Abstract
Studies related to partial pressure of carbon dioxide (pCO2) concentration linking with polychaete diversity in mangrove ecosystems are limited in time and space. Therefore, the present study was conducted during July 2017–June 2018 on a monthly interval and reported the concentration of pCO2 coupled with physico-chemical parameters in relation to polychaetes diversity in Pichavaram mangroves ecosystem, southeast coast of India. Totally, 41 species were identified and the most dominant species were Prionospio cirrifera, P. cirrobranchiata, P. sexoculata, Prionospio sp. and Capitella capitata. Among the stations, higher polychaete diversity was found in marine zone compared to other zones. The correlation reflected a significant positive linear relationship between dissolved inorganic carbon (DIC), dissolved organic carbon (DOC), particulate organic carbon (POC) and CO2 versus pCO2. The carbon species DIC, DOC, POC and pCO2 concentration ranged from 1100.1 to 2053.3(µmol/kg), 165.7–1954.0(µmol/kg), 4.5–89.2(µmol/kg) and 184.7–3763.1(µatm), respectively. Further, the statistical analyses revealed that there was a strong correlation among carbon species with distribution of polychaete species in various zones of mangroves and thus indicating pivotal role in occurrence of polychaetes in mangroves.
Similar content being viewed by others
1 Introduction
Urbanization, industrialization, afforestation and burning of fossil fuel are the major anthropogenic activities, responsible for the proliferation of greenhouse gas emission into the atmosphere. In recent years, the increasing concentration of CO2 (global warming) has resulted in rise of sea surface temperature (0.3–0.6 °C) and fall in seawater pH (0.1), referred to as ocean acidification, which are considered to be the most universal problem, particularly more vulnerable to marine ecosystem [1]. Of the various ecosystems, the mangroves, seagrass meadows and coral reef account for storing high rate (~ 70%) of organic carbon [2]. Among these three ecosystems, mangroves are said to be the most productive and biologically important ecosystem in tropical and subtropical region. Being productive, it also plays an important role in the global carbon cycle, and thus, estimated net primary production (NPP) of the world’s mangroves is about 218 ± 72 TgC per year [3, 4]. Consequently, the mangrove forests are the largest carbon sink, leading to reduction in carbon emissions [5]. True to its sense, several researchers pointed out that the mangroves are considered as the potential source of atmospheric CO2 and CH4 [6, 7].
Justifiably, the water columns of the mangroves ecosystem are rich in organic carbon derived from the mangrove litter. Despite its own high organic production, mangroves also receive considerable amount of organic carbon through freshwater influx during monsoon season. Added to it, discharges accruing from industrial effluents and shrimp ponds are the additional sources of CO2 to mangrove wetlands as stated by Purvaja [8] and Regnier et al. [9]. In his study, Kathiresan [10] compiled as many as 4011 species of fauna and flora to occur in the mangrove environment. Of this, 920 nos. belonged to floral species (23%) and 3091 to faunal (77%). Among the various faunal groups, benthic infauna are responsible for a significant amount of sediment secondary productivity, bioturbation and nutrient recycling [11, 12]. These organisms show numerous feeding behaviors at different levels of the complex mangroves food web, representing suitable bio-indicators of regional structure and environmental status and disturbances [13]. Of these benthic taxa, polychaetes play a pivotal role in decomposition of dead organic matter and break down of leaf litter in this productive ecosystem [14].
On the contrary, in recent years, mangrove forests are vulnerable to anthropogenic activities like tourism, urban development and natural disturbance [15, 16]. Under these circumstances, over the years, a large number of studies related to diversity of benthic fauna in mangrove ecosystem have been undertaken in India and elsewhere [14, 17]. Nevertheless, studies related to pCO2 concentration linking with benthic community in mangrove ecosystems are very limited, that too in Pichavaram mangroves skirting around ten fishing villages, vast extent of agricultural lands and aquaculture ponds besides being attracted by a large number of tourists regularly [18, 19]. Even though a suite of environmental parameters that determine the distribution of benthic organisms, temperature, pH and pCO2 are the most important environmental factors determining the distribution, physiological performance, morphology and behavior of marine invertebrates [20, 21]. Taking cognizance of the facts stated above, the present study was aimed to investigate the seasonal variation of pCO2 concentration and its influence coupled with environmental parameters on the polychaete diversity at Pichavaram mangrove environment.
