Abstract
The present work attempted to investigate the effect of SiO2 addition on the dielectric properties and microstructure of the ZnNiO2—ceramics. The content of added SiO2 is in the range 5–40 wt%, while that of the other additives keeps constant. The given samples were sintered at 1000–1300 °C for 120 min, with conventional ceramic processing method. By X-ray diffraction, Zinc silicate, Nickel silicate, Quartz, Nickel Zinc Oxide, Zinc Oxide and Nickel oxide were found in all the samples, and no extra phases were identified in the detection limit. Minimum water absorption was present in specimens fired at 1300 °C for 120 min. The DTA pattern shows a clear indication of five maxima happening at the point 104.1 °C, 112 °C, 109.5 °C, 106 °C, and 117.9 °C, respectively. SEM studies show that the increase of SiO2 wt% had significant influence on grain growth and microstructural characteristics of the sintered specimens. There are agglomerated fine particles of NiO, because it has high surface tension. The electrical properties of the samples varied with the increase content of SiO2. The capacitance (pF) and, dielectric constant (ɛ−), decrease when SiO2 content increase and with increasing frequency whereas, conductivity increased with increase content of SiO2.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Transition metals oxides are an important class of semiconductors, which have many applications such as magnetic storage media, solar energy transformation, electronics and catalysis, gas sensors, varistors, photocatalyst, electrochemistry, photoconductive and photothermal applications. These compounds are characterized by the in complement d-shell of the metal cations, which makes them the best material in consideration of the electrical properties [1]. SiO2 has excellent dielectric properties, very high resistivity of ~ 1018 cm and a large band gap of ~ 9 eV. Silica generally has served as the dielectric material for silicon-based microelectronic applications in decades, good resistance, electrical insulation, and good thermal stability. Also, fused silica used as good dielectric, insulating properties, and refractory materials or investment casting. Quartz is an insulator with band gaps ~ 6, used as substrates for supported catalysts. Their different applications are also shaped dependent [2,3,4]. Nickelous oxide nanoparticles, is an important metal oxide acts as a P-type semiconductor, has band gap ranged (3.6–4.0 eV) and high thermal stability, used in catalysis, gas sensing, battery cathodes and magnetic materials [5,6,7,8]. Pure stoichiometric NiO crystals are perfect insulators but it becomes a conductive substance by substituting Li for Ni at Ni sites or creating Ni vacancies [9, 10]. Also, NiO has excellent durability, electrochemical stability, low material cost, and large span optical density. ZnO oxide is a II–VI compound semiconductor with wurtzite structure [11, 12]. ZnO nanostructure have been explored a wide range applications in nanosclale devices such as, transparent conductors, solar cell windows, gas sensors, emission transistors, ultraviolet photo-detectors and surface acoustic wave devices because of their n-type conducting with an optical transparency in the visible range [13,14,15]. It has a wide band gap (3.37) eV in the near-UV spectral region, and a large free-exaction binding energy (60 meV) [16,17,18,19,20,21]. In this study, ZnO, NiO, and SiO2 were used as source materials to format ZnO–NiO–SiO2 compositions by using solid-state reaction method. The influences of sintering temperatures and SiO2 wt % on the crystal structures and dielectric properties of all compositions are developed.
2 Experimental procedure
The ceramic materials ZnO, NiO, and SiO2 were prepared by the solid-state reaction method. Reagent-grade raw materials of ZnO, NiO, and SiO2 with higher than 99.5% purity were used as starting materials, mixed according to the compositions. Batches were abbreviated as S1, S2, S3, S4, S5, and S6 (see Table 1). After ball-milled for 3 h with deionized water, dried, and ground, then the samples were calcined at 800 °C for 120 min. After calcinations, the samples were ground again, and then polyvinyl alcohol (PVA) as a binder was added. Two discs were used, the first one has 1.2 cm diameter and 0.2 cm thickness, and the second disc has 5 cm diameter and 0.2 cm thickness. These two discs were processed by a semi-dry press method under 70 kN. Small specimens were subjected to thermal treatment to select the proper maturing temperature for each mix. Three discs were always fired in muffle kiln with a rate of heating of 5 °C/min in the temperature range between 1000 and 1300 °C and for 120 min and cooled to room temperature freely. The sinterability of the different samples was determined in terms of physical properties. The sintering behavior was investigated by differential thermal analysis (DTA–TG) instruments, using a heating rate of 10 °C/min in air from room temperature up to 1000 °C. The crystal structures of different samples were analyzed using XRD patterns with using CuKa radiation. The microstructure of ZnO–NiO–SiO2 ceramics were observed by using scanning electronic micrograph (SEM) type Philips XL 30 provided with EDS. The dielectric properties and conductivity as a function of frequency of sintered samples were measured using (The PM 6304 program able automatic RCL meter).
