± 0.010 and initial NaOH/Na2CO3 concentration was 0.026 ± 0.000 mol/L (Table 3). As CO2 was sparged in the system, Na2CO3 and NaOH started to reduce and became 0 mol/L moles, whereas NaHCO3 increased from 0.035 ± 0.001 to 0.061 ± 0.003 mol/L at the end of 130 min.
Similarly, when seawater was used to sparge CO2, initial pH of 0.02 M Na2CO3 was 10.77 ± 0.031 which reduces to 7.75 ± 0.050 at the end of 100 min (Table 4). Also initial NaOH/Na2CO3 concentration of 0.028 ± 0.001 decreased to 0.00 and concentration of NaHCO3 increased from 0.030 ± 0.001 to 0.058 ± 0.004 mol/L, which is comparable to tap water system.
Salmon et al. used NaOH as sorbent for capturing CO2 [16]. When CO2 is sparged in NaOH, it leads to formation of intermediate species like OH¯, CO3¯ and HCO3¯. The amount of intermediates was determined by titration with HCl using two colour indicators phenolphthalein and methyl orange. The effect of flow rates at which CO2 is sparged and the initial concentration of CO2 on gas liquid mass transfer was studied. They concluded that alkaline solutions in either pure form (NaOH or Na2CO3) or mixture of NaOH and Na2CO3 can be used effectively for CO2 capture.
Actually, absorption of CO2 in aqueous medium depends on gas liquid equilibrium. Henry’s law constant H’ quantifies the concentration that maintains this gas liquid equilibrium. Cheng and Chou studied effect of two alkaline systems NaOH and Na2CO3 on partition of CO2 by calculating Henry’s law constant. The study was carried out in three phases. In phase I, H’ value for absorption of CO2 in neutral tap water was determined. The study was carried out at two different temperatures 27 and 37 degrees. In phase II, alkaline-buffering agents NaOH and Na2CO3 were used. And in phase III, alga was used for CO2 absorption [18]
When CO2 was sparged in 150-L reactor, the CO2 concentration varied from 600 to 2000 ppm. Initially, when the pH was more than 8, H’ value was as low as 2.2 atm/M and at this pH much gaseous CO2 is absorbed (1500 ppm) as the pH value ranged from 8 to 6, H’ varied from 2.2 atm to 65 atm and CO2 absorbing capacity gradually falls. When the pH decreases to pH range 4–5, the H’ is in the range of 90 to 94 atm/M and it absorbs very little CO2.
When NaOH was used as an alkaline buffer and CO2 was sparged for 2 h, the pH value ranged from 6.6 to 8.9. But when Na2CO3 was used as buffering system, the pH value ranged between 9.1 and 9.8. Adding Na2CO3 yielded H’ value from 16.3 to 21.3 atm/M. The CO2 concentration was 603 ppm and aqueous concentration of HCO3¯ was 4.085 mg/L. In NaOH buffering system, H’ value was between 27 to 59 atm/M and there was much decline in concentration of CO2. Thus, weak alkali Na2CO3 had greater buffering capacity than stronger alkali.
Also the difference in CO2 dissolution and holdup capacity of tap water, seawater and modified seawater BG11 medium (MSWBG11) was studied. It was observed that MSWBG11 medium that contained ten times higher Na2CO3 in the medium enabled maximum CO2 dissolution with concentration of 18.30 ± 1.17 mg/L after 3 h of CO2 sparging. Further on stopping the CO2 sparging, system was capable of holding DCO2 up to 4 h and was 3.34 ± 0.37 mg/L. Thus, seawater-based BG11 medium with ten times higher Na2CO3 in it (MSWBG11) made the best solution for capturing and holding maximum DCO2 in the system [9].