2 Materials and methods
2.1 Study area
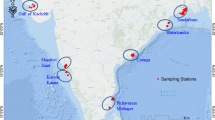
Pichavaram mangrove (Lat.11° 25′ N; Long.79° 47′ E) is situated amidst the Vellar and Coleroon estuarine complex. This mangrove has 51 islets of varying size with an area of 1200 ha, of which 40% is occupied by urban waterways (for fishing), 50% by mangrove forests and the rest by mud and sand flats [22]. This sprawling mangrove is criss-crossed by numerous channels and creeks linking other water bodies to this region. The tides are semidiurnal in nature with a range of 0.5–1.0 m. Besides this, a patchy occurrence of shrimp farms and agriculture lands with 2207 ha also borders this productive ecosystem [18]. The depth of the water here is generally low, varied from about 0.3 to 3.0 m.
To achieve the objectives of the study, five sampling stations, viz. St-1: freshwater zone; St-2: near Main Canal and Chinnavaikal; St-3: core mangrove zone; St-4: Main Canal linking Coleroon River; St-5: Marine Zone, were selected and monthly sampling was done in the predetermined locations from July 2017 to June 2018 (Fig. 1).
2.2 Collection of water and sediment sample
Water samples were collected using a 1-L sterile clean plastic high-density polypropylene bottle. Water parameters such as temperature, salinity, pH, dissolved oxygen (DO), oxygen saturation (DO%) and specific conductivity were recorded in situ using water quality monitoring system (Hydrolab Quanta Multi-Probe Meter); wind speed was measured using handheld anemometer. The water samples collected were preserved immediately in an icebox and brought to the laboratory. Total alkalinity (TA) was determined by following the standard method of Gran titration [23]. For this, the water sample was filtered through a cellulose acetate filter (0.45 μm) into 250-mL borosilicate bottles and then fixed with 100 μL of saturated mercury bi-chloride solution [24, 25]. During collection of water samples, all the bottles were overflowed for at least twice its volume to minimize contact with the atmosphere. Chlorophyll ‘a’ concentration was estimated by the method of Strickland and Parsons [26]. Dissolved organic carbon (DOC) was analyzed through Shimadzu TOC analyzer (Shimadzu TOC-VCPH). Particulate organic carbon (POC) was obtained on GF/F filters (0.45 µm), dried at 65 °C and then analyzed on elemental analyzer (PerkinElmer 2400). Data on carbonate (CO3), bicarbonate (HCO3), dissolved inorganic carbon (DIC) and partial pressure of carbon dioxide (pCO2) were calculated from the values of pH, water temperature and salinity using seacarb package available in ‘R’ software [27].
The sediment samples were collected using the stainless grab sampler; after collection, they were shade dried and the homogenized sediment samples were further subjected to soil texture by adopting the pipette method of Krumbein and Pettijohn [28] and total organic carbon (TOC) by following the chromic acid oxidation method [29].
2.3 Collection of benthic samples
Benthic fauna were collected using a long-armed Peterson grab, which covered an area of 0.1 m2. In each station, three replicate samples were collected at a depth range of 0.5–2.5 m and then passed through sieves with 0.5 mm mesh size. The organisms retained by the sieve were stored in clean plastic container, and samples were fixed with 5–7% formalin to which 4–5 drops of rose bengal (0.5 g/l) solution were added for enhanced visibility at the time of sorting. After a day or two, the benthic organisms were sorted, counted and identified up to major taxa, consulting standard literature (http://www.marinespecies.org/polychaeta/) [30, 31].
2.4 Calculation of diversity indices
For this, polychaete abundance data were subjected to the following univariate indices using PRIMER software (ver.7.0): Shannon diversity index (H′) [32]; Margalef’s species richness [33]; and Pielou’s evenness [34].
2.5 Statistical analysis
The data on physico-chemical parameters were shown as box plot. Scatterplot was also used for relating pCo2 versus carbon species using ggplot2 of ‘R’ software [35]. Similarly, the PCA-Bi-plot was drawn so as to find out the correlation between physico- chemical variables and sampling stations. Canonical correspondence analysis was also drawn to ascertain the relationship between polychaete diversity and environmental parameters (ran with the vegan library Ver. Vegan 2.4.4 [36]). All graphical and various multivariate analyses were performed using the statistical language ‘R Ver. 3.4.4’ (R Development Core Team 2018).