3 Results and discussion
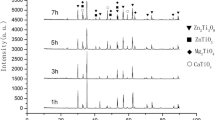
Results of density are shown in Fig. 1. A increase in density, firing shrinkage and decrease in water absorption were recorded with the rise in temperature. A maximum density, firing shrinkage and Minimum water absorption were attained in specimens fired at 1300 °C for 120 min. Maximum density in a sample (S1) containing, 40 mol% ZnO, 55 mol% NiO, and 5 mol% SiO2 fired at 1300 °C for 2 h. The content of pores and open governs the degree of densification reached at maturity. Minimum water absorption does not mean maximum densification. Also, the content of closed pores got affected to a greater extent the sinter ability of the ceramic samples produced. Usually, a percent not exceeding 98% was displayed by different mixes. Minimum water absorption in the sample (S2) which containing, 40 mol% ZnO, 50 mol% NiO, and 10 mol% SiO2 fired at 1300 °C for 2 h as shown in Fig. 2. Results of firing shrinkage as a function of the temperature of different mixes are given in Fig. 3. XRD analysis of sintered sample reveals to the formation of new phases and lattice constants of phase were change after sintering. Results of XRD patterns of different mixes sintered at 1300 °C are present in Fig. 4. It is easy to identify the main phase ZnO, Ni ZnO (Ni.8Zn.1O), NiO, zinc silicate and nickel silicate by fitting d spacing data. The results show that Zn2SiO4 and Olivine Ni2SiO4 generate during sintering, but the d-spacing data do not exactly match with those of JCPDS card. Then Zn2SiO4 and Ni2SiO4 crystallized at the ZnO boundary during furnace cooling and formed the potential barrier, and there is a little difference in d-spacing between Zn2SiO4 in the samples. Therefore, the crystallized Zn2SiO4 is a solid solution dissolving and other elements. SiO2 does not react completely with others at 1300 °C because of the evidence of SiO2 existing in the samples sintering at 1300 °C as shown in Fig. 4. It is easy to identify the main phase willemite Zn2(SiO4) (PDF card no. 01-070-1235) as the major phase, B: Liebenbergite Ni2SiO4 and Nickel-zinc oxide, zinc silicate and nickel silicate by fitting d spacing data. It is easy to identify the main phase willemite Zn2(SiO4) (PDF card no. 01-070-1235) as the major phase, B: Liebenbergite Ni2SiO4 and Nickel-zinc oxide, zinc silicate and nickel silicate by fitting d spacing data.
Figure 5 show the differential thermal analysis DTA thermogram of a sample [S2]. The thermogram shows an endothermic effect in which, the onset of the peak at 32.2 °C, the offset at 297.1 °C, the mix/min at 104.1 °C is equal to − 16.436 μv, and heat change is equal to − 1220.808 μVs/mg. Figure 6. Show the differential thermal analysis DTA thermogram of a sample S6. The thermogram shows an endothermic effect in which, the onset of the peak at 36.2 °C, the offset at 298.3 °C, the mix/min at 109.5 °C is equal to − 16.817 μv, and heat change is equal to − 1503.706 μVs/mg. Figure 7 shows the TG thermogram of sample S2, in which, the mass of the sample at 34.6 °C is equal to 9.496 mg, a mass of the sample at 84.5 °C is equal to 9.313 mg, and mass change is equal to − 0.18 mg. In addition, the mass of the sample at 84.5 °C is equal to 9.313 mg, a mass of the sample at 195.3 °C is equal to 8.965 mg, and mass change is equal to − 0.35 mg. In addition, the mass of the sample at 195.3 °C is equal to 8.965 mg, a mass of the sample at 352.3 °C is equal to 8.650 mg, and mass change is equal to − 0.31 mg. Figure 8. Shows the TG thermogram of sample S6, in which, a mass of the sample at 56.3 °C is equal to 9.056 mg, a mass of the sample at 207.5 °C is equal to 8.493 mg, and mass change is equal to − 0.56 mg. In addition, the mass of the sample at 207.5 °C is equal to 8.493 mg, a mass of the sample at 268.9 °C is equal to 7.975 mg, and mass change is equal to − 0.52 mg. In addition, the mass of the sample at 268.9 °C is equal to 7.975 mg, a mass of the sample at 344.2 °C is equal to 7.758 mg, and mass change is equal to − 0.22 mg.