In our present study, when NaOH was used as alkaline system and 0.01 M NaOH was dissolved in tap water, the initial pH was as high as 11.72 ± 0.012. After feeding CO2 for 110 min, the pH was decreased slightly to 10.58 ± 0.026 and amount of Na2CO3 produced was 0.040 ± 0.004 mol/L. But when seawater was used for dissolving 0.01 M NaOH, the initial pH was comparatively low, it was 10.5 ± 0.017. This might be due to buffering action of seawater. After feeding CO2 for 110 min, the pH became 8.24 ± 0.006 and amount of NaHCO3 was comparatively less 0.034 ± 0.006 mol/L.
But when Na2CO3 was used as alkaline system, 0.02 M was added to tap water; the pH was 11.31 ± 0.010. When CO2 was sparged for 110 min, it became 9.04 ± 0.032 and amount of NaHCO3 present in system was 0.053 ± 0.0031 mol/L. When seawater was used after addition of 0.02 M Na2CO3, the pH was 10.77 ± 0.031. After feeding CO2 for 100 minutes, it decreased to 7.75 ± 0.050 and amount of NaHCO3 was 0.058 ± 0.004 mol/L. This concentration of NaHCO3 using 0.02 M Na2CO3 was statistically proven to be best solution for CO2 sparging and was significantly different with other solutions using one-way ANOVA and Tukey’s test at level of significance of 0.05 (α = 0.05 and 0.01) (Fig. 2).
Thus, seawater along with Na2CO3 was found to be efficient system for capturing CO2 and converting it into NaHCO3. Hence, for further experiments of algal cultivation, Na2CO3 was used for CO2 sparging.
Our further objective was to utilize these CO2-sparged NaOH and Na2CO3 solutions for algal growth.
3.2 Use of CO2-enriched NaOH and Na2CO3 medium for algal growth
3.2.1 Use of CO2-sparged NaOH and Na2CO3 solutions for preparing MSWBG11 medium
CO2-sparged 0.01 M NaOH tap water solution and 0.02 M Na2CO3 seawater-based solution were used in the preparation of MSWBG11 medium. These media were used for the growth of algae Pseudanabaena limnetica. MSGBG11 medium contains 0.0018 mol/L of Na2CO3 which is far less than Na2CO3-enriched stock solutions obtained after CO2 sparging. Hence, the stock solution was diluted accordingly to obtain required moles of Na2CO3 for the preparation of medium.
However seawater-based Na2CO3 solution contained 0.058 ± 0.004 mol/L of NaHCO3. The stock was 1:5 times diluted and added to MSWBG11 medium. Both these media were used to study the growth of algae Pseudanabaena limnetica.
3.2.2 Effect of CO2-sparged alkaline solutions on the growth of algae
3.2.2.1 Effect of CO2-sparged NaOH medium on growth of P. limnetica
In case of control tank, DCO2 concentration and pH at day 0 were 2.07 ± 0.031 mg/L and 8.53 ± 0.097, respectively (Table 5). Initial inoculum added was 0.19 ± 0.021 g/L dried biomass weight. As days passed, algal biomass grew gradually to give dried biomass yield of 0.29 ± 0.031 g/L on day 6. Also DCO2 was decreased to1.50 ± 0.030 mg/L and pH became 10.16 ± 0.060. Maximum biomass yield of algae was obtained on day 15, i.e. 0.64 ± 0.035 g/L, which was confirmed by consumption of CO2, and DCO2 concentration of the system decreased from 2.07 ± 0.031 to 1.15 ± 0.050 mg/L on day 15. Also pH became alkaline from 8.53 ± 0.097 to 9.86 ± 0.051 on day 15.