3 Results
3.1 Physico-chemical characteristics of water samples
The monthly data were amalgamated to season, and the results are presented season wise. Overall, the depth in the sampling stations ranged from 0.5 (St-2) to 2.5 m (St-5). Water temperature (Fig. 2a) ranged between 26.8 °C (St-1; monsoon) and 33.5 °C (St-5; summer); salinity (Fig. 2b) from 22.3 ppt (St-1; monsoon) to 46.5 ppt (St-3; summer); pH (Fig. 2c) as 7.1 (core mangrove) and 8.3 (marine zone). DO (Fig. 2d) reached the peak value of 5.0 mg/l (St-5; monsoon) and trough of 1.6 mg/l (St-3; summer). Wind speed showed less value of 0.7 m/s (post-monsoon) and high value of 2.7 m/s (pre-monsoon). Chlorophyll ‘a’ (Fig. 3a) values were from 0.6 µg/l (St-5; summer) to 4.0 µg/l (St-1; monsoon). Alkalinity (Fig. 3b) showed a wide variation between 1221.3 µmol/kg (St-1; monsoon) and 2072.1 µmol/kg (St-1; summer); carbonate (Fig. 3c) values showed a minimum of 21.9 µmol/kg (St-3) during monsoon and a maximum of 252.8 µmol/kg (St-1) during summer season; bicarbonate (Fig. 3d) values varied from 955.6 (St-5; monsoon) to 1961.9 µmol/kg (St-3; summer).
3.2 Distribution pattern of DIC, DOC, POC and CO2
DIC (Fig. 4a) concentrations showed minimum (1100.1 µmol/kg; St-5) during monsoon season and maximum (2053.3 µmol/kg; St-3) in summer; similarly DOC (Fig. 4b) showed maximum (1954.0 µmol/kg; St-5) in summer and minimum (165.7 µmol/kg; St-1) in monsoon season; POC (Fig. 4c) concentration reached a peak during summer season and a trough during monsoon season. CO2 (Fig. 4d) showed a wide variation from 4.5 µmol/kg (St-1) in monsoon to 89.2 µmol/kg (St-3) in summer season.
3.3 pCO2 environmental controls
pCO2 concentration varied from 184.743 (St-5; monsoon) to 3763.083 µatm (St-3; summer). On the contrary, when pCO2 was linked against DIC, DOC, POC and CO2, a positive linear relation was found in all the stations [value pCO2 vs. DIC (R2 = 0.5, y = −2160 + 2.04x; Fig. 5a); pCO2 vs. DOC (R2 = 0.12, y = 453 + 0.493x; Fig. 5b); pCO2 vs. POC (R2 = 0.14, y = 509 + 1.12x; Fig. 5c); pCO2 vs. CO2 (R2 = 0.2, y = 446 + 17.8x; Fig. 5d)]. pH versus pCO2 showed a negative relationship in all the stations (Fig. 6).
3.4 Sediment characteristics
Invariably, sediment texture revealed that clay content was shown to be high compared to silt and sand in the stations studied; clay ranged between 26.8 and 68.3% followed by silt (26.5 and 63.7%) and sand (0.1 and 17.5%) (Fig. 7). Similarly, TOC varied greatly between 2.1 (St-4; monsoon) and 10.4 mgC/g (St-3; summer season) (Fig. 8).
3.5 Principal component analysis (PCA)
In order to ascertain the relationship between environmental variables with nature of sampling stations, PCA was drawn. The PCA plot revealed the distinct pattern of environmental variables across the sampling stations, which showed five large groups wherein each station followed different patterns of variation (Fig. 9). It showed 37.65% of the total variance exhibited with 22.62% and 15.03% of axes 1 and 2, respectively. Among the parameters, temperature, salinity, pH, DO, alkalinity, CO3, DOC, oxygen saturation, wind speed and sand were showing positive correlation with St-4 and St-5, while BOD, bicarbonate, chlorophyll, DIC, POC, pCO2, CO2, sediment TOC, silt and clay had negative with St-1 to St-3.
