Abstract
Uranium concentration has been estimated in 31 groundwater samples collected from the Nalbari district of Assam in pre-monsoon and post-monsoon season. Fourteen other water quality parameters have also been monitored to study their correlation with uranium. The uranium concentration varies from 0.3 to 7.1 µg/L with the mean value of 2.15 µg/L in pre-monsoon and 0.6–10.3 µg/L with the mean value of 2.75 µg/L in the post-monsoon season. The higher concentration of uranium in post-monsoon may be ascribed to the dissolution of uranium from soil sediments in the rainy season. It has been observed that the uranium content in both seasons is far lower than the WHO (2011) permissible limit of 30 µg/L. In both seasons, nitrate, sulphate, and especially phosphate show a positive correlation with uranium, which may be due to different agricultural activities. Agricultural nitrate and phosphate fertilization might be the carrier of uranium in groundwater through dissolution. For all life stage groups, the annual effective dose was appeared to be far below the WHO (2011) prescribed limit of 100 µSv/y. In consideration to adults, the ingestion dose for infants was turned out to be higher. The carcinogenic and non-carcinogenic risk was less than the permissible limits for both children and adults. Both dose value and risk indices were found higher in the post-monsoon season.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Groundwater is the main drinking water source in rural and urban India. Due to rapid urbanization, industrial development, enormous chemical fertilizers use, and other human activities, the groundwater becomes polluted. According to the World Health Organization, 80% of human disease occurs because of the deteriorating quality of drinking water. The primary concern about uranium contamination in drinking water is that uranium acts as chemically and radiologically toxic radioactive pollutants. The continuous exposure of uranium through drinking water leads to potential health risks to humans. Several studies have been done to estimate the probable health hazard due to the intake of groundwater contaminated with uranium [1,2,3,4,5,6]. Uranium originates from various anthropogenic activities such as mining, milling, mineral sands, and ores; fertilizer manufacturing [7,8,9,10] may be responsible for its groundwater presence.
Uranium occurs in various oxidation states, but hexavalent and tetravalent are the most dominant states of uranium. The hexavalent state is especially important in water due to its solubility because the tetravalent state is almost insoluble in water. The hexavalent form of uranium is commonly found in the form of uranyl ion UO22+. Uranium mainly enters human tissues through the air, water, food, and other accidental and occupational exposures. Uranium intake through air and water is typically low, but most input can be through drinking water because it depends on its source's circumstances. Water with uranium content above 30 µg/L [11] is not suggested for drinking purposes as on continuous intake; it can cause damage to internal organs like kidney [12, 13] and bones [14].
Physico-chemical parameters (pH, electrical conductivity, TDS, ORP, etc.) of water are essential to provide important firsthand unaltered information about how much the water is suitable for drinking purposes [8]. The pH is monitored to assess human and aquatic environment health, drinking water sources, irrigation sources, intake, and discharges of the industries. Total dissolved solids (TDS) are the small amounts of inorganic salts and organic matter solvated in water. The water’s capacity to oxidize the contaminants is termed as Oxidation Redox Potential (ORP). Though there is no standard for ORP by WHO, anything that shows ORP less than 550 mV is extraordinarily strong and is considered non-drinkable. Anions such as SO42−, NO3−, F− also play a vital part in determining the quality of drinking water. A high amount of sulphate can cause intestinal disorders and diarrhoea [15]. Problems like blue baby disease or methemoglobinemia can be caused by a nitrate content of more than 45 mg/L [16]. Diseases like dental and skeletal fluorosis can be caused by fluoride concentration of 3 mg/L or more [17].
Previous studies in countries like Finland, Jordan, the USA, and Sweden [18,19,20,21,22] have shown very high uranium concentration in groundwater. In contrast, countries such as Argentina, Canada, Italy, Kuwait, South Greenland [19, 23,24,25,26] have shown relatively low uranium concentration. In India, too, several studies have been executed to estimate the groundwater uranium concentration. Higher concentrations are reported from places like Kolar (Karnataka) [27], southwest Punjab and Bhatinda (Punjab) [8, 28], Peddagattu and Seripally (Andhra Pradesh) [29], Hyderabad (Telangana) [30], Sirsa (Haryana) [5], Madurai, Tamil Nadu [31] and Central Tamil Nadu [32]. On the other hand, studies carried out in places like Vishakhapatnam (Andhra Pradesh) [33], Jaduguda (Jharkhand) [1], Nadia (West Bengal) [34], and Patna (Bihar) [2] have estimated a lower concentration of uranium in groundwater. The uranium concentration in groundwater is also observed changing with seasons, as reported from India's different places [35,36,37]. Due to other heavy metals and major ions present in the groundwater, human health hazard risk has also been recorded [38,39,40,41,42,43].