In test 1 tank, where CO2-sparged NaOH solution was used for preparation of MSWBG11 medium, the initial DCO2 concentration was 2.47 ± 0.070 mg/L (Table 6) which was little higher than control tank (2.07 ± 0.031 mg/L). In Test tank, initial inoculum added was 0.19 ± 0.012 g/L and pH 8.63 ± 0.064, respectively. The highest biomass of algae was observed on 12th day and increase in the biomass was from 0.19 ± 0.012 to 0.58 ± 0.015 g/L which was slightly more than control tank but the increase was not significant. Growth of the algae was supported by consumption of CO2 and increase in the pH. On day 15, the DCO2 decreased from 2.47 ± 0.070 mg/L to 1.39 ± 0.105 mg/L, pH got alkaline from 8.63 ± 0.064 to 9.01 ± 0.067 which resulted in significant decrease in biomass yields. These results indicate that CO2-sparged NaOH solution can be used for algal growth.
3.2.2.2 Effect of CO2-sparged Na2CO3 medium on growth of P. limnetica
Further third set (test 2) was tested where CO2-sparged Na2CO3 solution was used as the source of carbonate for preparation of MSWBG11 medium. Growth conditions provided were same as given to above two tanks. In this experiment, the initial DCO2 of the system was 3.92 ± 0.046 mg/L which was substantially more than the other two tanks and pH was 7.82 ± 0.095. Algal inoculum added in the system on day 0 was 0.23 ± 0.046 g/L. As days passed, DCO2 reduced and pH started getting more alkaline indicating growth of algae in the system. As seen in Table 7 and Fig. 3, right from day 3 up to day 15, there is significant increase in biomass produced in CO2-sparged Na2CO3 medium over control. On day 15, algal biomass was maximum, i.e. 0.81 ± 0.046 g/L, where as in control tank the biomass produced is significantly less, 0.64 ± 0.035 g/L. At the end of 15 days, DCO2 was substantially decreased from 3.92 ± 0.046 mg/L to 1.23 ± 0.036 mg/L and pH was increased from 7.82 ± 0.095 to 9.54 ± 0.075 (Table 7).
Thus, the above experiments conclude that when CO2-sparged Na2CO3 solution is added to MSWBG11, it is sufficiently capable of holding maximum DCO2 as indicated by high DCO2 in third set, i.e. 3.92 ± 0.046 mg/L on day 0. Also it supports well the algal growth. In control tank and Tank 1 provided with NaOH medium, the biomass yields at the end of 15 days were 0.64 ± 0.035 g/L and 0.58 ± 0.015 g/L, respectively, which were very less compared to test 2 which yielded 0.81 ± 0.046 g/L of yield, thus proving that Na2CO3 solution when used to sparge CO2 suits best as a medium for maximum CO2 holding capacity and maximum algal yield. Hence, it was continued for further work.
Statistical analysis by one-way ANOVA proved there was significant difference in three sets Control, Test 1 and Test 2 at level of significance of 0.05. Further post hoc Tukey’s test proved there was significant difference in biomass yields of test 2 over Control and Test 1. (α = 0.05 and 0.01) (Fig. 3).
3.3 Semicontinuous mode of operation for high biomass yield of algae
Cultivation of algae can be done in various ways such as batch mode, fed-batch mode or continuous mode. Previous experiments were conducted in batch mode of operation where experiments were run for about 15 days to complete the growth cycle of algae. After 15 days, algal biomass started decreasing indicating stationary phase of algae.
To further increase the biomass production of algae, it was cultivated in semicontinuous mode of operation. Initially, the culture was allowed grow for 6 to 7 days till the log phase is achieved. The semicontinuous operation is initiated by removing some amount of algal culture and replacing it with fresh medium. By doing so, the algal culture remains in log phase continuously producing biomass. In the present work, it was necessary to standardise the amount of culture to be removed in semicontinuous operation in which 1 L (set A), 3 L (set B), 5 L (set C) of culture were harvested and the same volume of fresh media was added into each of the sets every day. Increase in the biomass yield in each set was evaluated gravimetrically on a daily basis.
