Abstract
We have study electronic, optoelectronic, linear and nonlinear optical; thermodynamic properties and UV–Vis Spectrum of Coronene and Coronene substituted with Chlorine using time-dependent density functional theory TD-DFT (TD-wB97XD, TD-B3LYP, TD-LSDA and TD-CCSD(T)). We quantified the effect of substitution of hydrogen atoms with Chlorine for a series of molecular properties relevant for molecular electronics and photonics. Results obtained with TD-B3LYP and TD-CCSD(T) are closer to experimental results reported in literature for Coronene substituted with Chlorine. Second hyperpolarizability (\( \gamma_{av} \)) values show that the molecules have very good optoelectronic, linear and non linear optical properties. Eg show that the molecules may have semiconductors properties and hence have applications in photonic, electronic and optoelectronic devices.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Recently, studies have shown that the production and consumption of energy might change if the research in organic electronics can improve on the efficiency and application in optoelectronic materials. Organic molecules have applications in photonic, linear and nonlinear optics, thin-film transistors (TFT), light emitting diodes (OLED) and thin-film photovoltaic cells (OPV). This is because organic molecules are relatively cheap, light and flexible and are in great abundant [1]. Equally, the physical, chemical, mechanical and electronic properties of these molecules can vary by adding specific functional groups at different positions and doping of the molecules with other atoms. Studies have proven that the homologous classes of polycyclic aromatic hydrocarbons in their crystalline state are among the materials which can be used in organic electronics [2]. For instance, oligoacenes and their derivatives, are constantly being used as active elements in various optoelectronic devices like in organic thin–film field–effect transistors [3], light–emitting diodes [4], photovoltaic cells [5], and liquid crystals [6].
Organic electronics based on acenes and heteroacenes is presently an interesting area of research [7,8,9,10,11]. Formation, migration, and dissociation of exciton, charge transport, charge collection at the electrodes, molecular packing in the bulk material, and absorption and emission properties are the main features controlling organic semiconductor devices [12, 13]. Consequently, a detailed understanding of the electronic structure of the molecular building blocks, the dependence of the microscopic optical properties on the molecular structure, and the differences between the isolated properties of the doped molecule when inserted in a macroscopic devices is of fundamental importance. Therefore, systematic quantum chemical calculations on doped molecule can significantly contribute to our understanding of the electronic and optical properties with promising optoelectronic applications since most of these properties pertain to the molecular structure.
As part of a more extensive research on organic molecules [14,15,16,17,18,19,20,21,22,23,24,25,26], and following our previous works on coronene (a polycyclic aromatic hydrocarbon of the circumacene class) [17], we present in this paper a comprehensive comparative theoretical study of Coronene and Coronene substituted with Chlorine usng TD-DFT ((TD-wB97XD, TD-B3LYP) and TD-LSDA and TD-CCSD(T)). The choice of this molecule was motivated by the availability of reliable experimental data for undoped coronene and theoretical results for doped coronene molecule. Equally, circumacenes are promising candidates for organic and molecular electronics [27]. Furthermore, as reported in literature, the modification of polycyclic aromatic hydrocarbons and conjugate pi-electron systems with strong electronegative substituent is an effective approach for converting p-type organic semiconductors to n-type [28,29,30]. The study was motivated by the fact that the electrical properties of semiconductor materials are significantly altered by presence of impurity atoms which are responsible for the development of transistors and has opened up the entire field of solid state device technology. Equally, n-type materials from polycyclic aromatic hydrocarbons can be obtain by attaching strong electron-withdrawing groups such as CN to the conjugated core, or by replacing some or all of the peripheral hydrogen atoms with halogen atoms (in particular F and Cl) [12, 29,30,31]. Functionalized coronene [32,33,34,35] and other organic compounds [35,36,37] are of profound interest nowadays for the fabrication of organic field effect transistors OFETs and organic thin film transistors (OTFTs) for better performance [37, 38]. Sanyal et al. 2013 investigated the effect of imide functionalization on the electronic, optical, and charge transport properties of coronene derivatives(coronene-5-diimide, coronene-6-diimide, and coronene-tetraimide) and their results suggested that these imide functionalized coronene have potential use in optoelectronic and field effect transistor devices [39].
They equally studied the energetics, photophysical properties, charge transfer integrals and reorganization energy of coronene and its various imide derivatives using DFT methods and their results showed that the introduction of imide functional group, an electron acceptor, strongly modulates the charge transfer behaviors of coronene [39]. Moreover, calculations on the electronic properties of solid coronene doped with potassium has demonstrated superconductivity as reported in literature [40,41,42].
There are many situations in which a judicious control of the types and amounts of imperfections can bring about specific characteristics desired in a system which can be achieved by proper processing techniques. The effects of imperfections or crystal defects and functionalization have some important properties of solids. The fact that such small amounts of impurity atoms can significantly alter the electrical properties of semiconductors and other organic materials is responsible for the development of transistor, light–emitting diodes [4] and photovoltaic cells [5] and has opened up the entire field of solid state device technology. Practically most of the semiconducting properties that led to this engineering are not found in a perfect crystal.