In the state of Assam, only a few studies have been performed. A study carried out in the state capital city Guwahati reported a relatively lower uranium concentration in the groundwater [44]. On the other hand, in Karbi Anglong district, uranium concentration higher than that of the WHO permissible limit (with a maximum value of 1.47–10.66 mg/L) in soil samples has been recorded by Kakati et al. [45]. It is, therefore, crucial to analyse the uranium concentration in groundwater of Assam, including the Nalbari district, as no study has been attempted to the best of our knowledge.
Considering the aspects mentioned above, particularly toxicity caused by uranium, the objective of the present work is to evaluate the concentration of uranium in the groundwater, which is primarily used for drinking. The study also estimates the radiation dose for different age groups and the carcinogenic and non-carcinogenic risk to the district's inhabitants, which may arise due to the consumption of water contaminated with uranium. Fourteen other physico-chemical parameters were also monitored to study the correlation with the analysed uranium concentration, if any. This study is expected to provide the baseline data on uranium present in the study area's groundwater.
2 Materials and method
2.1 Study area
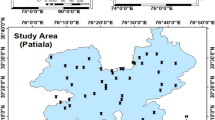
Nalbari district is one among 33 Districts of state Assam, India. It is located 51 km south towards the state capital Dispur at latitudes between 26°10ʹN and 26°47ʹN and 91°15ʹE and 91°38ʹE longitudes. It shares a border with Baksa district to the north, district of Kamrup to the south and east, and Barpeta on the west. Nalbari district occupies approximately 2257 km2 of area. The elevation of the district is in the range from 79 to 46 meters. The Nalbari district is located at the plains of Brahmaputra valley. The Brahmaputra's tributaries like Pagladiya, Nona, Buradia, Borolia, Tihu, etc., are wild, which causes floods in the area. These tributaries originate from the foothills of the Himalayas and flow into the Brahmaputra towards the southern part of the district.
Physiographically, the Nalbari district has two major regions, the northern alluvial region where the soil is deep reddish clayey alluvial soil, and southern flood plains of Brahmaputra river where recent age alluvial soil can be seen [46, 47]. A gentle slope can be observed from north to south towards the Brahmaputra. Throughout the district, the entire area is occupied by alluvial sediments of clay, sand, silt, boulders, and gravel of quartz, feldspars. The groundwater can be found in a semi-confined state, which belongs to a mono aquifer system. The depth of the system varies from 40 to 250m. The water level is almost directly dependent upon the area's total precipitation level in the aquifer system. The topmost thick alluvial layer of the ground has uniform porosity, and the permeability is observed to be in the range of 10–15% [46, 48]. A total of 68% of the district's land area is used for agricultural purposes; forest covers 3%, and other areas, including wasteland. The cultivable area covers 28.5%, and less than 0.5% of the land is used for industrial purposes. The district comprises rural areas where villages are right next to the agricultural lands [46, 48]. The climate of Nalbari is subtropical with semi-dry hot summer and cold winter. The humidity of the district hovers around 80%. From May to August, heavy rainfall occurs for which the people of the area experiences flood. Nalbari experiences annual average rainfall of about 1500 mm.
2.2 Collection and preservation of samples
A total of 31 samples were collected from different sources of the area based on a grid map of grid size 6×5 km2. The sampling locations are shown in Fig. 1 by numbers. The collection was done from tube-wells, wells, and local water supply reservoirs. A total of 28 samples were taken from tube-wells. The average borehole depth of tube-wells was around 18m. Two samples were taken from local water supply reservoirs; the water supply system's average borehole depth is 95m. In this case, we have collected the sample from the reservoirs, not from a household tap. A single sample is taken from a concrete well with a depth of around 15m. Sampling was done in the year of 2016; in the pre-monsoon samples collection was done in the month of May, and in the post-monsoon, the same has been done in October. A 0.45 µm membrane filtration system was used for the filtration of samples before analysis. From every source, groundwater was collected in two separate containers, one for the investigation of general parameters and the other for uranium analysis. To prevent wall adsorption and slower biological changes, 2 ml concentration HNO3 was added to 1 L sample to analyse uranium concentration.
2.3 Estimation of Uranium in samples
Uranium analysis was done in LF-2a LED fluorimeter (Quantalase Enterprises Pvt. Ltd., India). The calibration was done using a standard uranium solution of 1, 2, 5, 10, 15, 20, and 30 µg/L provided by the manufacturer. An internal standard addition method was used during analysis to prevent organic and inorganic interference present in the samples. 5% sodium pyrophosphate solution was prepared and acidified with orthophosphoric acid dropwise to adjust the pH range within 6.5–7.5. Further to convert different uranium species into a single form having identical fluorescence is done by adding fluorescent enhancing agents like sodium pyrophosphate to the sample. To refrain errors in pipetting, analytical balance and micropipettes were used. The limit of detection obtained using this method was 0.2 µg/L. Each sample was effectively analysed four times (n = 4). Statistical analyses included calculations of the mean and median value was calculated using Microsoft Excel software.