3.6 Canonical correspondence analysis (CCA)
Further, CCA analysis, which was done to find out the correlation between the environmental variables and polychaete abundance, (environmental polychaete species) showed axes 1 and 2 which explained total variance of 38.5% with maximum canonical values (0.701, 0.622, 0.541, 0.478, 0.407, 0.363 and 0.218). In axis 1, environmental parameters such as temperature, BOD, alkalinity, bicarbonate, DIC, POC, pCO2, CO2, sediment TOC, silt and clay were showing negative correlation with polychaete species Prionospio cirrifera, P. cirrobranchiata, Prionospio sp, Scolelepis lefebvrei, Spiophanes soederstromi and Orbiniid sp. In axis 2, parameters such as wind speed, DO, oxygen saturation, salinity, pH, carbonate, chlorophyll, DOC and sand had positive correlation with species Ancistrosyllis parva, Ancistrosyllis sp., Aonides oxycephala, Branchiomaldane vincenti, Capitella capitata, Diopatra dubia, Ficopomatus sp., Glycera sp., Heteromastus filiformis, Laeonereis ankyloseta, Laonice cirrata, Malacoceros indicus, Maldanid sp., Namalycastis sp., Nephtys dibranchis, Nereis sp., Notomastus aberrans, N. favela, N. latericeus, Notomastus sp., Platynereis dumerilii, Prionospio sexoculata, Pulliella annata, Scolelepis capensis, Sigambra nr. bassi, Spio filicornis, Spiophanes bombyx and Spiophanes sp. (Fig. 10).
CCA was also drawn to find out the relationship between the environmental parameters and benthic polychaete diversity components. The results of CCA analysis showed axes 1 and 2 explained total variance 93.6% and maximum canonical values (0.459, 0.411, 0.315, 0.263 and 0.238). The environmental parameters such as wind speed, pH, salinity, DO, carbonate, DOC and silt had strong correlation with diversity (H′), while other parameters, namely temperature, BOD, saturation, alkalinity, bicarbonate, chlorophyll, DIC, POC, CO2, pCO2, sediment TOC, sand and clay, had weak correlation with the evenness (J) and richness (d) (Fig. 11).
3.7 Biological entities
Polychaete density recorded in various stations was found to vary from 612 to 5725 nos/m2 with maximum noticed during summer and minimum during monsoon season. Altogether 41 species of benthic polychaetes were recorded and abundant among them were Prionospio cirrifera, P. cirrobranchiata, P. sexoculata, Prionospio sp, C. capitata and Orbiniid sp.
3.8 Diversity indices
Species diversity (H’) varied from 1.57 (St-3) to 3.04 (St-5); species richness (d) fluctuated between 4.54 and 5.13 with maximum in St-3 and minimum in St-4; with respect to Pielou’s species evenness (J’), it ranged between 0.44 and 0.90 with maximum in St-5 and minimum in St-3 (Table 1).
4 Discussion
The continued emission of carbon dioxide into the atmosphere has a great impact on aquatic carbonate chemistry. Researchers across the world evince keen interest in focusing on climate change aspects to study the behavior of organisms in response to the marine low pH/elevated pCO2. Anthropogenic activities, agricultural runoff and aquaculture farm discharges are known to influence greatly the health of aquatic and terrestrial environments [37]. As stated earlier, mangroves act as sink for sequestering carbon, due to biomass burning, forest degradation and deforestation [38]. The distribution of benthic fauna in mangroves in relation to water quality has been described quantitatively by Guerreiro et al. [39]. Various environmental variables, such as substrate nature, texture, temperature, dissolved oxygen, salinity, sedimentation rate, organic carbon, and anthropogenic pollutants, are reported to influence the distribution of benthic organisms [40].
The depth was observed ranging between 0.5 and 2.0 m depth in study area. The water temperature assumes greater significance as it regulates various abiotic and biotic activities of an aquatic ecosystem [41]. Presently, the water temperature varied between 26.8 and 33.5 °C. The maximum temperature was recorded during summer (St-5), and minimum was recorded during monsoon season (St-1). It is attributed from the observation on water temperature that during non–monsoon seasons, the water gets more warmed compared to monsoon season. Temperature variation could also be an effect of the tropical climate and anthropogenic discharges in this area. Benthic researchers elsewhere also stated that the freshwater and less air temperature during monsoon season will have a greater influence on polychaete diversity patterns in the mangrove ecosystems [42, 43]. True to its sense, the minimum density of polychaetes was recorded during monsoon and maximum during dry seasons.