Our main objective is to see how some of the properties of coronene like electronic, optoelectronic, linear and nonlinear optical properties and UV–Vis Spectrum can be improved by doping the molecule with chlorine. This is because since chlorine atom has a high electron affinity, large dipole moment and effective delocalization of the pi – electrons, the introduction of the chlorine atom to the coronene may be a good choice to construct a high acceptor molecule. Also, since fluorine atom has been widely introduce into organic semiconductors material to synergistically improve the molecular energy level, optical absorption and carrier mobility properties for photovoltaic applications, due to it high electronegativity and small atomic radius [30, 43,44,45,46], we think that chlorine atom can do same. Equally, Kan et al. 2018 [47] showed that the chlorination of small-molecule acceptor for organic solar cells reduces the optical band gap, the energy loss, increases the efficiency of the molecule and broaden the effective absorption range.
2 Computational methodology
The electronic structure, the structural parameters and the vibrational analysis of Coronene and Coronene substituted with Chlorine were optimized with the aid of the Gaussian 09 program package [48]. Following our previous studies on coronene, the geometry optimization was carried out using the RHF, WB97XD and B3LYP hybrid exchange–correlation functional in combination with the correlation-consistent polarized Valence Double Zeta basis-set [17]. In this work, geometric optimizations of the electronic structure of the molecules were fully optimized using TD-DFT (TD-wB97XD, TD-B3LYP, TD-SVWN (LSDA) and TD-CCSD(T)) with correlation-consistent polarized Valence Double Zeta basis set. The TD-DFT was performed using the route section in the Gaussian 09 by define the Nstates (the number of excited states) with the method used. In all cases, structural optimizations were performed without any symmetry constraints and the optimized geometric structures were confirmed as correct minima by the vibrational frequency analyses. These optimized stationary points characterized by harmonic vibrational frequency analyses confirm that local minima were found due to the absence of imaginary vibrational frequencies. The minimum energy configurations of chlorinated coronene obtained by complete peripheral Chlorine substitution and by substituting 6 hydrogen atoms with 6 chlorine atoms maintained the planar geometry of the substituted Coronene molecule. The adiabatic electron affinities and ionization energies were calculated using the total energy differences. The vertical ionization energies (IEV) and electron affinities (EAV) were evaluated at the relaxed geometry of the neutral molecule. This enabled us to calculate the quasi-particle corrected energy gap which is defined in the self consistent field scheme as: EQpg = IEV − EAV = (EN+1 − EN) − (EN − EN−1) [49, 50], where EN is the total energy of the N -electron system.
The EHOMO, ELUMO and related properties such as electronegativity \( \varsigma \), chemical hardness \( \xi \) and chemical softness \( \vartheta \) were also determined. The quantities \( \varsigma \), \( \xi \) and \( \vartheta \) were better discussed in terms of expressions given in literature [14, 51, 52]. The IP and EA of the molecules were calculated using the equations IP = − EHOMO and EA = − ELUMO found in literature [15, 16, 53].
Thus, the values of \( \varsigma \) and \( \xi \) according to Pearson, were evaluated using the following relations [54]: \( \varsigma = \frac{IP + EA}{2} \) and \( \xi = \frac{IP - EA}{2}. \) The chemical softness \( \vartheta \), which describes the ability of an atom or group of atoms to receive electrons [55] was estimated by using the equation: \( \vartheta = \frac{1}{\xi } = \frac{2}{IP - EA}. \) The chemical softness is proportional to the magnitude of the electrical conductivity. The electrical conductivity of organic materials usually exhibits a thermally activated behavior. Experimental data obtained on high-quality single crystals indicate that the appearance of an activated transport is in many instances more likely due to the presence of structural disorder and chemical defects rather than corresponding to the intrinsic signature of polaron formation. The chemical hardness of the molecules gives us information on how the electron cloud can be deformed. From the maximum hardness principle, which states that the most stable systems are correlated with higher chemical hardness and therefore correspond to larger HOMO–LUMO energy gaps. Low hardness, which correlates to small HOMO–LUMO energy gaps correspond to easily deformable electron cloud.
3 Results and discussion
3.1 Optimized geometric structure of coronene (C24H12) and coronene substituted with chlorine
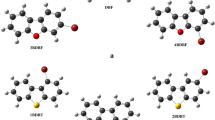
The optimized structure of coronene (C24H12), that obtained when 6 hydrogen atoms are substituted by 6 chlorine atoms (C24H6Cl6) and the complete peripheral chlorine substitution (C24Cl12) are shown in Fig. 1a–c respectively. We observed that the planar geometry of the molecule did not change compared to the original planar molecular structure for complete peripheral and partial chlorine substituted coronene.
3.2 Dipole moment, first molar hyperpolarizability and second molecular hyperpolarizability
The values of the dipole moment, first molar hyperpolarizability and second molecular hyperpolarizability of C24H12, C24H6Cl6 and C24Cl12 are reported in Table 1.
The first molecular hyperpolarizability \( \beta_{mol} \) can be calculated from the Gaussian output file by using equation found in literature [12, 14, 15, 52, 55,56,57,58]. The \( \beta_{\text{mol}} \) value of the molecules was determined using the equation below given in literature [14, 15, 19,20,21, 59].
The values of \( \beta_{\text{mol}} \) for the molecules are small compared to that of Urea given in literature [20, 21, 57, 58, 60]. Due these relatively small values of \( \beta_{\text{mol}} \), we decided to calculate the second order hyperpolarizability.