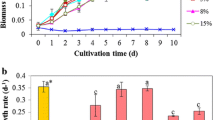
In set A, initial inoculum was 0.30 ± 0.058 g/L which was allowed to grow for 6 days till the biomass increased to 0.71 ± 0.023 g/L which was considered as day 0 for starting semicontinuous mode of operation. Daily 1 L of culture was harvested and analysed for biomass yield. Same amount of fresh medium was added into the tank to compensate for the lost harvested culture. It was found that in initial 10 days biomass of 0.71 ± 0.023 g/L increased to 1.18 ± 0.049 g/L and further it went on increasing from 10th till 60th day till which the experiment continued. The final biomass that was obtained at the end of 60 days was 2.62 ± 0.032 g/L.
However, in set B, the initial biomass of 0.82 ± 0.021 g/L was gradually increased to 1.43 ± 0.36 g/L at the end of 30 days. But the biomass produced at the end of 30 days in set B was significantly lesser than biomass in set A (Fig. 4). The same trend was found to be continued, on 60th day the biomass produced in set A (2.61 ± 0.032 g/L) which was significantly higher than set B (1.60 ± 0.025 g/L).
Similarly, in set C where 5 L of media was removed every day, the initial biomass was 0.81 ± 0.040 g/L, and at the end of 30 days, it negligibly increased to 1.08 ± 0.015 g/L which was significantly less than biomass produced in set A. The condition was still worst on day 60, and the biomass produced was still reduced to 0.76 ± 0.012 g/L (Table 8, Fig. 4).
These results indicated that replacing 1 L of culture with fresh media is the suitable way to replenish the lost nutrient into media and also the biomass harvested in 1 L culture can be overcome by exponentially growing culture. On the other hand in set B and C where 3 L and 5 L of culture were harvested, biomass was not able to grow at that fast rate to cope up the loss of culture. (Table 8). This was statistically proven by performing one-way ANOVA and Tukey’s test at α = 0.05 and 0.01 (Fig. 4).
In one of the studies, it was reported that A. braunii when cultivated in batch cultures, the maximum biomass concentration (Xm) was 1588 ± 11 mg L−1 using 20 mM of NaNO3. A fed-batch process with the addition of 20 mM NaNO3 each 48 h from the first to the sixth cultivation day reached Xm = 2753 ± 7 mg L−1. The semicontinuous process was effective in eradicating the lag phase of algae allowing it to grow to maximum biomass of Xm = 2399 ± 5 mg L−1. As seen in our experiment semicontinuous mode of operation increased the biomass gradually beyond 18 days till 60th day and reached maximum biomass of 2.62 ± 0.032 g/L [19]. It could be concluded from the data that semicontinuous mode of operation is not only optimum for mass scale production of algae but can also be used to feed CO2-rich medium in system every day to compensate for the loss of CO2 in the environment due to high temperatures in tropical environments.
3.4 Using Na2CO3-sparged MSWBG11 medium in semicontinuous mode of operation
In this experiment, CO2 gas coming from Bio-CNG plant was directly sparged in MSWBG11 (Test1). This CO2-sparged MSWBG11 medium was used for replacing the medium in a semicontinuous mode of operation. Experiment was conducted in 60-L photobioreactor that was inoculated with approximately 0.35 g/L of culture and agitated with 4LPM of ambient air. Set-up was kept in partial shade near Bio-CNG vicinity of CNG station plant in the premises of Primove Engineering Pvt. Ltd., Pune. DCO2 concentration (mg/L), % CO2 consumption, pH and dried biomass yield (g/L) were analysed and calculated daily.
In one set, only MSWBG11 medium with no sparged CO2 was used as the source of medium for semicontinuous mode and this was considered as control tank. In both sets, i.e. test and control, algal biomass was initially cultivated for 10 days till biomass attains log phase. In control set, initial inoculum added was 0.35 ± 0.072 g/L which increased to 0.77 ± 0.052 g/L at the end of 10 days. DCO2 at day 0 was 1.82 ± 0.012 mg/L which reduced to 1.68 ± 0.070 mg/L at the end of 10 days. Initial pH of 8.75 ± 0.050 increased to 8.91 ± 0.036 after 10 days. However, in Test tank, initial inoculum of 0.34 ± 0.096 g/L increased to 0.77 ± 0.066 g/L at the end of 10 days. Initial DCO2 and pH of the system were 2.08 ± 0.060 mg/L which reduced to 1.28 ± 0.085 mg/L after 10 days indicating algal culture grew consuming DCO2 of the system.