Fluctuation in pH can also have major effects on organisms through various processes like physiological and biological activities. The variation in pH could be attributed to the influence of freshwater, reduction in salinity, photosynthetic activity. The present observation agrees well with the previous works of Pravinkumar et al. [63], Murugesan et al. [14, 44] and Silambarasan et al. [45] who reported the peak during summer and trough during monsoon season. Salinity is a key entity, which regulates the benthic faunal composition in the marine and coastal environments as described earlier by Kinne [46] and Sridhar et al. [47]. Generally, the higher salinity is due to greater evaporation and the lower is due to the greater dilution by rainfall and surface runoff. Similar summer maxima and monsoonal minima were reported earlier by Silambarasan et al. [45]; Murugesan et al. [14] at Pichavaram mangroves. Dissolved oxygen plays an important role in the distribution of marine biota. In natural environment, high organic wastes resulted in decrease in average dissolved oxygen concentration; moreover, the solubility of dissolved oxygen decreases with increasing temperature and salinity of water [48]. Similar observation with higher value in monsoon and lower in summer seasons was made by Morgan et al. [49] and Sigamani et al. [50].
Equally, alkalinity is a yet another important factor in determining the ability of the estuary to neutralize acidic nature from rainfall or wastewater. During this study, alkalinity level fluctuated between 1221.3 µmol/kg (St-1; monsoon) and 2072.1 µmol/kg (St-1; summer). Carbonate and bicarbonate values showed a minimum during monsoon season and maximum during summer season. Justifiably, on carbonate and bicarbonate range, a few researchers elsewhere stated that the frequent increase in alkalinity level in water might be due to anthropogenic activities and other decomposition processes [52, 53]. The present range of alkalinity is in close agreement with the previous assessment made by Abdo [51]; Toma [52]; Thasneem et al. [53].
The high concentration of chlorophyll ‘a’ recorded during monsoon season might be due to land runoff from the rivers, causing low salinity, higher turbidity and less availability of light as stated by Kumar et al. [54]. Earlier reports also indicated that freshwater discharges from the rivers during monsoon season led to higher concentration of chlorophyll ‘a’ [54,55,56], and thus, the range of chlorophyll recorded presently is justifiable.
DIC, DOC and POC are important attributes in biogeochemical carbon cycling between land and sea [57]. Through PCA plot, it was inferred that DIC level was strongly influenced by the pH and pCO2. Similarly, DOC and POC showed significant inter-correlation among themselves. In the present investigation, DIC showed minimum in marine zone during monsoon and maximum in core mangrove zone in summer. Justifiably, high DOC was observed (1954.0 µmol/kg) during summer and low (165.7 µmol/kg) in monsoon season; POC concentration also peaked during summer season and troughed during monsoon season; pCO2 varied widely from 184.7 (monsoon) to 3763.1 µatm (summer). DIC, DOC, POC and CO2 exhibited exponential decrease with salinity due to significant contribution through river runoff and inputs from wastewater discharges during monsoon. The high pCO2 and the low pH concentrations were recorded in core mangrove station, and accordingly, a negative relationship was found between them. Partial pressure of carbon dioxide versus DIC, DOC, POC and CO2 had positive linear relationship. The results of present observation are in close agreement with the previous assessment made by Marescaux et al. [58] and Hutchins et al. [59].
Similarly, total organic carbon also plays an important role in the accumulation and release of different micro-nutrients which reflects more accurately the level of organic matter. It is well-known fact that the sediment texture determines the distribution of total organic carbon content, which in turn influences the abundance of benthic fauna. The mangroves play a significant role in storing the large amounts of organic carbon [60]. The distribution of total organic carbon (TOC) closely followed the trends of distribution of sediment texture. High total organic carbon was found in the clay soil content, and seasonally, summer registered the maximum and monsoon the minimum. The summer maxima are attributed to large amount of dead organic matter settled at the bottom, which would have triggered the elevated level of TOC [61, 62]. Similar observation was made earlier by Pravinkumar [63] and Murugesan et al. [14, 44] at Pichavaram mangroves.
Polychaetes are veritable indicators of the environmental health of various ecosystems including benthic ecosystems. True to its sense, the dominant species with high abundance recorded were Prionospio cirrifera, P. cirrobranchiata, P. sexoculata, Prionospio sp, in Spionidae and C. capitata in Capitellidae, in St-1 and St-3 owing to high organic load. Similar species composition with greater abundance was reported earlier by Khan et al. [64]; Dean [65]; Sigamani et al. [50, 66]; Murugesan et al. [14] in the region where organic enrichment is more, and thus, their findings lend support to the results of the present study.
