Abstract
The potential of using granite dust as reinforcement into polybenzoxazine matrix was investigated. In this article, novel granite powder waste-reinforced bisphenol-A aniline-based benzoxazine composites were prepared using solution blending technique by varying the content of granite powder from 10 to 40 wt%. The effect of the granite powder content on the thermal, structural, dimensional, and morphological properties of granite powder/polybenzoxazine composites have been investigated. Thermogravimetric analysis (TGA) was performed on the composite samples. Char yield of the composites increased from 44.55 to 67.70% for increasing filler content from 10 to 40wt%, whereas 22% for pure polybenzoxazine. The maximum weight loss temperatures of the composites were analyzed from derivative of thermogravimetric analysis (DTG). Limiting oxygen index (LOI) values also increased as granite powder content increased. Structural properties of benzoxazine, polybenzoxazine, and composites were observed with Fourier-transform infrared spectroscopy (FTIR). Dimensional stability of the composites was investigated through the water absorption test up to 30 days. The composites exhibited zero percent water absorption. Micro-hardness of the composites increased from 17.45 to 78.66% for increasing filler content from 10–40 wt% when compared with pristine polybenzoxazine. The morphological analysis using scanning electron microscopy (SEM) showed the distribution of filler, aggregate formation, compatibility between polybenzoxazine and granite powder. Overall, the importance of using granite powder waste as reinforcement in polybenzoxazine composites revealed from results of the structural, dimensional, and morphological analysis along with the improvement in thermal properties and micro-hardness.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
A million tonnes of stone production take place throughout the world every year, which causes the generation of massive waste. Granite slurry waste is a product obtained while shaping granite rocks. This is mainly composed of quartz, small content of mica, feldspar, amphiboles, and other elements [1]. The importance of the work is to utilize granite stone waste which is anti-biodegradable and causing various problems if it mixes into the air or when it is left on the ground. Currently, industrial waste-reinforced polymer composites are gaining researchers and industrialist’s attention [1,2,3]. Yet little research done on the use of waste granite powder as the filler in thermosets has been published so far. Pawar M.J. et al. reported about granite powder, jute fiber, and carbon fiber, granite powder-reinforced epoxy composites [4, 5]. Shujit et al. presented in a paper about polyester composites reinforced with granite powder scrap [6]. Wear testing was performed on glass fiber and granite powder-reinforced epoxy composites by Nallusamy and Karthikeyan [7]. Mathavan, J.J., and Amar, P. analyzed wear characteristics of polymer composite reinforced with granite powder for wind turbine blade [8]. Therefore, if the granite powder (GNT) is used as a filler, it would be advantageous to minimize the cost of the composites and it will be beneficial in granite powder waste management [9]. GNT is used for the preparation of tiles; bricks and fly ash magnesium oxychloride cement; and filler for roads [10,11,12].
The benzoxazine resin is having excellent molecular design flexibility, near-zero volumetric change upon curing, high heat resistance, chemical resistance, low dielectric constant, excellent thermal and mechanical properties, very low melt viscosity, low flammability [13,14,15,16,17]. Even though all these properties of polybenzoxazine (PBZ) lead to many applications such as electronic packaging materials, coatings, composites, and adhesives, its brittleness and high curing temperatures are having few disadvantages of PBZ which restricts to some more applications [17].
The granite powder has high compressive strength (greater than 200 MPa) and availability, because of that it is drawing researcher’s attention as a filler in thermosetting composites [28]. The granite powder has been reinforced with polypropylene, polyester, and more with epoxy resins [6, 27, 29]. However, challenges associated with granite particles as a filler are incompatibility between polymer and GNT, tendency to form aggregates during processing, and low resistance to moisture [18]. These challenges are expected to overcome by reinforcing GNT into polybenzoxazine due to low melt viscosity, molecular design flexibility, and moisture resistant properties of benzoxazine.