2.4 Estimation of Physico-chemical parameters in groundwater samples
The measurement of pH, TDS, EC (electrical conductivity), ORP was done using a portable electrode-based multiparameter kit. EDTA complexometric titration method was employed to determine the total hardness and calcium in the groundwater samples. Hence the hardness of magnesium is simply the difference between the values of total hardness and calcium hardness. The total alkalinity was found by the H2SO4 titration method using methyl orange as an indicator, which was due to bicarbonate alkalinity only. AgNO3, in the presence of the K2CrO4 indicator in Mohr’s method, gives reddish-brown precipitation of AgCl, which has been used for chloride estimation. The phosphate, nitrate, and sulphate concentrations were determined respectively by the stannous chloride method, screening method, and turbidimetric method. The fluoride concentration was measured using colorimetrically with SPADNS reagent. A UV-Visible spectrophotometer (Hitachi 3210) was used for the above techniques. American Public Health Association (APHA) standard protocols have been followed in the analysis of all the parameters [49].
2.5 Radiation dose evaluation for various age groups
The net annual effective dose of radiation (\(D_{{\text{R}}}\)) for various age groups due to consumption of drinking water was estimated using the equation given below [50],
\(A_{{\text{U}}}\) = Activity concentration of uranium (Bq/L). Unit conversion factor, \(1\mu g/L = 2.528 \times 10^{ - 2} Bq/L\) was used to calculate the value of \(A_{{\text{U}}}\). \(DWI = DRI\) s [51] prescribed daily water intake rate for various age groups (L/day), \(FDC = IAEA\) [52] suggested factor of dose conversion for definite age groups (Sv/Bq).
2.6 Carcinogenic risk assessment
USEPA approved equations (Equations 2 and 3) [53] were used to evaluate the excess cancer risk (\(ECR\)),
Where \(R_{{\text{F}}} =\) risk factor (L/Bq), r = risk coefficient value for uranium mortality, 1.13×10-9 Bq-1, \(C_{{\text{R}}}\) = consumption rate of water (L/day) , \(E_{{\text{P}}}\) = period of exposure. For adults, \(C_{{\text{R}}}\) = 4.05 L/day [8] and \(E_{{\text{P}}}\) = 23725 days. For children, \(C_{{\text{R}}}\) = 2 L/day [11] and \(E_{{\text{P}}}\)= 3650 days [54].
2.7 Non-carcinogenic risk assessment
Hazard quotient (\(H_{{\text{Q}}}\)) is used to express the non-carcinogenic risk. The ratio of lifetime average daily dose (\({\text{LADD}}\)) and the reference dose (\(R_{{\text{f}}} {\text{D}}\)) gives the net \(H_{{\text{Q}}}\). The value of \(R_{{\text{f}}} {\text{D }}\) is 4.53 µg/kg/day. Any \(H_{{\text{Q}}}\) value of less than 1 is regarded as safe from chemical toxicity point of view [55].
Where \(C_{{\text{U}}}\)= uranium concentration (µg/L), \(C_{{\text{R}}}\)= consumption rate of water (L/day), \(F_{{\text{E}}}\)= frequency of exposure (~350 days per year) [53], \(D_{{\text{E}}}\)= duration of total exposure (years), \(E_{{\text{L}}}\)= expectancy of life (years), \(W_{{\text{B}}}\)= body weight (kg).
Therefore, using the value of \(C_{{\text{R}}}\) = 4.05 L/day, \(D_{{\text{E}}}\) = 65 years [11], \(E_{{ {\text{L}}}}\) = 65 years, \(W_{{\text{B}}}\) = 53 kg [56] (for adults) and \(C_{{\text{R}}}\) = 2 L/day, \(D_{{\text{E}}}\) = 10 years, \(E_{{ {\text{L}}}}\)= 10 years, WB = 32.7 kg (for children) [54].
3 Results and discussions
3.1 Estimation of uranium and other water quality parameters
In Table 1, the statistical summary of uranium and other water quality parameters of pre-monsoon and post-monsoon samples of the Nalbari district was reported. The uranium concentration in groundwater is found to be 0.3–7.1 µg/L and 0.6–10.3 µg/L in the pre-monsoon and post-monsoon, respectively. The comparatively higher uranium concentration in post-monsoon may be attributed to uranium dissolution from soil sediments in the rainy season (Fig. 2). The maximum concentration 10.3 µg/L was found in the sample collected from Bhunukuchi under Barkhetri block (lat: 26°25ˊ44˝, long: 91°29ˊ06.9˝; sampling point number 12 in the study area map, Fig. 1) in post-monsoon. The variation observed in the uranium concentration may be due to the different geographical locations with different meteorological phenomena. All samples of the study area show uranium concentration well under the WHO prescribed limit of 30 µg/L during both seasons.