The utility of univariate and multivariate statistical methods has been in vogue since many years toward understanding and assessing the status of ecological quality [67]. The principal component analysis, which was performed to set a well-defined distinction between environmental parameters and stations, revealed significant correlation for St-4 and St-5 with environmental parameters such as temperature, salinity, pH, saturation, wind speed, DO, alkalinity, CO3, DOC and sand, while stations St-1, St-2 and St-3 showed significant correlation with other environmental parameters. Similar variables’ combinations were observed earlier by Medeiros et al. [68] in eastern Canadian Arctic; Mukherjee et al. [69] in Hooghly estuarine region; and Sigamani et al. [50] in Vellar–Coleroon estuarine system.
CCA results also clearly indicated the significant parameters, showing positive correlation with the polychaetes species, were wind speed, salinity, DO, saturation, pH, chlorophyll, carbonate, DOC and sand. Similarly, the correlation made between environmental parameters against diversity components revealed that the parameters such as, wind speed, pH, salinity, DO, carbonate, DOC and silt had significant positive correlation with diversity, while other parameters were showing weak correlation with evenness (J) and richness (d). True to this, earlier reports by Lamptey and Armah [70]; Sivaraj et al. [71]; Murugesan et al. [14] proven that stable environmental parameters like increased pH, temperature, salinity, DO and high intensity of light penetration during summer are responsible for its positive relationship with diversity. Further, CCA results clearly indicated the significant association of the polychaete species with many environmental parameters. For example, temperature, salinity, alkalinity, bicarbonate, chlorophyll, DIC, POC, pCO2, CO2 and sediment TOC were showing strong correlation with Prionospio cirrifera, P. cirrobranchiata, Prionospio sp. and Orbiniid sp. Similar combinations of variables influencing benthic polychaetes were reported earlier by Andem et al. [72]; Zabbey and Arimoro [73].
Diversity index is an ecologically powerful tool, which is applied to measure the species biodiversity in an ecosystem [74]. Researchers elsewhere reported that high pCO2/low pH plays negative role as reduction in polychaete diversity and increase in species richness values [75, 76]. In the present study, a marked seasonal variation in the Shannon diversity was found with maximum diversity (3.0; St-5) during summer season and minimum (1.6; St-3) during monsoon season. Similar range of diversity values was recorded earlier by Khan et al. [77] in Vellar–Uppanar estuary; Pravinkumar et al. [63] at Pichavaram; and Sigamani et al. [50] at Vellar–Coleroon.
5 Conclusion
The present study yielded quite interesting findings on the understanding of physico-chemical parameters and pCO2 concentration on mangroves-associated polychaetes. Among the variables, temperature, pH, DO, salinity, alkalinity, carbon species, TOC and sediment characteristics are also proved to be the important expressive parameters influencing abundance and distribution of polychaete diversity. Further, the univariate and multivariate statistical analyses done for both physico-chemical and biological variables also drawn meaningful conclusions. Thus, our study concludes that pCO2 concentration in the mangrove waters exerted a greater role on the distribution of benthic biota in general and polychaete diversity in particular as evidenced in CCA analysis. This study also opened up new avenues for furthering this study to a greater extent, wherein an important beam of light will be thrown in the context of role of pCO2 on the polychaete distribution in the mangrove environment all along the coast.