After 10 days, the semicontinuous operation was initiated in both control and test tanks, i.e. every day, 1L of culture from both tanks was replaced with same volume of media. In control tank (Table 9), 1L of culture was replaced with same volume of fresh MSWBG11 medium (with no CO2 sparged in it), whereas in test tank 1L of culture was replaced with same volume of CO2-sparged fresh MSWBG11 medium containing DCO2 concentration of 7–8 mg/L.
In control tank, it was observed that as semicontinuous mode of operation started, initial DCO2 of 1.68 ± 0.070 mg/L observed at 7:00 h increased to 1.70 ± 0.015 mg/L at 11:00 h after addition of fresh medium. As culture grew throughout the day, DCO2 was consumed and its concentration reduced to 1.61 ± 0.012 mg/L till evening at 19:00 h. Growth of the culture was confirmed by increase in dried weight biomass from 0.77 ± 0.052 g/L to 0.85 ± 0.036 g/L at end of day 1. As DCO2 was consumed by algae, there was decrease in the pH from 8.91 ± 0.036 on day 0 to 8.95 ± 0.068 on day 1. Slowly as days passed and fresh medium was added, DCO2 gradually increased from 1.74 ± 0.130 mg/L on day 1 to 2.02 ± 0.042 mg/L at the end of 8 days and pH of the system became more alkaline from 8.95 ± 0.068 to 9.42 ± 0.081 after 8 days. Biomass yield gradually increased from 0.85 ± 0.036 g/L on day 1 to maximum on day 8, i.e., 1.46 ± 0.066 g/L.
In test tank (Table 10), initial DCO2 of 1.28 ± 0.085 mg/L increased to 1.42 ± 0.140 mg/L on addition of CO2-enriched fresh MSWBG11 medium to the system after 1 day. On addition to DCO2, pH got alkaline from 9.14 ± 0.040 on day 0 at 7:00am to 9.58 ± 0.075 at 7:00 pm on day 1 and culture grew from 0.77 ± 0.066 g/L to 0.80 ± 0.070 g/L in a day. As days passed, DCO2 concentration gradually increased from day 1 to day 8, DCO2 on addition of media at day 1 was 1.42 ± 0.140 mg/L which slightly increased to 1.55 ± 0.120 mg/L on day 8, DCO2 was continuously available for the growth of algae and algal biomass increased from 0.80 ± 0.070 g/L on day 1 to 1.62 ± 0.070 g/L after 8 days of semicontinuous mode of operation. Significant difference in the biomass yields between Control and Test was proven by using one-way ANOVA (α = 0.05) (Fig. 5).
Also Yadav et al. set up a small-scale pilot reactor at Oil and Natural Gas Corporation, Hazira, Surat, India, to capture CO2 from vent gas. It consisted of a cylindrical CO2 absorber of volume 0.25 m3. The pH of fresh medium was adjusted to 11 by using 0.1 g/L of NaOH and was stored in storage tank. The medium was transferred to column using peristaltic pump. CO2 containing vent gas was tapped from exhaust stack at Hazira plant and was purged into absorption column at a flow rate 5.1 L/min. The gas was bubbled from bottom to ensure a long contact time between alkaline solution and gas. The pilot carbonation column was able to bring down the initial CO2 concentration of 30 vol% to 15 vol%, and the pH of medium was decreased from 11 to 6.8 or 7. This CO2-sparged medium was then allowed to flow into algal raceway pond of capacity 0.2m3. The growth of microalgae consortium was measured as increase in OD from 0.001 to 0.402. Due to algal growth, the pH of pond rose from 8 to 10. This high pH medium was reused for absorbing CO2 in absorption column [20].