In this paper, thermal, structural, dimensional, and morphological properties of granite powder-reinforced bisphenol-A aniline-based polybenzoxazine (BPA-PBZ/GNT) composites were evaluated along with micro-hardness. The granite powder content in the resin varied from 10 wt% to 40 wt%. Curing of the composites took place in a vacuum oven. The raw granite powder (without any treatment) was used as filler. Granite particles have higher melting point temperature, high oxidation resistance, and excellent strength over other materials such as silica. Studies proved that improvement of mechanical and thermal properties is possible by reinforcing fumed silica into polybenzoxazine [19]. Therefore, this study is expected to improve thermal and physical properties by using granite powder as filler in polybenzoxazine matrix. The composites expected to exhibit a substantial increase in char yield, LOI, micro-hardness over pristine polybenzoxazine, and the study of structural, morphological, and dimensional properties give great information about the composites.
2 Materials and methods
2.1 Materials
Bisphenol-A was supplied by Antares Chem Private Limited, Mumbai. Paraformaldehyde for synthesis 96%, aniline for synthesis 99%, 1,4-dioxane pure 99% were procured from Amaravathi Scientifics and Lab equipments, Guntur. Granite slurry waste was collected from CNR granites, Amaravati, India. Here, granite powder is obtained as the dust from the granite sawmill while cutting various granite rocks.
2.2 Synthesis of bisphenol-A aniline benzoxazine
The bisphenol-A aniline benzoxazine material is prepared using BPA, aniline, and paraformaldehyde at 1:2:4 molar ratio and dioxane used as the solvent. Bisphenol-A and aniline solution are stirred in a round bottom vessel at room temperature for not less than 30 min. Then, the temperature of the mixture reduced to below 5 °C with the help of an ice tub to add paraformaldehyde and dioxane. Then, the reaction vessel was heated for 1 hour at 60 °C and the temperature increased to 120 °C followed by constant stirring for 5 h at 300 rpm. An orange-colored benzoxazine monomer residue was obtained which is used as matrix material.
2.3 Filler preparation
Granite slurry waste obtained while sizing the granite rocks was collected from CNR granites, Amaravati, India. It was dried under sunlight for a month and further dried for 48 h in an oven at 200 °C to remove moisture. Then, oven-dried granite powder was sieved with 425 µ mesh to maintain the uniform size of the particles and the same is used as filler.
2.4 Methods
Degradation or weight loss of the composite samples was investigated using thermogravimetric analyzer (TGA) model Hitachi STA-7200 instrument. The samples taken to investigate were in the range of 10 to 20 mg, heated from 30 to 800 °C under nitrogen purging of 20 ml/min at a heating rate of 20 o C /min. The maximum weight loss temperature of the composites was analyzed from derivatives of thermogravimetric analysis (DTG). FTIR spectra for the composites were recorded from 4000 to 400 Cm−1 on an Agilent Cary 630 FTIR spectrometer instrument. Micro-hardness of the composites was tested using digital Vickers micro-hardness tester HVS1000B, Daksh quality systems Pvt. Ltd., Ahmadabad, India, equipped with an inbuilt printer, hardness conversion facility, automatic digital hardness, reading with a load range from 10 gm up to 1000 gm with the latest LED light facility for crystal clear vision, automatic dwell time selection, and magnification 100x, 400 x. The samples were tested at 100 gf and 10 s of constant load and dwell time. The indentation was measured by a micro-metric eyepiece of 400 objective lens magnification. The micro-hardness of the samples reported was the average of four readings.
The composites of dimensions 35 mm dia and 4 mm thick were prepared to evaluate the water uptake of the composites. The composite specimens were oven-dried at 70 °C till the weight of the composites reached to constant weight, before the insertion of the samples into distilled water. The values tabulated are the average of three samples. The samples were taken out every 24 h and wiped surface water with cotton, re-checked the weight of each sample, and immersed back immediately into the distilled water. Water absorption of the composites was measured using the formula as follows.
The morphology of the composites (BPA-PBZ/GNT) and interfacial adhesion between GNT and PBZ were examined by scanning electron microscopy (TESCAN, VEGA 3 SBH, CZECH Republic) at an acceleration voltage of 10 kV.