The ORP of the samples was found in the range of ( − 27)–68 mV in the pre-monsoon season, whereas ( − 31)–23 mV in post-monsoon, respectively. The total hardness recorded for the samples in both the seasons was found within the permissible limit. The total hardness varied from 40 to 151 mg/L in pre-monsoon and 58–175 mg/L in post-monsoon. The calcium concentration varied from 29 to 124 mg/L and 52–139 mg/L in pre-monsoon and post-monsoon, respectively. One sample, each from pre and post-monsoon, exceeded the WHO safe limit (100 mg/L) of calcium concentration. The concentration of magnesium was observed between 6 and 52 mg/L in pre-monsoon and 7–36 mg/L in post-monsoon. The evaporation effect of water in the pre-monsoon season for which the number of ions increases in groundwater may be held responsible for the greater values of hardness and magnesium and calcium concentration. The samples' total alkalinity ranged from 64 to 126 mg/L and 68–144 mg/L during pre-monsoon and post-monsoon season. The chloride ion concentration was observed to vary in the range of 8–46 mg/L in pre-monsoon, and 15–48 mg/L in post-monsoon; both the ranges were within the WHO prescribed limit of 250 mg/L[11]. A moderately high nitrate concentration was recorded in the water samples with the range of 5.8–56.7 mg/L and 7.7–68.3 mg/L in pre-monsoon and post-monsoon, respectively. This reasonably high nitrate concentration may be attributed to the region's agricultural practices as agricultural lands primarily cover the study area. The fluoride concentration was also found within the safe limit of 1.5 mg/L. The concentration of sulphate varied from 2.9 to 15.4 mg/L in pre-monsoon and 8.3–28.3 mg/L in the post-monsoon season. The phosphate concentration in pre-monsoon was found to be 0.01–0.63 mg/L, and in post-monsoon, the same was 0.04–0.54 mg/L
3.2 Correlation analysis
Pearson’s correlation matrix was done for 15 sets of variables and stated in Table 2a and b for both the seasons. A significant positive correlation of phosphate with uranium present in the region's groundwater may be accredited to the agricultural practices done here. It can be summarized that heavy usage of chemical fertilizers in the paddy fields enhances the leaching of phosphate through the soil, which then precipitates as UO2(HPO4)22− [59] in the groundwater. It is reported that phosphate fertilizers contain natural radioactivity and are a credible uranium source in the environment [60,61,62]. Nitrate holds a mild but crucial positive correlation with uranium in both seasons. In addition to this, a good positive correlation of nitrate with TDS is observed. This finding suggests that nitrate may take part an important role in the dissolution of uranium from minerals like uranyl nitrate (UO2 (NO3)2) [34, 63]. Hence it can be said that agricultural nitrate and phosphate fertilization might be the carrier of uranium. This region of Assam is well known for rice cultivation since rice is the most widely consumed food crop in Assam. Therefore, extensive use of fertilizers such as urea, ammonium sulphate, NPK in the agricultural lands might be another reason for uranium in the groundwater of the region. Uranium exhibits a positive correlation with ORP, which signifies the favourable condition for uranium transport and release in aquifers [2, 64, 65]. A minor but positive correlation of sulphate with uranium suggests that SO42− ions may be an additional complexing agent for uranyl (UO22+) ions in groundwater [34, 66, 67]. Sulphate shows a strong positive correlation with EC, TDS, total hardness, calcium, total alkalinity, chloride, and nitrate, which confers the parameters' interdependency. A good positive correlation of chloride with total alkalinity, EC, TDS, calcium, magnesium, and total hardness has also been observed. Similarly, a good positive correlation for magnesium is also noticed with total alkalinity, EC, TDS, total hardness, and calcium. Therefore, the magnesium ions largely influence the ionic conductance of the groundwater of the study area.
3.3 Radiation dose evaluation of uranium for various age groups
The age-dependent net annual dose of uranium was calculated and given in Table 3. The higher dose values in the case of 0.6–1 years old compared to 0–0.5 years infants may be accredited to a higher water consumption rate of 0.6–1 years old group. Though infants consume less water than adults, the annual dose for infants is more compared to adults because of the difference of smaller organ weights and their metabolism, resulting in higher radiosensitivity [3]. During pregnancy and lactation periods, females receive a higher radiation dose, which may be attributed to increased water intake. However, all males show a higher radiation dose as compared to females. The higher amount of water intake by males may be the reason for this. The annual effective dose of all life stage groups was within the recommended level of 100 µSv/y by WHO [11].
3.4 Assessment of carcinogenic risk
The excess cancer risk (ECR) was computed and outlined in Table 4. For children, the ECR was ranged between 0.006×10−5 and 0.148×10−5 in pre-monsoon and 0.013×10−5 and 0.215×10−5 in the post-monsoon season. For adults, in pre-monsoon ECR varied from 0.082×10−5 to 1.949×10−5 with the mean value of 0.592×10−5 and in post-monsoon 0.165×10−5–2.827×10−5 with the mean value of 0.754×10−5 respectively. The ECR values for both children and adults during both seasons were low compared to the AERB prescribed limit of 1.67×10−4 [55]. Based on the maximum groundwater uranium concentration, it can be summarized from Table 4 that there could be 2–3 cases of cancer per one hundred thousand adult population on continuous uranium exposure.