4 Conclusions
In the current research, indigenous halophilic strain Pseudanabaena limnetica (Lemm.) Komárek was used for CO2 sequestration studies along with mass cultivation. CO2 sequestration studies were carried out in previously designed, constructed and operationally optimized 60-L flat-panel photobioreactor system for growth of P. limnetica. MSWBG11 medium was the nutrient medium for the growth of the algae.
To enhance maximum CO2 dissolution and holdup, different alkaline systems were studied in seawater and tap water out of which 0.02 M Na2CO3 in seawater was the best solutions which could hold maximum CO2. To improve the algal biomass productivity, these solutions were used as source of carbonate. To further enhance the biomass, the system was operated in a semicontinuous mode where 1 L of culture replaced with 1 L of fresh medium everyday showed maximum algal biomass yield even after 60 days operation. Further to sequester CO2 continuously so that it could be used for industrial application, semicontinuous system was set up at premises of Bio-CNG plant. Here, the gas released from Bio-CNG plant as by-product containing CO2 was sparged in Na2CO3-containing medium and 1 L of old culture was replaced with this CO2-enriched medium every day and proved to be helpful in enhancing the biomass production.
References
Dunn RJH , Stanitski DM , Gobron N , Willett KM (eds) (2020) Global climate [in “State of the Climate in 2019"]. Bull Amer Meteor 101(8):S9--S127https://doi.org/10.1175/BAMSD-20-0104.1
Cheah WY, Ling TC, Juan JC, Lee DJ, Chang JS, Show PL (2016) Biorefineries of carbon dioxide: from carbon capture and storage (CCS) to bioenergies production. Bioresour Technol 215:346–356. https://doi.org/10.1016/j.biortech.2016.04.019
Pires JC (2017) COP21: the algae opportunity? Renew Sustain Energy Rev 79:867–877. https://doi.org/10.1016/j.rser.2017.05.197
Singh J, Dhar DW (2019) Overview of carbon capture technology: microalgal biorefinery concept and state-of-the-art. Front Mar Sci 6:29. https://doi.org/10.3389/fmars.2019.00029
Whitton BA (2012) Ecology of Cyanobacteria II: Their Diversity in Space and Time. Springer, Dordrecht. https://doi.org/10.1007/978-94-007-3855-3
Vuppaladadiyam AK, Yao JG, Florin N, George A, Wang X, Labeeuw L, Jiang Y, Davis RW, Abbas A, Ralph P, Fennell PS, Zhao M (2018) Impact of flue gas compounds on microalgae and mechanisms for carbon assimilation and utilization. Chemsuschem 2018(11):334–355. https://doi.org/10.1002/cssc.201701611
Chisti Y (2013) Constraints to commercialization of algal fuels. J Biotechnol 167(3):201–214. https://doi.org/10.1016/j.jbiotec.2013.07.020
Zheng Q, Martin GJ, Kentish SE (2016) Energy efficient transfer of carbon dioxide from flue gases to microalgal systems. Energy Environ Sci 9:1074–1082. https://doi.org/10.1039/C5EE02005K
Magar C, Rambhiya S, Deodhar M (2019) Evaluation of CO2 Removal efficiency of Pseudanabaena limnetica (Lemm.) Komárek grown in Na2CO3 enriched seawater medium in 60 L airlift flat panel photobioreactor. J Environ Sci Technol 12:186–196
Rippka R, Deruelles J, Waterbury JB, Herdman M, Stanier RY (1979) Generic assignments, strain histories and properties of pure cultures of cyanobacteria. J Gen Microbiol 111:1–61
Magar CS , Deodhar MA (2018) Operational strategies for cost effective mass cultivation of halophilic microalgalstrain Pseudanabaena limnetica in 1000 L flat panel photobioreactor. J Pet Environ Biotechnol, 9https://doi.org/10.4172/2157-7463.1000380