References
Anderson LG, Jörgen EK, Ericson Y, Humborg C, Semiletov I, Sundbom M, Ulfsbo A (2017) Export of calcium carbonate corrosive waters from the East Siberian sea. Biogeosciences 14:1811–1823. https://doi.org/10.5194/bg-14-1811-2017
Macreadie PI, Allen K, Kelaher BP, Ralph PJ, Skilbeck CG (2012) Paleoreconstruction of estuarine sediments reveal human-induced weakening of coastal carbon sinks. Glob Chang Biol 18:891–901. https://doi.org/10.1111/j.1365-2486.2011.02582.x
Bouillon S, Borges AV, Castañeda-Moya E, Diele K, Dittmar T, Duke NC, Kristensen E, Lee SY, Marchand C, Middelburg JJ, Rivera-Monroy VH (2008) Mangrove production and carbon sinks: a revision of global budget estimates. Global Biogeochem Cycles 22:1–12. https://doi.org/10.1029/2007GB003052
Fuchs H, Magdon P, Kleinn C, Flessa H (2009) Estimating aboveground carbon in a catchment of the Siberian forest tundra: combining satellite imagery and field inventory. Remote Sens Environ 113:518–531. https://doi.org/10.1016/j.rse.2008.07.017
Pham VH, Luu VD, Nguyen TT, Koji O (2017) Will restored mangrove forests enhance sediment organic carbon and ecosystem carbon storage? Reg Stud Mar Sci 14:43–52. https://doi.org/10.1016/j.rsma.2017.05.003
Ray R, Baum A, Rixen T, Gleixner G, Jana TK (2018) Exportation of dissolved (inorganic and organic) and particulate carbon from mangroves and its implication to the carbon budget in the Indian Sundarbans. Sci Total Environ 621:535–547. https://doi.org/10.1016/j.scitotenv.2017.11.225
Purvaja R, Ramesh R (2001) Natural and anthropogenic methane emission from coastal wetlands of south India. Environ Manage 27:547–557. https://doi.org/10.1007/s002670010169
Purvaja GR (1995) Interactions between greenhouse gas, nutrients, major and trace elements in mangrove ecosystems of south India. Ph.D. thesis, Anna University, Madras, India
Regnier P, Friedlingstein P, Ciais P, Mackenzie FT, Gruber N, Janssens IA, Laruelle GG, Lauerwald R, Luyssaert S, Andersson AJ, Arndt S (2013) Anthropogenic perturbation of the carbon fluxes from land to ocean. Nat Geosci 6(8):597–607. https://doi.org/10.1038/ngeo1830
Kathiresan K (2018) Mangrove forests of India. Curr Sci 114:976–981. https://doi.org/10.18520/cs/v114/i05/976-981
Graf G, Rosenberg R (1997) Bioresuspension and biodeposition: a review. J Mar Syst 11:269–278. https://doi.org/10.1016/S0924-7963(96)00126-1
Coblentz KE, Henkel JR, Sigel BJ, Taylor CM (2014) Technical note: the use of laser diffraction particle size analyzers for inference on infauna-sediment relationships. Estuar Coast 38:699–702. https://doi.org/10.1007/s12237-014-9837-y
Jumars P, Fauchald K (1979) The diet of worms: a study of polychaete feeding guilds. Oceanogr Mar Biol Annu Rev 17:193–284
Murugesan P, Sarathy PP, Muthuvelu S, Mahadevan G (2018) Diversity and distribution of polychaetes in mangroves of east coast of India. In: Sharma S (ed) Mangrove ecosystem ecology and function. IntechOpen, London. https://doi.org/10.5772/intechopen.78332
Upadhyay VP, Ranjan R, Singh JS (2002) Human–mangrove conflicts: the way out. Curr Sci 83:1328–1336
Buitre MJ, Zhang H, Lin H (2019) The mangrove forests change and impacts from tropical cyclones in the Philippines using time series satellite imagery. Remote Sens 11:688. https://doi.org/10.3390/rs11060688
Venkataraman K, Wafar MVM (2005) Coastal and marine biodiversity of India. Indian J Mar Sci 34:57–75
Khan AS, Ramachandran A, Usha N, Punitha S, Selvam V (2012) Predicted impact of the sea-level rise at Vellar–Coleroon estuarine region of Tamil Nadu coast in India: mainstreaming adaptation as a coastal zone management option. Ocean Coast Manag 69:327–339. https://doi.org/10.1016/J.OCECOAMAN.2012.08.005
Gnanamoorthy P, Selvam V, Ramasubramanian R, Nagarajan R, Chakraborty S, Burman PK, Karipot A (2019) Diurnal and seasonal patterns of soil CO2 efflux from the Pichavaram mangroves, India. Environ Monit Assess 191:258. https://doi.org/10.1007/s10661-019-7407-2