3.5 Assessment of non-carcinogenic risk
The LADD values and \(H_{Q}\) were evaluated and reported in Table 4. LADD values for children ranged from 0.018 to 0.416 µg/kg/day in pre-monsoon and 0.03–0.604 µg/kg/day in post-monsoon. For adults, in pre-monsoon LADD values ranged between 0.022 and 0.520 µg/kg/day and in post-monsoon 0.044–0.755 µg/kg/day. According to WHO [11], the prescribed limit of LADD is 1 µg/kg/day. The \(H_{Q}\) values for children in both seasons varied from 0.004 to 0.092 and 0.008–0.133, with the mean value of 0.028 and 0.036. For adults, \(H_{Q}\) ranged from 0.005 to 0.115 with mean value of 0.035 and 0.011–0.167 with mean value of 0.044, respectively. Therefore, the \(H_{Q}\) values for all the samples in both seasons were less than the AERB recommended limit of 1 [55], and hence the water is safe from the chemical toxicity effect.
3.6 Comparison of uranium concentration with previous studies
The uranium concentration obtained from the previously reported studies in different states of India and worldwide is compared with the present study and shown in Table 5a and b. Uranium concentration in groundwater worldwide shows large variations. In Vishakhapatnam, Bhangare et al. recorded a uranium concentration of 0.6–12.3 µg/L [33]. Patra et al. reported that, around the uranium mining sites in Jaduguda, Jharkhand, the concentration of uranium ranged from 0.03 to 11.6 µg/L [1]. In Nadia, West Bengal, uranium concentration ranged from 0.21 to 20.9 µg/L in groundwater samples, reported by Das et al. [34]. Similarly, a uranium concentration of 0.1–14.5 µg/L was found in drinking water samples in Patna, Bihar, reported by Kumar et al. [2]. All the studies mentioned above obtained low uranium concentration values under the WHO limit of 30 µg/L [11] and found comparable with the present study. Similarly, lower values were observed for Guwahati, Assam (0.08–5.32 µg/L), reported by Talukdar et al. (1983) [44].
However, higher uranium concentration has been reported in southwest Punjab (0.5–579 µg/L) [8], Kolar, Karnataka (0.3–1442.9 µg/L) [27], Bhatinda, Punjab (11.7–113.7 µg/L) [28], Peddagattu/Seripally, Andhra Pradesh (0.6–521.15 µg/L) [29], Gaya, Bihar (0.1-238.2 µg/L) [6], Madurai, Tamil Nadu (0.2–156.84 µg/L) [31], Central Tamil Nadu (0.79–71.93 µg/L) [32] . Further, in other countries like Argentina (0.04-11.0 µg/L) [23], Ontario, Canada (0.05–4.21 µg/L) [24], Italy (0.02–5.2 µg/L) [19], Kuwait (0.02–2.48 µg/L) [25], South Greenland (0.5–1.0 µg/L) [26] has been reported concentration of uranium in lower side and found comparable with the current study. From the comparison with the previous studies as discussed above, the uranium concentrations in Nalbari, Assam is on the very lower side.
4 Conclusions
The range of concentration of uranium in groundwater observed in the present study was considerably lower than the WHO permissible value of 30 µg/L. The concentration is also observed to change with seasons and is found to be higher in post-monsoon. The disbandment of uranium in groundwater from soil sediments during the rainy season may hold responsible for the same. Uranium exhibits a positive correlation with phosphate, nitrate, and sulphate in both seasons, which may be due to the region's different agricultural activities. So, it is needful to mention that in the future, nitrate, sulphate, and phosphate concentration in groundwater may exacerbate uranium dissolution in the study area as they are good carriers of uranium. Correlation study shows that parameters are less or more correlated with each other, which implies the ions' interdependence in the groundwater system of the region. Similarly, the annual effective dose was also recorded within the WHO safe limit of 100 µSv/y for all the different age groups. The carcinogenic and non-carcinogenic risk due to water consumption was much lesser than the permissible limits for both children and adults. Hence, the water can be considered to be safe for use. The study indicates that the drinking water around the Nalbari district of Assam does not need any attention from the policymakers regarding uranium and other minerals. It appears instructive to regularly monitor uranium to determine alterations in uranium in Assam's groundwater for a unique variation.