Wangbiao G, Jun C, Yanmei S, Santosh K, Kubar Ameer A, Caifeng G, Zhanshan Q (2019) Developing a CO2 bicarbonation absorber for promoting microalgal growth rates with an improved photosynthesis pathway. RSC Advances 2019, Issue 5, Page 2332 to 2876. doi: https://doi.org/10.1039/c8ra09538h
Vega F, Cano M, Camino S, Gallego LM, Fernández EP, Navarrete B (2018) Solvents for carbon dioxide capture carbon dioxide chemistry, capture and oil recovery, Iyad Karamé, Janah Shaya and Hassan Srour IntechOpenhttps://doi.org/10.5772/intechopen.71443
Cai Y, Wang W, Li L, Wang Z, Wang S, Ding H, Zhang Z, Sun L, Wang W (2018) Effective capture of carbon dioxide using hydrated sodium carbonate powders. J Mater 11:183
Shim J-G, Lee DW, Lee JH, Kwak N-S (2016) Experimental study on capture of carbon dioxide and production of sodium bicarbonate from sodium hydroxide. Environ Eng Res 21(3):297–303
Salmón IR, Cambier N, Luis P (2018) CO2 capture by Alkaline solution for carbonate production: a comparison between a packed column and a membrane contactor. Appl Sci 2018(8):996
Alshatteri AH (2019) Determination of a mixture of sodium carbonate and sodium hydroxide by using double indicator method https://doi.org/10.13140/RG.2.2.36565.27363
Wen-Hsi C, Ming-Shean C (2016) Optimization of gas-water absorption equilibrium of carbon dioxide for algae liquors: selection of alkaline buffering chemicals. Int J Photoenergy. 2016, Article ID 2562638, 5 p
Bresaola MD, Morocho-Jácome AL, Matsudo MC, Monteiro de Carvalho JC (2019) Semi-continuous process as a promising technique in Ankistrodesmus braunii cultivation in photobioreactor. J Appl Phycol. https://doi.org/10.1007/s10811-019-01774-0
Yadav A, Choudhary P, Atri N, Teir S, Mutnuri S (2016) Pilot Plant at Hazira, India, for capture of carbon dioxide and its biofixation using microalgae. Environ. Sci. Pollut. Reshttps://doi.org/10.1007/s11356-016-6479-6
Acknowledgements
The authors acknowledge the Department of Biotechnology (DBT), New Delhi, for its financial support to this research project under the call ‘Mass Cultivation of Algae for Cost-Effective Biofuel Production'. The grant agreement number was BT/PR12347/PBD/26/476/2015.Authors are thankful to Primove Engineering Pvt. Ltd., Pune, Maharashtra, for providing us with gas released from Bio-CNG plant as by-product and space for setting up our photobioreactors at their Bio-CNG plant. Authors are also thankful to Principal Dr. B. B. Sharma and Kelkar Education Trust of V. G. Vaze College Mulund East, Mumbai-400081, for their support to carry out this research work.
Funding
This research work was funded by the Department of Biotechnology (DBT), Ministry of Science and Technology, Government of India, New Delhi, under Grant agreement No. BT/PR12347/PBD/26/476/2015.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author(s) declare that they have no competing interests.
Consent to participate
All authors have contributed equally and voluntarily in the conduct of study and preparation of the manuscript.
Consent for publication
The article is sent for publication upon consent from all authors.
Availability of data and material:
Data generated during the conduct of this study have been included in the manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rambhiya, S.J., Magar, C.S. & Deodhar, M.A. Using seawater-based Na2CO3 medium for scrubbing the CO2 released from Bio-CNG plant for enhanced biomass production of Pseudanabaena limnetica. SN Appl. Sci. 3, 276 (2021). https://doi.org/10.1007/s42452-021-04271-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-021-04271-7