SEM micrographs of ZnO–NiO–SiO2-based ceramics with different sintering temperature. It can be seen that the microstructure consisted of only two phases; ZnO grain (bulk phase) and intergranular layer (second phase) consisted of Ni- and Si-rich phase located at the boundaries as indicated by EDAX. The microstructure was more densified with increasing sintering temperature. All of these compositions led to dense ceramic bodies after sintering at 1000 °C or 1300 °C, with microstructures similar to Figs. 9, 10 and 11. Also, the microstructure of ZnO–NiO–SiO2 sintered specimens ‘show uniformly sized grains of average size less than 10 pm. Liquid phase sintering can produce smaller grains than that formed by solid state process. It can be concluded that the effect of SiO2 addition was demonstrated not only by the decrease of the sintering temperature but also by densification of ZnO–NiO–SiO2 ceramics. SEM of mix S2 is shown in Fig. 9. There are different shades, the main bulk formed of grains of ZnO with smaller white nodal shaped grains, lying intergranular. EDAX of these grains indicates that they are a mixture of Ni and Zn. The decrease of grain size is attributed to the precipitation of secondary phase in the grain boundaries and nodal points. SEM of sample S2 shows the segregation of the intergranular phase in patches. This is evident from the different shades displayed varying from dark grey–light grey and white. The samples were of a very dense microstructure. Although some pores were present, they were very small and separated from one another. SEM micrographs in Fig. 10. show the microstructure of the Sample S3 after sintering at 1000 ◦C for 2 h. shows evidence of the existence of a liquid phase as well as relatively small grains, with an average size clearly below the micron (similar to the particles sizes of the starting materials) i.e. figure shows the microstructure of the as-prepared ZnO–NiO–SiO2 micro-composite (mix S3). The ZnO grains are homogeneous, NiO, SiO2 particles are small and distributed at the boundary of the ZnO grains. Elementary composition analysis by EDAX confirms that the Ni and Si contents are higher near the grain boundary than in the matrix (ZnO), indicating the formation of a Ni-rich phase along the ZnO grain boundaries. The SEM of the sample (S5) after sintering at 1000 °C for 2 h present in Fig. 9. Shows evidence of the presence of the liquid phase as well as relatively small grains, with an average size clearly below the micron (similar to the particle size of the starting materials. SEM of sample S5, after sintering at 1100 °C for 2 h shows the segregation of the intergranular phase in patches. This is evident from the different shades displayed varying from dark grey – light grey and white. The samples were of a very dense microstructure. Although some pores were present, they were very small and separated from one another. The SEM of sample S5 fired at 1200 °C/2 h clearly indicates the morphology of the particles to be roughly spherical and homogeneous. Some of the particles are agglomerates, and crystalline phase silica beads formed dense aggregates of spheres. SEM of sample S5 sintered at 1300 °C for 2 h reveals the randomly distributed aggregated micro rods of particles and the average diameter of micro rods ~ 1 to 2 µm.