The second order hyperpolarizability \( \gamma_{av} \) which is a microscopic property of the molecules was also determined and calculated \( \gamma_{av} \) values in atomic unit were converted into SI units using conversion factors given in literature [17, 56]. The values of \( \gamma_{av} \) were determined by using the equation [17, 61, 62] \( \gamma_{av} = \frac{{\gamma_{xxxx} + \gamma_{yyyy} + \gamma_{zzzz} + 2\gamma_{xxyy} + 2\gamma_{yyzz} + 2\gamma_{xxzz} }}{5} \)
The results showed that \( \gamma_{xxxx} \,and\;\gamma_{yyyy} \) which are the tensor components of \( \gamma \) in the x and y directions respectively gave the highest contribution in the calculation of \( \gamma_{av} \) and these tensor components are approximately the same. From Table 1, it is observed that all \( \gamma_{av} \) values are negative which indicates that the contributions to \( \gamma_{av} \) is due to the pi electrons except for the molecule C24H12 that we obtained a positive value at the TD-CCSD(T) level. This implies that in calculating the \( \gamma_{av} \) of the molecule C24H12 at the TD-CCSD(T) level, the contribution was due to the pi and sigma bonds with the sigma bond giving the highest contribution. The TD-B3LYP method gave the highest values of \( \gamma_{av} \) for the molecule C24H12 compared to that obtained using the wB97XD, TD-SVWN and TD-CCSD(T) while that of the TD-CCSD(T) was the largest for the C24H6Cl6 molecule. We also observed that with the complete peripheral chlorine substitution of the hydrogen atoms in Coronene (C24Cl12), the \( \gamma_{av} \) values are greater than those obtained from C24H6Cl6 and C24H12 with that of C24H6Cl6 greater than that of C24H12 at all the levels of study. For instance, the \( \gamma_{av} \) value of C24Cl12 obtained by using the TD-SVWN (LSDA) is approximately 6.5 times larger than that of C24H6Cl6 and that of C24H6Cl6 is approximately 2.6 times greater than that of C24H12. It implies that as the number of peripheral Chlorine atoms substitution increases, the \( \gamma_{av} \) value also increases. The large values of \( \gamma_{av} \) permit us to conclude that these molecules have very good linear and nonlinear optical effects and hence can be used in linear and nonlinear materials which may have electronic, optoelectronic and photonic applications. Hence, these molecules may have all or some of the following effects: second harmonic generation, optical rectification, electro-optic Pockel effect, electric induced second harmonic generation and the electro-Kerr effect.
3.3 Ionization potential IP, electron affinity EA, chemical hardness \( \xi \), chemical softness \( \vartheta \), and electronegativity \( \varsigma \), HOMO–LUMO energy band gap E g, work function EF, Kohn–Sham HOMO–LUMO energy band gap \( E_{g}^{KS} \), quasiparticle energy corrected gap E Qpg, total Electronic Energy E 0 and molecular orbital diagrams of the molecules C24H12 and C24H6Cl6
The ionization potential, electron affinity, chemical hardness, chemical softness, electronegativity and total Electronic Energy of coronene, when 6 hydrogen atoms are substitute with 6 chlorine atoms and the complete peripheral chlorine substitution obtained by using TD-wB97XD, TD-B3LYP, TD-SVWN (LSDA) and TD-CCSD(T) are shown in Table 1. The IP and AE values were obtained using formulae given in literature [24, 49, 53, 63]. The ionization energy values and electron affinity values obtained directly from the Kohn–Sham eigenvalues are given in brackets. The cc-pVDZ basis set which is approximately equal to the commonly used 6-31G basis set was employed in all the calculations. Thereafter, we compared our theoretical results with experimental results given in literature. The IPv results for coronene are closed to the experimental results of the vertical ionization potential (7.29 eV) found in literature [64], the value of 7.34 eV reported by Boschi and Schmidt [65] and to the experimental results of the adiabatic ionization potential given in literature (7.64 eV) [66], 7.29 eV [67] and (7.29 + - 0.03)eV [31, 68]. Our estimated EAv value of coronene are close to the experimental adiabatic electron affinity values (0.47 + − 0.09)eV) [31] and the theoretical values (0.47 eV and 0.54 eV) [69] reported in literature and are the same with those previous reported by using RFH, wB97XD and B3LYP methods with the cc-pVDZ [17].
We equally observed that \( \xi \), \( \vartheta \) and \( \varsigma \) values of coronene remain unchanged at all the levels of theory. It implies the electrical conductivity of the molecule also remain unchanged since the chemical softness \( \xi \) is proportional to the magnitude of the electrical conductivity.