References
Patra AC, Mohapatra S, Sahoo SK, Lenka P, Dubey JS, Tripathi RM, Puranik VD (2013) Age-dependent dose and health risk due to intake of uranium in drinking water from Jaduguda, India. Radiat Prot Dosim 155:210–216
Kumar D, Singh A, Jha RK (2018) Spatial distribution of uranium and basic water quality parameter in the capital of Bihar and consequent ingestion dose. Environ Sci Pollut Res 25:17901–17914
Sahoo SK, Mohapatra A, Chakrabarty A, Sumesh CG, Jha VN, Tripathi RM, Puranik VD (2009) Distribution of uranium in drinking water and associated age-dependent radiation dose in India. Radiat Prot Dosim 136(2):108–113
Duggal V, Rani A, Mehra R, Saini K, Bajwa B (2017) Assessment of age-dependent radiation dose and toxicity risk due to intake of uranium through the ingestion of groundwater from Northern Rajasthan, India. Toxicol Environ Chem 99:516–524
Duggal V, Sharma S, Saini K, Bajwa BS (2017) Assessment of carcinogenic and non-carcinogenic risk from exposure to uranium in groundwater from Western Haryana, India. J Geol Soc India 89:663–668
Kumar D, Singh A, Kumar P, Jha RK, Sahoo SK, Jha V (2020) Sobol sensitivity analysis for risk assessment of uranium in groundwater. Environ Geochem Health 42(6):1789–1801
Rana BK, Dhumale MR, Lenka P, Sahoo SK, Ravi PM, Tripathi RM (2016) A study of natural uranium content in groundwater around Tummalapalle uranium mining and processing facility, India. J Radioanal Nucl Chem 307:1499–1506
Bajwa BS, Kumar S, Singh S, Tripathi RM, Sahoo SK (2015) Uranium and other heavy toxic elements distribution in the drinking water samples of SW-Punjab, India. J Radiat Res Appl Sci 10:13–19
Tripathi RM, Sahoo SK, Mohapatra S, Patra AC, Lenka P, Dubey JS, Jha VN, Puranik VD (2012) an assessment of radiological scenario around uranium mines in singhbhum east district, Jharkhand. India. Radiat Prot Dosim 150(4):458–464
Adithya VS, Chidambaram S, Tirumalesh K, Thivya C, Thilagavathi R, Prasanna MV (2016) Assessment of sources for higher uranium concentration in ground waters of the Central Tamilnadu, India. IOP Conf Ser Mater Sci Eng 121, p. 012009. IOP Publishing Ltd
WHO (World Health Organization) (2011) Guidelines for drinking water quality. WHO, Geneva, pp 241–252
Limson Zamora M, Tracy BL, Zielinski JM, Meyerhof DP, Moss MA (1998) Chronic ingestion of uranium in drinking water: a study of kidney bioeffects in humans. Toxicol Sci 43:68–77
Arzuaga X, Rieth SH, Bathija A, Cooper GS (2010) Renal effects of exposure to natural and depleted uranium: a review of the epidemiologic and experimental data. J Toxicol Environ Health B 13:527–545
Kurttio P, Komulainen H, Leino A, Salonen L, Auvinen A, Saha H (2005) Bone as a possible target of chemical toxicity of natural uranium in drinking water. Environ Health Perspect 113:68
Kaur T, Bhardwaj R, Arora S (2017) Assessment of groundwater quality for drinking and irrigation purposes using hydrochemical studies in Malwa region, southwestern part of Punjab, India. Appl Water Sci 7:3301–3316
Jain C, Bandyopadhyay A, Bhadra A (2010) Assessment of ground water quality for drinking purpose, District Nainital, Uttarakhand, India. Environ Monit Assess 166:663–676
Adimalla N, Venkatayogi S, Das SVG (2019) Assessment of fluoride contamination and distribution: a case study from a rural part of Andhra Pradesh. India. Appl Water Sci 9:94
Prat O, Vercouter T, Ansoborio E, Fichet P, Perret P, Kurttio P, Salonen L (2009) Uranium speciation in drinking water from drilled wells in southern Finland and its potential links to health effects. Environ Sci Technol 43:3941–3946
UNSCEAR (United Nations Scientific Committee on the Effect of Atomic radiation) (2000) United Nations general assembly. Vol. 1, Annex B, United Nations 84–140.