SEM of sample S2, a S2 annealed at 1000 °C for 2 h X = 2000, b S2 annealed at 1000 °C for 2 h X = 20,000, c S2 at annealed at 1100 °C, X = 2000 shows more dense microstructure (crystalline phase), d S2 at annealed at 1100 °C, X = 10,000, e EDXA of sample S2 annealed at 1100 °C showing, the distribution of different elements in the intergranular accumulation, f S2 annealed at 1200 °C, X = 2000, g S2 annealed at 1200 °C, X = 10,000, h S2 annealed at 1300 °C, X = 2000, i S2 annealed at 1300 °C, X = 10,000, shows intraganular Ni, Si rich phase, more homogeneous and more dense microstructure
SEM of sample S5. a Annealed at 1000 °C for 2 h, thermally etched, general view X = 2000, b annealed at 1000 °C for 2 h, X = 20,000. c Annealed at 1100 °C for 2 h, thermally etched, general view X = 2000. d Annealed at 1100 °C for 2 h, thermally etched, general view X = 20,000. e At 1200 °C for hour 2 h, thermally etched, general view X = 2000. f At 1200 °C for 2 h, crystalline phase silica beads formed dense aggregates of spheres (X = 20,000). g at 1300 °C for 2 h, thermally etched, general view X = 2000. h at 1300 °C for 2 h showing glassy phase between the ZnO grains X = 20,000 (crystalline phase)
The dielectric properties of ZnO–NiO–SiO2 ceramic system were studied in the frequency range from 50 Hz to 5 MHz at room temperature. Figure 12a–d show the variation of the dielectric constant as a function of frequency for all samples. Obviously, the dielectric constant shows a decreasing trend for all the samples. The decrease is rapid at a lower frequency and slower and stable at the higher frequency. The decrease of dielectric constant with increasing frequency is a normal dielectric behavior which is also observed by other researchers [22,23,24,25,26,27]. This behavior may be due to the present of interfacial polarization and electron hopping takes place, [28], which exists in non-homogeneous dielectric and was explained by Koops and Maxwell–Wagner model [29,30,31]. Therefore, at a lower frequency the dielectric constant is maximum. For different mixes sintered at 1000 °C and 1100 °C for 2 h, the increase mol% SiO2 from the sample (S1–S6) leading to a maximum dielectric constant at all frequency related to S5. Generally the dielectric constant for all samples decreases with increasing frequency. This decrease may be due to the increased intergranular porosity resulting from discontinuous grain growth and the absence of the space charge polarization at the higher frequency. Also when annealed at 1200 °C and 1300 °C for 2 h. The increase mol% SiO2 from a sample (S1–S6) leading to a maximum dielectric constant at all frequency related to S3. This observation may be attributed to a combined contribution to the dielectric constant due to electric, ionic, interfacial polarization at low frequencies. At higher frequency, there are space charge polarizations in these materials. The results of dielectric loss as a function of frequency at room temperature of different groups sintered at 1000 °C, 1100 °C, 1200 °C and 1300 °C for 2 h are graphically plotted in Fig. 13a–d, all mixes exhibit decrease relation demonstrated by the value (ε−). The dielectric loss increase as the content of SiO2 increase from 5 to 40 mol% reaches a maximum value 3.14312 × 107 at log f = 2.3979(250 Hz) (0.25 kHz) in the sample (S3)annealed at 1300 °C for 2 h. The relation between conductivity and frequency for different mixes of different groups are shown in Fig. 14a–d. It increases with increasing frequency at room temperature. This may be attributed to the increase in the number of dipoles. The increase of frequency raised the conductivity because it increases the ionic response to the field again this is related to intergranular material at the field again this is related to intergranular material at any particular temperature. These effects are associated with polarization currents arising from trapping states of various kinds and densities. We can deduce that the increase of the percentage of SiO2 and frequency in the different samples leading to increasing of resistivity. ZnNiSiO4 are ceramic semiconductor materials, these components have specific resistance, it used as protection of electric and electronic devices.