LUMO–HOMO Energy band gap Eg, work function EF, Kohn–Sham HOMO–LUMO energy band gap \( E_{\text{g}}^{\text{KS}} \) and work function EF of the molecules C24H12, C24H6Cl6 and C24Cl12 are given in Table 1 and the molecular orbital diagrams are shown in Fig. 2. The Eg value determined for coronene is the same as that obtained in our previous studies by using RHF, wB97XD and B3LYP methods with the cc-pVDZ [17] and smaller than the theoretical value reported in literature (4.3 eV) [31] obtained using B3LYP/def2-TZVP method. The Egap and EF value did not change at all levels of study. From Table 2, it is observed that when coronene is doped with Cl (when we substitute 6 atoms of hydrogen with 6 atoms of chlorine), the Eg decreases while EF values increases. Comparing the Eg values of C24H6Cl6 and C24Cl12 with that of coronene when doped with an atom of Cl (C24H11Cl), with an atom of F (C24H11F) [31], with the peripheral substitution of all hydrogen atoms by fluorine atoms C18F12 [30] and Co(coronene) [70] given in literature, our studies reveal that there is increase conductivity. From our previous studies, we observed that the Eg values of C24H6Cl6 and C24Cl12 are less than that of Coronene doped with B, N, and BN [17]. As reported in literature, the modification of polycyclic aromatic hydrocarbons with strong electronegative substituent is an effective approach for converting p-type organic semiconductors to n-type [28,29,30]. Typically, n-type materials based on polycyclic aromatic hydrocarbons are obtained by attaching strong electron-withdrawing groups such as CN to the conjugated core, or by replacing the peripheral hydrogens with halogen atoms (in particular F and Cl) [29, 30] hence, C24H6Cl6 and C24Cl12 can be used as n-type semiconductors. From our results, the calculated Eg value at all levels of theory show that charge transfer occurs within these molecules. The associated Eg of the doped C18H6Cl6 molecule is 2.59 eV which is within the range of electrochemically and optically Eg values of thin films (2.08–2.77 eV) as documented in literature [71] while the Eg value of C24Cl12 molecule is 1.46 eV smaller than the Eg values of thin films and that of some inorganic semiconductors. Futhermore, we observed that our Eg values for the doped coronene are lower than that of potassium doped coronene [72] reported in literature. Hence we can conclude that these molecules have very good electronic, optoelectronic and photonic applications.
The work function EF of the molecules reported was obtained from the Fermi energy [17, 73] \( E_{F} = E_{\text{HOMO}} + \frac{{E_{\text{gap}} }}{2} \), where HOMO is the energy of the highest occupied molecular orbital and \( E_{\text{gap}} \) is the band gap of the system. The EF value for C24H12 is − 7.52 eV, − 7.23 eV for C24H6Cl6 and − 6.42 eV for C24Cl12 molecule for all the methods. In general, our study reveal that the complete substitution of the peripheral hydrogen atoms with chlorine in the coronene (C24Cl12) molecule gives rise to a decrease in ionization energy, electron affinity, total electronic energy, electronegativity, LUMO–HOMO energy gap, chemical hardness and an increase in the chemical softness, second order hyperpolarizability, excitation wavelength, absorption coefficient and the Fermi energy. Due to the large chemical softness of the molecules, it implies the molecules have a high electrical conductivity since the chemical softness is proportional to the magnitude of the electrical conductivity with C24Cl12 being a better conductor compared to C24H12 and C24H6Cl6. The order of increase conductivity is as follows: the conductivity of C24Cl12 greater than that of C24H6Cl6 and that of C24H6Cl6 is greater than that of C24H12. From the above results, we observed an improvement in the electronic, optoelectronic, linear and nonlinear optical properties of chlorine substituted coronene with those of C24Cl12 being more pronounced compare to those of C24H6Cl6.
The HOMO and LUMO molecular orbital diagrams of the molecules are shown in Fig. 2. The HOMO and LUMO molecular orbital diagrams of the molecules C24H18, C18H6Cl6 and C24Cl12 obtained using TD-wB97XD, TD-B3LYP, TD-SVWN (LSDA) and TD-CCSD(T) methods with the cc-pVDZ basis set are the same.
3.4 Excitation energy, oscillator strength and UV spectrum
The computed first singlet–singlet permitted excitation energies of unsubstituted and chlorinated coronene as obtained by TD-DFT at the TD-wB97XD/cc-pVDZ, TD-B3LYP/cc-pVDZ, TD-SVWN (LSDA)/cc-pVDZ and TD-CCSD(T)/cc-pVDZ level of theory are reported in Table 2. Only the values of the first 6 excitation energies of the excited states with corresponding oscillator strength f and all the excited energies with oscillator strength different from zero were reported. TD-CCSD(T)/c-pVDZ method gave the largest excited energies values with the corresponding oscillator strength compared to the other methods for the molecules C24H12 and C24H6Cl6.
The spectra are reported in Figs. 3, 4 and 5. The singlet–singlet excitation energies and the electronic spectra in the visible/near-UV region (UV–vis spectra) were obtained by performing TD-DFT calculations at the TD-wB97XD/cc-pVDZ, TD-B3LYP/cc-pVDZ, TD-SVWN (LSDA)/cc-pVDZ and TD-CCSD(T)/cc-pVDZ levels as employed for the electronic ground-state. The frequency-space implementation of TD-DFT based on the linear response of the density-matrix was used. Here the pole strengths correspond to the oscillator strengths and the poles of the linear response function correspond to vertical excitation energies [30]. The required number of transitions and electronic excitations are limited to the low-energy part of the spectrum. Our restriction is based on the first 42 singlet–singlet roots which are sufficient to cover the optical window for each molecule in all the cases. The calculated UV–Vis (vertical excitation) spectra of C24H12 obtained with the TD-wB97XD/cc-pVDZ, TD-B3LYP/cc-pVDZ, TD-SVWN (LSDA)/cc-pVDZ and TD-CCSD(T)/cc-pVDZ methods are shown in Fig. 3. Figure 4 gives the TD-wB97XD/ cc-pVDZ, TD-B3LYP/ cc-pVDZ, TD-SVWN (LSDA)/ cc-pVDZ and TD-CCSD(T)/cc-pVDZ UV-Vis Spectra of C24H6Cl6 while the TD-wB97XD/ cc-pVDZ, TD-B3LYP/ cc-pVDZ, TD-SVWN (LSDA)/ cc-pVDZ and TD-CCSD(T)/cc-pVDZ UV-Vis Spectra of C24Cl12 are given in Fig. 5.