3 Results and discussion
3.1 Preparation of granite powder/polybenzoxazine composites
Benzoxazine monomer which is in solid form was taken into four round bottom flasks and dioxane of appropriate proportion added to it to dissolve the benzoxazine. The proper dissolving of benzoxazine happened by stirring the content for 30 min. Granite powder particles of four weight ratios such as 10, 20, 30, and 40 wt% were added to the matrix in a round bottom flasks and stirred overnight for the homogeneous mixing and thereby wetting of the filler. The mixtures were gently poured into four different Petri dishes of 100 mm dia. The Petri dishes were kept at 50 °C in an oven overnight, to evaporate the solvent. Curing of the samples was done at the rate of 20 °C/hour from 100 to 220 °C and then cooled to ambient temperature [20]. The cured composites were tough and appeared in dark gray color with glassy finish. The granite powder-reinforced bisphenol-A aniline-based polybenzoxazine composites were abbreviated as BPA-PBZ/GNT 10, BPA-PBZ/GNT 20, BPA-PBZ/GNT 30, and BPA-PBZ/GNT 40 based on the weight percentage of the granite powder.
3.2 Fourier-transform infrared spectroscopy (FTIR)
The structure of benzoxazine monomer was confirmed through FTIR. The FTIR plot of bisphenol-A aniline-based benzoxazine monomer and polymer is depicted in Figs. 1, 2 [21].
The characteristic absorption peak at 941 cm−1 indicates the oxazine ring [22]. Also, 1230 cm−1 indicates the C–O–C asymmetric as well as symmetric stretching vibrations. C–N–C asymmetric and symmetric stretching vibration observed at 1118–1157 cm−1 and around 822 cm−1 confirmed the structure of the benzoxazine ring. The sharp and very strong bands at 1494 cm−1 and the bands with medium intensity at 1597 cm−1 correspond to the tri-substituted structure of the benzene ring with in-plane as well as the out-of-plane bending mode of C-H. The bands at 753 and 691 cm−1 are assigned to the monosubstituted benzene in the skeleton of bisphenol-A. There is an OH peak at 3412 cm−1, and it may be due to the ring-opening mechanism of benzoxazine monomer on a small scale.
The curing behavior of benzoxazine was analyzed by FTIR spectra. The characteristic absorption bands at 941 cm−1 are due to the formation of benzoxazine structure, symmetric and asymmetric stretching of C–O–C, and CH2 wagging at 1325 cm−1, and tri-substituted benzoxazine ring at 1494 and 822 cm−1 disappeared completely, due to complete opening of the ring. The strong absorption bands assigned to the asymmetric stretching of the C–N–C shift around 1120 cm−1. The new absorption band is visible around at 1702, 1491 cm−1, which are ascribed to intermolecular hydrogen-bonded phenolic OH and tetra-substituted benzoxazine ring [23, 24]. These show that the ring-opening cross-linking of the bisphenol-A aniline-based benzoxazine produced linkage of Mannich bridge and phenolic hydroxyl groups. FTIR spectra of 10 to 40 wt% granite dust-filled polybenzoxazine composites are represented in Fig. 3. The presence of bands at 690 cm−1, 580 cm−1 was due to the presence of Si–O (quartz), rich content of iron oxides in granite dust, and Swaminathan et al. reported that there was no change in the structure of granite powder at higher temperatures up to 900 °C [25]. The comparison of FTIR spectra of benzoxazine monomer, polymer, and composites showed that 941 cm−1, 1325 cm−1, 1494 and 822 cm−1 are also disappeared completely in Fig. 3 due to complete oxazine ring opening by formations of composites.
3.3 Scanning electron microscopy (SEM)
Morphology of the GNT/PBZ composites was investigated using scanning electron microscopy (SEM) as depicted in Fig. 4. This revealed substantial compatibility between the PBZ matrix and GNT filler because of stirring the benzoxazine and granite filler mixtures overnight for the wettability of the filler. Strong compatibility between GNT and PBZ is vital for the improvement of thermal properties and hardness of the composites. The dispersion of granite particles into the matrix was observed from the SEM images and concluded that granite particles were well distributed throughout the matrix. Additionally, the penetration of benzoxazine into the microstructure of granite powder was observed which causes the PBZ to become mechanically interlocked through granite powder irregularities. The granite powder tends to form aggregates when mixed with polymers, [18], but here from the SEM analysis, aggregates formation was not observed, that is there was no visible accumulation of matter found, which may be due to low A-stage viscosity of the polybenzoxazine resin.