Gedeon R, Smith B, Amro H, Jawadeh J (1994) Natural radioisotopes in groundwaters from the Amman-Zarka basin Jordan. Hydrochemical and regulatory implications. Applications of tracers in arid zone hydrology. Wallingford, UK: IAHS Press; IAHS Publication 232
Cothern CR, Lappenbusch WL (1983) Occurrence of uranium in drinking water in the US. Health Phys 45:89–99
Selden I, Lundholm C, Edlund B, Hogdahl C, Ek Britt-Marie, Bergstroma BE et al (2009) Nephrotoxicity of uranium in drinking water from private drilled wells. Environ Res 109:486–494
Bomben AM, Equillor HE, Oliveira AA (1996) Ra-226 and natural uranium in Argentina bottled mineral waters. Radiat Prot Dosim 67:221–224
OMEE, Ontario Ministry of Environment and Energy (1996) Monitoring data for uranium1990-1995. Ontario Drinking Water Surveillance Program, Toronto, Ontario
Bou-Rabee F (1995) Estimating the concentration of uranium in some environmental samples in Kuwait after the 1991 Gulf War. Appl Radiat Isot 46:217–220
Brown A, Steenfelt A, Kunzzenorf H (1983) Uranium districts defined by reconnaissance geochemistry in South Greenland. J Geochem Explor 19:127–45
Babu MNS, Somashekar RK, Kumar SA, Shivanna K, Krishnamurthy V, Eappen KP (2008) Concentration of uranium levels in ground water. Int J Environ Sci Tech 5:263–266
Singh J, Singh L, Singh G (1995) High U-contents observed in some drinking waters of Punjab, India. J Environ Radioact 26:211–222
Raghavendra T, Srilatha K, Mahender C, Elander M, Vijayalakshmi T, Himabindu V, Prasad V, Savithri PP, Datta D, Arunachalam J (2014) Distribution of uranium concentration in groundwater samples from the Peddagattu/ Nambapur and Seripally regions using laser fluorimetry. Radiat Prot Dosim 158(3):325–330
Balbudhe AY, Srivastava SK, Vishwaprasad K, Srivastava GK, Tripathi RM, Puranik VD (2011) Assessment of age-dependent uranium intake due to drinking water in Hyderabad India. Radiat Prot Dosim 148(4):502–506
Thivya C, Chidambaram S, Thilagavathi R, Prasanna MV, Singaraja C, Adithya VS, Nepolian M (2015) A multivariate statistical approach to identify the spatio-temporal variation of geochemical process in a hard rock aquifer. Environ Monit Assess 187:552
Adithya VS, Chidambaram S, Keesari T, Mohokar HV, Prasanna MV (2017) Occurrence of uranium in groundwater along the lithological contacts in central Tamilnadu, India: an isotope hydrochemical prespective. Expo Health 11:277–290
Bhangare RC, Tiwari M, Ajmal PY, Sahu SK, Pandit GG (2013) Laser fluorimetric analysis of uranium in water from Vishakhapatnam and estimation of health risk. Radiat Prot Environ 36:128–132
Das A, Das SS, Roy Chowdhury N, Joardar M, Ghosh B, Roychowdhury T (2020) Quality and health risk evaluation for groundwater in Nadia district, West Bengal: an approach on its suitability for drinking and domestic purpose. Groundw Sustain 10:100351
Sahu M, Sar SK, Dewangan R, Baghel T (2019) Health risk evaluation of uranium in groundwater of Bemetara district of Chhattisgarh state, India. Environ Dev Sustain 1-20
Sharma T, Sharma A, Kaur I, Mahajan RK, Litoria PK, Sahoo SK, Bajwa BS (2019) Uranium distribution in groundwater and assessment of age dependent radiation dose in Amritsar, Gurdaspur and Pathankot districts of Punjab, India. Chemosphere 219:607–616
Sahu M, Sar SK, Baghel T, Dewangan R (2020) Seasonal and geochemical variation of uranium and major ions in groundwater at Kanker district of Chhattisgarh, central India. Groundw Sustain Dev 10:100330
Ahamad A, Madhav S, Singh P, Pandey J, Khan AH (2018) Assessment of groundwater quality with special emphasis on nitrate contamination in parts of Varanasi City, Uttar Pradesh. India. Appl Water Sci 8(4):115
Yadav SK, Ramanathan AL, Kumar M, Chidambaram S, Gautam YP, Tiwari C (2020) Assessment of arsenic and uranium co-occurrences in groundwater of central Gangetic Plain, Uttar Pradesh. India Environ Earth Sci 79:154
Ahamad A, Raju NJ, Madhav S, Khan AH (2020) Trace elements contamination in groundwater and associated human health risk in the industrial region of southern Sonbhadra, Uttar Pradesh India. Environ Geochem Health 42(10):3373–3391
Ahamad A, Raju NJ, Madhav S, Gossel W, Wycisk P (2019) Impact of non-engineered Bhalswa landfill on groundwater from Quarternary alluvium in Yamuna flood plain and potential human health risk, New Delhi, India. Quat Int 507:352–369
Sharma S, Kaur J, Nagpal AK, Kaur I (2016) Quantitative assessment of possible human health risk associated with consumption of arsenic contaminated groundwater and wheat grains from Ropar Wetand and its environs. Environ Monit Assess 188(9):506
Prasad M, Kumar GA, Sahoo SK, Ramola RC (2019) Health risks associated with the exposure to uranium and heavy metals through potable groundwater in Uttarakhand state of India. J Radioanal Nucl Chem 319:13–21
Taukdar BC, Chaudhary PK, Pathak KM (1983) On uranium contamination in water. Indian J Pure Appl Phys 21:381–382