The UV spectrum is made up of large bands which correspond to the superposition of the electronic, vibrational and rotational transitions. The electron transitions are highly energetic, the vibrational transitions are weakly energetic and the rotational transitions are more weakly energetic. The spectra presented in Figs. 3, 4 and 5 present one or two maximum which correspond to the absorption coefficient corresponding to a maximum wavelength, which is more or less as a result of the electron delocalization for organic molecules. The spectra of C24H12 and C24H6Cl6 present two maximum wavelengths while the spectra of C24Cl12 show one maximum wavelength. Epsilon on the y-axis is a constant which is proportional to the absorption coefficient. From our spectra, we observed that the value of epsilon is higher for C24Cl12 compare to that of C24H6Cl6 and C24H12 at all level of theory. This implies the wavelength and absorption coefficient are higher when coronene is completely substituted by chlorine at the peripheral.
4 Conclusion
In this work we have investigated theoretically the electronic, optoelectronic, linear and non linear optical properties and UV–Vis Spectrum of Coronene and Coronene substituted with Chlorine. By means of TD-wB97XD, TD-B3LYP, TD-SVWN (LSDA) and TD-CCSD(T) calculations we have quantized the effect of Coronene substituted completely and partially with Chlorine on a series of key molecular properties of interest for solid-state applications. We observed that the partial substitution of 6 peripheral hydrogen atoms with 6 Chlorine atoms in coronene molecule gives rise to increase second order hyperpolarizability, chemical softness, Fermi energy while the ionization energy, electron affinity, chemical hardness, LUMO–HOMO energy gap and dipole moment decrease. The second order hyperpolarizability of the molecule C24H6Cl6 obtained using the TD-wB97XD, TD-B3LYP, TD-SVWN (LSDA) and TD-CCSD(T)) method is 2.67, 2.63, 2.62 and 2.70 times greater than its corresponding value of coronene respectively. Equally, \( E_{\text{g}}^{\text{KS}} \) and Eg values of C18H6Cl6 are smaller than their corresponding values obtained with C24H12 molecule. Since most of the above quantities change by approximately the same amount, the corresponding quasi-particle corrected energy gap is observed to vary slightly following chemical substitution for all the methods. Hence, the partial or complete substitution of hydrogen atoms in coronene by chlorine atoms or other atoms may give rise to interesting electronic, optoelectronic, linear and nonlinear optical properties of this molecule.
The large values of \( \gamma_{av} \) and Eg permit us to conclude that these molecules have very good linear and nonlinear optical effects and hence can be used in linear and nonlinear materials which may have electronic, optoelectronic and photonic applications.
References
Forrest SR (2004) The path to ubiquitous and low-cost organic electronic appliances on plastic. Nature 428:911
Anthony JE (2008) The larger acenes: Versatile organic semiconductors. Angew Chem. Int. Ed. Eng. 47(3):452
Halik M, Klauk H, Zschieschang U, Kriem T, Schmid G, Radlik W, Wussow K (2002) Fully patterned all-organic thin film transistors. Appl Phys Lett 81:289
Kan Y, Wang L, Duan L, Hu Y, Wu G, Qiu Y (2004) Highly-efficient blue electroluminescence based on two emitter isomers. Appl Phys Lett 84:1513
Yoo S, Domercq B, Kippelen B (2004) Efficient thin-film organic solar cells based on pentacene/C60 heterojunctions. Appl Phys Lett 85:5427
Shiyanovskaya I, Singer KD, Percec V, Bera TK, Miura Y, Glodde M (2003) Charge transport in hexagonal columnar liquid crystals self-organized from supramolecular cylinders based on acene-functionalized dendrons. Phys. Rev. B 67:035204
Anthony JE (2006) Chem Rev 106(12):5028
Murphy AR, Frechet MJ (2007) Functionalized acenes and heteroacenes for organic electronics. Chem Rev 107:1066
Huang L, Rocca D, Baroni S, Gubbins KE, Nardelli MB (2009) Molecular design of photoactive acenes for organic photovoltaics. J. Chem. Phys. 130:194701
Karak S, Reddy VS, Ray SK, Dhar A (2009) Organic photovoltaic devices based on pentacene/N, N′-dioctyl-3,4,9,10-perylenedicarboximide heterojunctions. Org Electron 10:1006
De Angelis F (2010) Direct versus indirect injection mechanisms in perylene dye-sensitized solar cells: a DFT/TDDFT investigation. Chem Phys Lett 493:323