3.4 Thermogravimetric analysis (TGA)
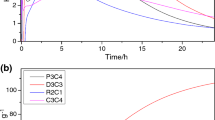
BPA-PBZ/GNT composites thermal stability, char yield, and degradation temperatures were analyzed using thermogravimetric analysis, and the supporting plots are depicted in Fig. 5. The temperatures of 5% and 10% weight loss (T5, T10), amount of char yield (CY) and estimated char yield (when independently decomposed) at 800 °C, and LOI are shown in Table 1. As the GNT content increased, char yield of the composites also increased, and this is 22% for the neat resin and 67.70% for the highly filled system. Polybenzoxazine individually recorded less char yield, whereas the GNT/PBZ composites recorded an increase in char yield for the increase in GNT content. From this, it was obvious that the addition of granite powder char yield of the composites is also increasing substantially. Shujith, C.P. et al. reported an increase in char yield to 62% for the addition of up to 50 wt% of granite powder scrap in polyester resin, respectively, as compared to nearly 0% for unfilled polyester resin at 550 °C temperature cell [6]. The T5 values of 10, 20, 30, and 40 wt% granite powder-filled composites were 240.16, 242.27, 245.35, and 280.41, respectively. The weight loss temperature of composites increased with increase in GNT content due to high resistivity of filler as well as polybenzoxazine to the temperature. Even though T5, T10 values of composites increase with respect to increase in GNT content, decrease in T5, T10 values is observed in comparison of pure polybenzoxazine and P(BPA-BZ)/GNT 10 due to less degradation of temperature of polybenzoxazine over GNT and less influence of 10 wt% of GNT over 90 wt% of polybenzoxazine, but the rate of weight loss of granite powder-filled composites is found to be decreased as compared to pure polybenzoxazine due to higher temperature resistivity of granite powder. The initial weight loss of the composites started at about 200 °C, and from 200 °C to 350 °C initial weight loss because degradation of benzoxazine starts at about 220 °C as reported by Sarawut et al., and 350–550 °C secondary major degradation and 550–800 °C final degradation of the composites occurred [20]. From Fig. 5, it is clear that the degradation of the composites occurred in two steps because of presence of GNT particles, whereas single step degradation for pure PBZ within the temperature range 200–550 °C. The initial lower decomposition of composites was because of the hydrated water evaporation of granite powder crystal components as GNT powder consists completely crystal components [6]. The TGA of granite powder was tested up to 800 °C based on the limitation of the equipment available, and no major degradation observed up to 800 °C. The maximum weight loss temperatures (Tmax) of the composites were analyzed from DTG plot as shown in Fig. 6. The increase in granite powder content slightly reduced the Tmax of the composites, i.e., from 396 to 392 °C when compared with the pure polybenzoxazine, and interestingly Tmax remains same for all the composites irrespective of the raise in granite powder content due to high temperature resistance of granite powder. Limiting oxygen indices (LOI) of the composites at 800 °C were calculated using the relation between LOI and char yield [26], i.e.,
where CY is the char yield of respective composites at 800 °C.
LOI is the percentage of oxygen that supports the combustion of the polymer under nitrogen and oxygen purging. The composite’s LOI values increased with an increase in filler content due to an increase in char yield because the char yield of the composite is directly proportional to LOI.
3.5 Water absorption
Water absorption test is conducted from 1 to 30 days continuously on granite powder filled polybenzoxazine composites with GNT varying from 10 to 40 wt%. The water absorption of the composites remains the same irrespective of the weight percent of the filler. BPA-PBZ/GNT composites recorded 0% water absorption due to water resistant or low water absorption matrix material polybenzoxazine as reported by Sarawut, R. et al. [20].