Kakati RK, Kakati L, Ramachandran TV (2013) Measurement of uranium, radium and radon exhalation rate of soil samples from Karbi Anglong district of Assam, India using EDXRF and Can technique method. APCBEE Procedia 5:186–191
Central Ground Water Board, I. (2013, September). Ground Water Information Booklet Nalbari District, Assam. Retrieved from http://cgwb.gov.in/District_Profile/Assam/Nalbari.pdf
Soil and land use Survey of India, (2009, April). Inventory of Soil Resources of Nalbari District, Assam Using Remote Sensing and GIS Techniques. Retrieved from https://slusi.dacnet.nic.in/srm/srmabstracts/SRM_42_Nalbari.pdf
Central Ground Water Board, I. (2013, September). Ground Water Information Booklet of Kamrup and Kamrup Metro District, Assam. Retrieved from http://cgwb.gov.in/District_Profile/Assam/Kamrup.pdf
APHA (2005) Standard methods for the examination of water and wastewater. American Public Health Association (APHA), Washington, DC, USA
Bronzovic M, Marovic G (2005) Age-dependent dose assessment of 226Ra from bottled water intake. Health Phys 88(5):480–485
DRIs (Dietary reference Intakes) (2005) Dietary reference intakes for water potassium sodium, chloride and sulphate. Panel on dietary reference intakes for electrolytes and water. In: Standing Committee on the Scientific Evaluation of Dietary References Intake Food and Nutrition Board, Institute of Medicine National Academies Press, Washington, DC,USA (www.npa.edu)
International Atomic Energy Agency (2011) Radiation protection and safety of radiation sources: International Basic Safety Standards. Interim Edition, General Safety Requirements Part 3, No. GSR Part 3 (Interim), IAEA, Vienna
USEPA (United States Environmental Protection Agency) (1999) Cancer risk coefficients for environmental exposure to radionuclides. Federal Guidance Report No. 13. EPA 402-R-99-001. Washington, DC
Sharma S, Kaur J, Nagpal AK, Kaur I (2016) Quantitative assessment of possible human health risk associated with consumption of arsenic contaminated groundwater and wheat grains from Ropar Wetland and its environs. Environ Monit Assess 188:506
AERB, Dae, 2004 Drinking water specifications in India Atomic Energy Regulatory Board Mumbai, India
Dang HS, Jaiswal DD, Parameswaran M, Krishnamony S (1994) Physical, anatomical, physiological and metabolic data for reference Indian Man—A proposal. Bhabha Atomic Research Centre, Mumbai
WHO (World Health Organization) (2004) Guidelines for drinking water quality, 3rd edn. WHO, Geneva
WHO (World Health Organization) (2006) Guidelines for drinking water quality recommendations (3rd edn., vol 1, pp 515) Geneva, WHO
Thivya C, Chidambaram S, Keesari T, Prasanna MV, Thilagavathi R, Adithya VS, Singaraja C (2016) Lithological and hydrochemical controls on distribution and speciation of uranium in groundwaters of hard-rock granitic aquifers of Madurai District, Tamil Nadu (India). Environ Geochem Health 38:497–509. https://doi.org/10.1007/s10653-015-9735-7
Liesch T, Hinrichsen S, Goldscheider N (2015) Uranium in groundwater — Fertilizers versus geogenic sources. Sci Total Environ 536:981–995
Roselli C, Desideri D, Meli MA (2009) Radiological characterization of phosphate fertilisers: Comparison between alpha and gamma spectroscopy. Microchem J 91(2):181–186
Wetterlind J, Richer De Forges AC, Nicoullaud B, Arrouays D (2012) Changes in uranium and thorium contents in topsoil after long-term phosphorus fertilizer application. Soil Use Manag 28:101–107
Nolan J, Weber KA (2015) Natural uranium contamination in major US aquifers linked to nitrate. Environ Sci Technol Lett 2(8):215–220
Baik MH, Park CK, Cho WJ (2007) Migration and retardation properties of uranium through a rock fracture in a reducing environment. J Nucl Fuel Cycle Waste Technol (JNFCWT) 5:113–122
Kale A, Bandela N, Kulkarni J (2019) Assessment of chemo-radiological risk of naturally occurred uranium in groundwater from Beed district, India. J Radioanal Nucl Chem 323:151–157
Selvi BS, Vijayakumar B, Rana BK, Ravi PM (2016) Distribution of natural Uranium in groundwater around Kudankulam. Radiat Protect Environ 39:25–29
Almeida RM, Lauria DC, Ferreira AC, Sracek O (2004) Groundwater radon, radium and uranium concentrations in Regiao dos Lagos, Rio de Janeiro State Brazil. J Environ Radioact 73(3):323–334
Funding
Board of Research in Nuclear Sciences (BRNS), Department of Atomic Energy, Government of India, (BRNS Project Ref. No. 36(4)/14/12/2014-BRNS/1124-12 dated 17 July 2014)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Saikia, R., Chetia, D. & Bhattacharyya, K.G. Estimation of uranium in groundwater and assessment of age-dependent radiation dose in Nalbari district of Assam, India. SN Appl. Sci. 3, 21 (2021). https://doi.org/10.1007/s42452-020-04071-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-020-04071-5