Bredas J-L, Beljonne D, Coropceanu V, Cornil J (2004) Charge-transfer and energy-transfer processes in pi-conjugated oligomers and polymers: a molecular picture. Chem Rev 104:4971
Sancho-Garca JC (2007) Assessment of density-functional models for organic molecular semiconductors: the role of HartreeFock exchange in charge-transfer processes. Chem Phys 331:321
Ejuh GE, Tchangnwa Nya F, Djongyang N, Ndjaka JMB (2018) Study of some properties of quinone derivatives from quantum chemical calculations. Optic. Quant. Electron. https://doi.org/10.1007/s11082-018-1603-0
Ejuh GW, Tchangnwa Nya F, Yossa Kamsi RA, Ndjaka JMB (2018) Investigation of the Electronic, optoelectronics, Linear and nonlinear optical properties of the molecules Heptacene ([7]acene) (C30H18) and [7]acene doped with potassium atom (C30H9K9). Poly. Bullet. 75(2):537
Ejuh GW, Ottou Abe MT, Tchangwa Nya F, Ndjaka JMB (2018) Prediction of electronic structure, dielectric and thermodynamical properties of Flurbiprofen by Density Functional Theory calculation. Karbala J. Mod. Sci. 4:12
Ejuh GW, Tchangnwa Nya F, Ottou Abe MT, Fankam Fankam JB, Ndjaka JMB (2017) Electronic structure, physico-chemical, linear and non linear optical properties analysis of coronene, 6B-, 6 N-, 3B3N- substituted C24H12 using RHF, B3LYP and wB97XD methods. Optic. Quant. Electron. 49(11):382
Taura L S, Ndikilar C E, Geh W. Ejuh G W, Muhammad A 2018. RHF and DFT Study of the Molecular and Electronic Properties of \( \left( {SiO_{2} } \right)_{n} \) and \( \left( {GeO_{2} } \right)_{n} \) Nanoclusters. Mod. Appl. Sci. 12(9) 1913
Tchangnwa Nya F, Ejuh GW, Ndjaka JMB (2017) Theoretical study of optoelectronic and thermodynamic properties of molecule 4- [2- (2-N, N-dihydroxy amino thiophene) vinyl] benzanamine: influence of hydroxyl position. Mater Lett 202:89
Ejuh GW, Nouemo S, Tchangnwa Nya F, Ndjaka JMB (2016) Modeling of the electronic, Optoelectronics, photonic and thermodynamics properties of 1, 4–bis (3-carboxyl–3-oxo-prop-1-enyl) benzene molecule. J Iran Chem Soc 13(11):2039
Ejuh GW, Nouemo S, Tchangnwa Nya F, Ndjaka JMB (2016) Computational determination of the Electronic and Nonlinear Optical properties of the molecules 2-(4-aminophenyl) Quinoline, 4-(4-aminophenyl) Quinoline, Anthracene Anthraquinone Phenanthrene. Mater Lett 178:221
Tadjouteu Assatse Y, Ejuh GW, Yossa Kamsi RA, Tchoffo F, Ndjaka JMB (2019) Theoretical studies of nanostructures modeled by the binding of Uracil derivatives to functionalized (5,5) carbon nanotubes. Chem Phys Lett 731:136602
Veved A, Ejuh GW, Djongyang N (2019) Effect of HfO2 on the dielectric, optoelectronic and energy harvesting properties of PVDF. Optic. Quant. Electron. 23(10):330
Yossa Kamsi RA, Ejuh GW, Tchoffo F, Mkounga P, Ndjaka JMB (2019) Electronic Structure, Spectroscopic (IR, Raman, UV-Vis, NMR), Optoelectronic, and NLO Properties Investigations of Rubescin E (C31H36O7) Molecule in Gas Phase and Chloroform Solution Using Ab Initio and DFT Method. Adv. Conden. Matt. Phys. ID. https://doi.org/10.1155/2019/4246810
Fonkem C, Ejuh GW, Tchangnwa Nya F, Yossa Kamsi RA, Ndjaka JMB (2019) Theoretical study of optoelectronic properties of the molecule 2-cyano-3-[4-(diphenylamino) phenyl] acrylic acid. J. Iranian Chem. Soc. https://doi.org/10.1007/s13738-019-01790-4
Veved A, Ejuh GW, Djongyang N (2020) Study of the optoelectronic and piezoelectric properties of ZrO2 doped PVDF from quantum chemistry calculations. Chin. J. Phys. 63:213
Pérez-Jiménez AJ, Sancho-Garca JC (2009) Assessment of double-hybrid energy functionals for π-conjugated systems. J Am Chem Soc 131:14857
Delgado MCR, Pigg KR, Da Silva Filho D A, Gruhn NE, Sakamoto Y, Suzuki T, Osuna RM, Casado J, Hernandez V, Navarrete JTL, Martinelli NG, Cornil J, Sanchez-Carrera RS, Coropceanu V, Bredas J-L (2009) Impact of peruorination on the charge-transport parameters of oligoacene crystals. J Am Chem Soc 131(4):1502
Tang MT, Bao Z (2011) Halogenated materials as organic semiconductors. Chem Mater 23(3):446
Cardia R, Malloci G, Serra G, Bosin A, Cappellini G (2016) Electronic and optical properties of functionalized polyaromatic hydrocarbons: a computational investigation on perfluorinated circumacenes. Proc. SPIE 9895 Org Photon II. https://doi.org/10.1117/12.2229744
Sancho-García JC, Pérez-Jiménez AJ (2014) Theoretical study of stability and charge-transport properties of coronene molecule and some of its halogenated derivatives: a path to ambipolar organic-based materials. J. Chem. Phys. 141:134708