3.6 Micro-hardness
Vickers micro-hardness test results of the composites are shown in Fig. 7. A significant increase in micro-hardness of the BPA-PBZ/GNT materials was found compared to pristine polybenzoxazine. The composite’s hardness was found to rise from the observed value of 353.93 MPa for the PBZ to the values of 415.71, 509.08, 560.17, and 632.35 MPa with the respective GNT contents of 10, 20, 30, and 40% by weight, which is about 17.45–78.66% increase in MPa. This was due to the prevention of the deformation of polybenzoxazine by GNT particles. Therefore, micro-hardness of the composites increased with the rise of GNT content. The hardness of granite powder-reinforced epoxy composites also increased with an increase in filler content [27]. Pawar et al. reported improvement of hardness from 372.7 to 431.5 MPa for the reinforcement of granite powder from 8 to 24 wt% into epoxy matrix, respectively [4]. Hence, the hardness of the granite powder-reinforced polybenzoxazine composites is more than that of epoxy-based composites.
4 Conclusions
The polybenzoxazine was prepared by using the solvent method and industrial waste such as granite powder effectively used as reinforcement material. The effect of the granite powder content on micro-hardness and thermogravimetric, morphological, structural properties of BPA-PBZ/GNT materials was examined. The properties of granite powder-reinforced polybenzoxazine composites were concluded as follows. Along with increase in GNT content char yield of the composites increased that is 22% for the neat resin and 67.70% for the highly filled system. From this, it was obvious that the addition of granite powder char yield of the composites increases substantially. Moreover, degradation temperatures at 10% and 15% weight loss were reported. The limiting oxygen indices values also increased with increase in granite powder. The water absorption of the composites was recorded as zero percent irrespective of filler content and the number of days immersed in water. Significant improvement in micro-hardness was recorded with the increase in filler content that is from 17.45–78.66% in HV. The morphology of the composites was studied using SEM and concluded that there was good compatibility between granite powder and polybenzoxazine. Therefore, these materials are best suited for marine applications due to its zero percent water absorption and high hardness.
References
Kareem AA (2013) Mechanical properties of granite powder as a filler for polycarbonate toughened epoxy resin. Int J Pharm Sci 3:254–257
Ramakrishna HV, Padmapriya S, Rai SK (2007) Flexural, compression, chemical resistance and morphology studies on granite powder filled epoxy and acrylonitrile butadiene styrene (ABS) toughened epoxy matrices. J Appl Polym 104:171–177
Ramakrishna HV, Padmapriya S, Rai SK (2006) Effect on the mechanical properties and water absorption of granite powder composites on toughening epoxy with unsaturated polyester and unsaturated polyester with epoxy resin. J Appl Polym 25:17–32
Pawar MJ, Patnaik A, Ravindra N (2015) Investigation on mechanical and thermo-mechanical properties of granite powder filled treated jute fiber reinforced epoxy composite. Polym Compos 38:1–13
Pawar MJ, Patnaik A, Ravindra N (2016) Mechanical and thermo-mechanical analysis based numerical simulation of granite powder filled polymer composites for wind turbine blade. Fiber Polym 17:1078–1089
Shujit CP, Muhammed YM, Abdul G, Rajib CD (2017) Study of thermal properties of granite powder (scrap) reinforced polyester resin composite. J Adv Chem Eng 7:1–5
Nallusamy S, Karthikeyan A (2017) Synthesis and wear characterization of reinforced glass fiber polymer composites with epoxy resin using granite powder. J Nano Res-Sw 49:1–9
Mathavan JJ, Amar P (2020) Analysis of wear properties of granite dust filled polymer composite for wind turbine blade. Results Mater 5:1–4
Baskaran R, Sarojadevi M, Vijayakumar CT (2014) Utilization of granite powder as filler for vinyl ester resin. M P J 9:39–44