References
Sunu SS, Prabhu E, Jayaraman V, Gnanasekar KI, Seshagiri TS, Gnanasekaran T (2004) Electrical conductivity and gas sensing properties of MoO3. Sens Actut B 101:161–174
Li Chunzhao, Benicewicz Brian C (2005) Synthesis of well-defined polymer brushes grafted onto silica nanoparticles via surface reversible addition–fragmentation chain transfer polymerization. Macromolecules 38:5929–5936
Yang H, Zhang S, Yang W, Chen X, Zhuang Z, Xu J, Wang X (2004) Molecularly imprinted sol-gel nanotubes membrane for biochemical separations. J Am Chem Soc 26(13):4054–4055
Guo H, Qian H, Sun S, Sun D, Yin H, Cai X, Liu Z, Wu J, Jiang T, Liu X (2011) Hollow Mesoporous silica nanoparticles for intracellular delivery of fluorescent dye. Chem Central J 5:1
Chakrabarty S, Chatterjee K (2009) Synthesis and characterization of nano-dimensional nickelous oxide (NiO) semiconductor. J Phys Sci 13:245–250
Borgstrom M, Blart E, Boschloo G, Mukhtar E, Hagfeldt A, Hammarstrom L, Odobel F (2005) Sensitized hole injection of phosphorus porphyrin into NiO: toward new photovoltaic devices. J Phys Chem B 109:22928–22934
Qi Y, Qi H, Lu C, Yang Y, Zhao Y (2009) Photoluminescence and magnetic properties of b-Ni(OH)2 nanoplates and NiO nanostructures. J Mater Sci: Mater Electron 20:479–483
Krish KSR, Liberati M, Grazioli VM, Turchini S, Luches P, Vateri S, Carbone C (2007) Magnetic linear dichroism studies of in situ grown NiO thin films. J Magn Magn Mater 310:8
Vincent CA, Bonion F, Lizzari M, Scrosati B (1987) Modern batteries, 1st edn. Edward Arnold, London
Bosman AJ, Crevecoeur C (1966) Mechanism of the electrical conduction in Li-doped NiO. Phys Rev 144:763
Lu J, Ye Z, Wang L, Huang J, Zhao B (2003) Structural, electrical and optical properties of N-doped ZnO films synthesized by SS-CVD. Mater Sci Semicond Process 5:491
Reynolds DC, Look DC, Jogai B (1996) Solid State Commun 99:873
Ogale SB (2005) Thin films and heterostructures for oxide electronics. Springer, New York
Nickel NH, Terukov E (2005) Zinc oxide—a materialm for micro- and optoelectronic applications. Springer, Amsterdam
Jagadish C, Pearton SJ (2006) Zinc oxide bulk, thin films, and nanostructures. Elsevier, New York
Thomas DG (1960) Excitons and the absorption edge of ZnO. J Phys Chem Solids 15:86
Mang A, Reimann K, Rübenacke S (1995) Zinc oxide: fundamentals, materials and device technology. Solid State Commun 94:251
Reynolds DC, Look DC, Jogai B, Litton CW, Cantwell G, Harsch WC (1999) ZnO bulk thin flims and nanostructure. Phys Rev B 60(23):40
Xiu FX, Yang Z, Mandalapu LJ, Liu JL (2006) p-type ZnO films with solid-source phosphorus doping by molecular-beam epitaxy. Appl Phys Lett 88:052106
Ghosh CK, Chattopadhyay KK (2007) Effect of Co doping on the static dielectric constant of ZnO nanoparticles. J Appl Phys 101:124911
Reynolds DC, Look DC, Jogai B, Litton CW, Cantwell G, Harsch WC (1999) Phys Rev B 60:2340
Chenari HM, Hassanzadeh A, Golzan MM, Sedghi H, Talebian M (2011) Frequency dependence of ultrahigh dielectric constant of novel synthesized SnO2 nanoparticles thick films. Curr Appl Phys 11:409–413
Venkataraman BH, Varma KBR (2004) Frequency-dependent dielectric characteristics of ferroelectric SrBi2Nb2O9 ceramics. Solid State Ionics 167:197–202
Ravinder D, Mohan GR, Nitendarkishan P, Sagar DR (2000) High frequency dielectric behaviour of aluminium-substituted lithium ferrites. Mater Lett 44:256–260
Chandra P, Bajal JSJ (1985) Less Common Metals 107: 51
Ravinder D (1993) Effect of sintering temperature on the dielectric behaviour of lithium ferrite. Phys Status Solidi (a) 139:K69
Reddy R (1999) A V, Ranga Mohan G, Ravinder D and Boyanov B S. J Mater Sci 34:3169
Abdullah MA, Yusoff AN (1996) Complex impedance and dielectric properties of an Mg-Zn ferrite. J Alloys Compd 233:129
Koops CG (1951) On the dispersion of resistivity and dielectric constant of some semiconductors at audio frequencies. Phys Rev 83:121–124
Maxwell JC (1988) Electricity and magnetism, vol 1. Oxford University Press, Oxford, p 328
Wagner KW (1913) Zur theorie der unvollkommenen dielektrika. Ann. Phys. (Leipzig) 40:817
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares that have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Desouky, O.A. The effect of SiO2 addition on dielectric properties and microstructure of ZnNiO2: based ceramics. SN Appl. Sci. 2, 136 (2020). https://doi.org/10.1007/s42452-019-1891-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-019-1891-4