Morisset S, Rougeau N, Teillet-Billy D (2017) Influence of a graphene surface on the first steps of the hydrogenation of a coronene molecule. Chem Phys Lett 679:225
Kowalzik P, Rathgeber S, Karthauser S, Waser R, Schnaebele N, Raimundo J-M, Gingras M (2012) Columnar self-assembly of a 3D-persulfurated coronene asterisk. The dominant role of aryl-sulfur bonds. New J Chem 36:477
Ito S, Wehmeier M, Brand JD, Kubel C, Epsch R, Rabe JP, Mullen K (2000) Synthesis and self-asembly of functionalized hexa–peri-hexabenzocoronenes. Chem Eur J 6(23):4327
Wang C, Dong H, Hu W, Liu Y, Zhu D (2012) Semiconducting π-conjugated systems in field-effect transistors: a material odyssey of organic electronics. Chem Rev 112:2208
Kang MJ, Doi I, Mori H, Miyazaki E, Takimiya K, Ikeda M, Kuwabara H (2011) Alkylated dinaphtho[2,3-b:2′, 3′-f]thieno[3,2-b]thiophenes (Cn–DNTTs): organic semiconductors for high performance thin–film transistors. Adv Mater 23:1222
Fichou DJ (2000) Structural order in conjugated oligothiophenes and its implications optoelectronic in devices. Mater. Chem. 10:571
Allard S, Forster M, Souharce B, Thiem H, Scherf U (2008) Organic semiconductors for solution–processable field-effect transistors (OFETs). Angew Chem Int Ed 47:4070
Sanyal S, Manna AK, Pati KS (2013) Effect of imide functionalization on the electronic, optical, and charge transport properties of coronene: a theoretical study. J Phys Chem C 117(2):825
Kosugi T, Miyake T, Ishibashi S, Arita R, Aoki H (2011) Ab initio electronic structure of solid coronene: differences from and commonalities to picene. Phys. Rev. B 84:020507
Roth F, Bauer J, Mahns B, Buchner B, Knupfer M (2012) Electronic structure of undoped and potassium-doped coronene investigated by electron energy-loss spectroscopy. Phys. Rev. B 85:014513
Fedorov I, Zhuravlev Y, Berveno V (2012) Properties of crystalline coronene: dispersion forces leading to a larger van der Waals radius for carbon. Phys Status Solidi B 249:1438
Zhang M, Guo X, Ma W, Ade H, Hou J (2015) A large-bandgap conjugated polymer for versatile photovoltaic applications with high performance. J Adv Mater 27:4655
Zheng Z, Awartani OM, Gautam B, Liu D, Qin Y, Li W, Bataller A, Gundogdu K, Ade H, Hou J (2017) Efficient charge transfer and fine-tuned energy level alignment in a THF-processed fullerene-free organic solar cell with 11.3% efficiency. J Adv Mater 29(5):1604241
Dai S, Zhao F, Zhang Q, Lau TK, Li T, Liu K, Ling Q, Wang W, Lu X, You W, Zhan X (2017) Fused nonacyclic electron acceptors for efficient polymer solar cells. J Am Chem Soc 139(3):1336
Fan Q, Su W, Wang Y, Guo B, Jiang Y, Guo X, Liu F, Russell TP, Zhang M, Li Y (2018) Synergistic effect of fluorination on both donor and acceptor materials for high performance non-fullerene polymer solar cells with 13.5% efficiency. Sci China Chem. 61:531
Kan B, Feng H, Yao H, Chang M, Wan X, Li C, Hou J, Chen Y (2018) A chlorinated low-bandgap small-molecule acceptor for organic solar cells with 14.1% efficiency and low energy loss. Sci China Chem. 61:1307
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA Jr, Peralta JE, Ogliaro F, Bearpark MJ, Heyd J, Brothers EN, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell AP, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam NJ, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann KM, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich J, Daniels AD, Farkas O, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2010) Gaussian 09, revision C.01. Gaussian Inc., Wallingford
Yossa Kamsi RA, Ejuh GW, TadjouteuAssatse Y, Tchoffo F, Ndjaka JMB (2019) Computational study of reactivity and solubility Rubescin D and E in gas phase and in solvent media using Hatre-Fock and DFT method. Chin. J. Phys. 60:1
Malloci G, Cappellini G, Mulas G, Satta G (2004) Quasi-particle effects and optical absorption in small fullerene-like gap clusters. Phys. Rev. B 70:205429
Fonkem C, Ejuh GW, Tchangnwa Nya F, Yossa Kamsi RA, Tadjouteu Assatse Y, Ndjaka JMB (2020) A Density Functional Theory (DFT) study of the doping effect on 2-cyano-3- [4 (diphenylamino) phenyl] acrylic acid. Chin. J. Phys. 63:207
Geerlings P, De Proft F, Langenaeker W (2003) Conceptual density functional theory. Chem Rev 103(5):1793
Yossa Kamsi RA, Ejuh GW, Nkounga P, Ndjaka JMB (2020) Study of the molecular structure, electronic and chemical properties of Rubescin D molecule. Chin. J. Phys. 60:104
Pearson RG (1988) Absolute electronegativity and hardness: application to inorganic chemistry. Inorg Chem 27:7