Romualdo RM, Heber SF, Gelmires AN, de Helio LL, Heber CF (2005) Use of granite sawing wastes in the production of ceramic bricks and tiles. J Eur Ceram 25:1149–1158
Monteiro SN, Pecanha LA, Vieira CMF (2004) Reformulation of roofing tiles body with addition of granite waste from sawing operations. J Eur Ceram 24:2349–2356
Ying L, Hongfa Y, Lina Z, Jing W, Chengyou W, Yongshan T (2013) Compressive strength of fly ash magnesium oxychloride cement containing granite wastes. Constr Build Mater 38:1–7
Ghosh NN, Kiskan B, Yagci Y (2007) Polybenzoxazines-new high performance thermosetting resins: synthesis and properties. Prog Polym Sci 32:1344–1391
Yagci Y, Kiskan B, Ghosh NN (2009) Recent advancement on polybenzoxazine-a newly developed high performance thermoset. J Polym Sci Part A Polym Chem 47:5565–5576
Ishida H (2011) Overview and historical background of polybenzoxazine research. In: Ishida H, Agag T (eds) Handbook of benzoxazine resins. Elsevier, USA, pp 3–69
Ohashi S, Ishida H (2017) Various synthetic methods of benzoxazine monomers. In: Ishida H, Froimowicz P (eds) Advanced and emerging polybenzoxazine science and technology, pp 3–8. Elsevier, USA
Kiskan B, Ghosh NN, Yagci Y (2011) Polybenzoxazine-based composites as high performance materials. Polym Int 60:167–177
Balakrishna SS, Girish H, Mohan Kumar GC, Narendranath S (2016) Analysis on mechanical and dynamic behaviour of granite epoxy composites with cast iron particulates as filler. Indian J Adv Chem Sci S1:122–126
Isala D, Chanchira J, Tsutomu T, Sarawut R (2014) High thermal and mechanical properties enhancement obtained in highly filled polybenzoxazine nanocomposites with fumed silica. Compos B Eng 56:197–206
Sarawut R, Chanchira J, Sunan T (2013) Introduction to commercial benzoxazine and their unique properties. In: Sarawut R, Chanchira J, Sunan T (eds) Alloys and composites of polybenzoxazines. Springer, Singapore, pp 1–27
Das DLJ, Rajeev R, Rajeev RS, Santhosh Kumar KS (2013) Synthesis, characterization, curing and thermal decomposition kinetics of bisphenol-a based polybenzoxazine. Int J Sci Technol Res 2:146–155
Dunkers J, Ishida H (1995) Vibrational assignment of N, N-bis (3, 5-dimethyl-2-hydroxybenzyl) methylamine in the fingerprint region. Spectrochim Acta 51:855–867
Garea SA, Lovu H, Nicolescu A, Calin D (2007) Thermal polymerization of benzoxazine monomers followed by GPC, FT-IR and DETA. Polym Test 26:162–171
Yousefi A, Lafleur PG (1997) Kinetic studies of thermoset cure reactions: a review. Polym Compos 18:164
Swaminathan D, Balasubramani G, Thirunavukkarasu R (2009) Utilization of granite and marble sawing powder wastes as brick materials. Carpath J Earth Env 4:147–160
Ranganathan T, Beaulieu M, Zilberman J, Kenneth DS, Phillip RW, Richard JF, Bryan EC, Todd E (2008) Thermal degradation of deoxybenzoin polymers studied by pyrolysis-gas chromatography/mass spectrometry. Polym Degrad Stab 93:1059–1066
Jorge AVG, Diego ATC, Gislane DJO, Maria DLDSR, Marcelo AM (2014) Mechanical properties of epoxy resin based on granite stone powder from the sergipe fold-and-thrust belt composites. Mater Res 17:878–887
Arivumangai A, Felixkala T (2014) Strength and durability properties of granite powder concrete. J Civ Eng Res 4:1–6
Awad AH, Ahmed WAG, Ayman AAE, Ramadan E, Mohamed HA (2020) The influence of adding marble and granite dust on the mechanical and physical properties of PP composites. J Therm Anal Calorim 140:2615–2623
Acknowledgements
The authors would like to acknowledge the funding allotted by Vignan’s Foundation for Science Technology and Research, Guntur, India, and the support provided by Professors and research scholars of Chemical Engineering and Mechanical Engineering departments. The author would also like to thank the researchers/academicians whose works have been cited directly or indirectly in this paper.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Garigipati, R.K.S., Malkapuram, R. Characterization of novel composites from polybenzoxazine and granite powder. SN Appl. Sci. 2, 1545 (2020). https://doi.org/10.1007/s42452-020-03333-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-020-03333-6