Henríquez-Román JH, Padilla-Campos L, Páez MA, Zagal JH, María Rubio A, Rangel CM, Costamagna J, Cárdenas-Jirón GJ (2005) The influence of aniline and its derivatives on the corrosion behaviour of copper in acid solution: a theoretical approach. Mol. Struct. 757:1
Reis H, Papadopoulos MG, Calaminici P, Jug J, Koster AM (2000) Calculation of macroscopic linear and nonlinear optical susceptibilities for the naphthalene, anthracene and meta-nitroaniline crystals. J. Chem. Phys. 261:359
Sajan D, Joe H, Jayakumar VS, Zaleski J (2006) Structural and electronic contributions to hyperpolarizability in methyl p-hydroxy benzoate. J Mol Struct 785:43
Nagalakshmi R, Krishnarkumar V, Hagemann H, Muthunatesan S (2011) Polarized Raman and hyperpolarizability studies of Hydroxyethylammonium (l) tartrate monohydrate for quadratic nonlinear optics. J Mol Struct 988:17
Alyar H (2013) A review on nonlinear optical properties of donor-acceptor derivatives of naphthalene and azanaphthalene. Rev. Adv. Mater. Sci. 34:79
Wu K, Snijders JG, Lin C (2002) Reinvestigation of hydrogen bond effects on the polarizability and hyperpolarizability of urea molecular clusters. J. Phys. Chem. B 106(35):8954
Champagne B, Spassova M (2009) Theoretical investigation on the polarizability and second hyperpolarizability of polysilole. Chem Phys Lett 471:111
Wang C, Yuan Y, Tian X, Sun J, Yuan J (2016) The effects of exact exchange of density functionals on the evaluation of second hyperpolarizabilities of streptocyanines using sum-over-states method. Comput. Theor. Chem. 1085:40
Ejuh GW, Ottou Abe MT, Ghislain T, Ndjaka JMB (2018) Ab Initio and DFT studies on the donor-acceptor molecules 1,2,3-trihydroxy-9,10-anthraquinone; 1-(methylamino) anthraquinone; 2-phenyl quinoxaline and 2-(4-aminophenyl) quinoxaline. Mater. Focus 7(1):37
Clar E, Schmidt W (1976) Correlations between photoelectron and phosphorescence spectra of polycyclic hydrocarbons. Tetrahedron 32(21):2563
Boschi R, Schmidt W (1972) Photoelectron spectra of polycyclic aromatic hydrocarbons. Tetrahedron Lett 13(25):2577
Kuroda H (1964) Ionization potentials of polycyclic aromatic hydrocarbons. Nature 201:1214
Clar E, Robertson JM, Schlogl R, Schmidt W (1981) Photoelectron spectra of polynuclear aromatics. Applications to structural elucidation: “circumanthracene”. J Am Chem Soc 103(6):1320
Schröder D, Loos J, Schwarz H, Thissen R, Preda DV, Scott LT, Caraiman D, Frach MV, Böhme DK (2001) Single and double ionization of corannulene and coronene. Helv Chim Acta 84:1625
Duncan MA, Knight AM, Negishi Y, Nagao S, Nakamura Y, Kato A, Nakajima A, Kaya K (1999) Production of jet-cooled coronene and coronene cluster anions and their study with photoelectron spectroscopy. Chem Phys Lett 309:49
Kandalam AK, Kiran B, Jena P, Li X, Grubisic A, Bowen KH (2007) Ground state structures and photoelectron spectroscopy of [Co-m(coronene)](-) complexes. J. Chem. Phys. 126:084306
Chen-Jen Y, Jenekhe AS (1995) conjugated aromatic polyimines. 2. Synthesis, structure, and properties of new aromatic polyazomethines. Macromol. 28:1180
Kosugi T, Miyake T, Ishibashi S, Arita R, Aoki H (2011) Electronic structure of solid coronene: differences and commonalities to picene. Cond-Mat. Sup-Con, (2011), arXiv:1105.0248(2)
Chang-Guo Z, Nichols JA, Dixon DA (2003) Ionization potential, electron affinity, electronegativity, hardness, and electron excitation energy: molecular properties from density functional theory orbital energies. J Phys Chem A 107(20):4184
Acknowledgements
We are thankful to the Council of Scientific and Industrial Research (CSIR), India for financial support through Emeritus Professor scheme (Grant No21(0582)/03/EMR-II) to late Prof. A.N. Singh of the Physics Department, Bahamas Hindu University, India which enabled him to purchase the Gaussian Software. We are most grateful to Late Emeritus Prof. A.N. Singh for donating this software to Prof. G. W. Ejuh.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict interest
The authors declare that there is no conflict of interest as concern this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ejuh, G.W., Tchangnwa Nya, F., Djongyang, N. et al. Theoretical study on the electronic, optoelectronic, linear and non linear optical properties and UV–Vis Spectrum of Coronene and Coronene substituted with Chlorine. SN Appl. Sci. 2, 1247 (2020). https://doi.org/10.1007/s42452-020-3028-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-020-3028-1