Widdicombe S, Spicer JI (2008) Predicting the impact of ocean acidification on benthic biodiversity: What can animal physiology tell us? J Exp Mar Bio Ecol 366:187–197. https://doi.org/10.1016/J.JEMBE.2008.07.024
Doney SC, Fabry VJ, Feely RA, Kleypas JA (2009) Ocean acidification: the other CO2 problem. Ann Rev Mar Sci 1:169–192. https://doi.org/10.1146/annurev.marine.010908.163834
Selvam V, Ravichandran KK, Gnanappazham L, Navamuniyammal M (2003) Assessment of community-based restoration of Pichavaram mangrove wetland using remote sensing data. Curr Sci 85:794–798
Gran G (1952) Determination of the equivalence point in potentiometric titrations, Part II. Analyst 77:661. https://doi.org/10.1039/an9527700661
Cai W-J, Wang Y (1998) The chemistry, fluxes, and sources of carbon dioxide in the estuarine waters of the Satilla and Altamaha Rivers, Georgia. Limnol Oceanogr 43:657–668. https://doi.org/10.4319/lo.1998.43.4.0657
Jiang LQ, Cai WJ, Wanninkhof R, Wang Y, Lüger H (2008) Air-sea CO2 fluxes on the U.S. South Atlantic bight: spatial and seasonal variability. J Geophys Res 113:C07019. https://doi.org/10.1029/2007jc004366
Strickland JD, Parsons TR (1972) A practical handbook of seawater analysis, 2nd edn. Bulletin of the Fisheries Research Board of Canada, Nanaimo, p 167
Gattuso JP, Magnan A, Billé R, Cheung WW, Howes EL, Joos F, Allemand D, Bopp L, Cooley SR, Eakin CM, Hoegh-Guldberg O (2015) Contrasting futures for ocean and society from different anthropogenic CO2 emissions scenarios. Science 349:aac4722. https://doi.org/10.1126/science.aac4722
Krumbein WC, Pettijohn FJ (1938) Manual of sedimentary petrography. Appleton-Century-Crofts, New York
El Wakeel SK, Riley JP (1957) The determination of organic carbon in marine muds. ICES J Mar Sci 22:180–183. https://doi.org/10.1093/icesjms/22.2.180
Fauvel P (1953) The fauna of India including Pakistan, Ceylon, Burma and Malaya. Annelida: Polychaeta. The Indian Press Ltd., Allahabad
Day JH (1967) A monograph on the polychaeta of Southern Africa. Part II sedentaria. British Museum of Natural History, London, pp 459–878
Shannon CE, Wiener W (1949) The mathematical theory of communication. University of Illinois Press, Urbana
Margalef R (1958) Information theory in ecology. Int J Gen Syst 3:36–71
Pielou EC (1966) The measurement of diversity in different types of biological collections. J Theor Biol 13:131–144
Wickham H, Chang W, Henry L, Pedersen TL, Takahashi K, Wilke C, Woo K (2018) ggplot2: create elegant data visualisations using the grammar of graphics. R Package Version 3.1.0. https://CRAN.R-project.org/package=ggplot2
Oksanen J, Blanchet FG, Friendly M, Kindt R, Legendre P, McGlinn D, Minchin PR, O’Hara RB, Simpson GL, Solymos P, Stevens MH (2017) Vegan: community Ecology Package. R Package Version 2(4):4
Smith VH, Tilman GD, Nekola JC (1998) Eutrophication: impacts of excess nutrient inputs on freshwater, marine, and terrestrial ecosystems. Environ Pollut 100:179–196. https://doi.org/10.1016/S0269-7491(99)00091-3
Hamilton SE, Friess DA (2018) Global carbon stocks and potential emissions due to mangrove deforestation from 2000 to 2012. Nat Clim Chang 8:240–244. https://doi.org/10.1038/s41558-018-0090-4
Guerreiro J, Freitas S, Pereira P, Paula J, Macia A (1996) Sediment macrobenthos of mangrove flats at Inhaca Island, Mozambique. Cah Biol Mar 37:309–327
Magno MC, Bergamin L, Finoia MG, Pierfranceschi G, Venti F, Romano E (2012) Correlation between textural characteristics of marine sediments and benthic foraminifera in highly anthropogenically-altered coastal areas. Mar Geol 318:143–161. https://doi.org/10.1016/J.MARGEO.2012.04.002
Radhika CG, Mini I, Gangadevi T (2004) Studies on abiotic parameters of a tropical fresh water Lake–Vellayani Lake, Thiruvananthapuram District, Kerala. Pollut Res 23:49–63
Musale AS, Desai DV (2011) Distribution and abundance of macrobenthic polychaetes along the south Indian coast. Environ Monit Assess 178:423–436. https://doi.org/10.1007/s10661-010-1701-3
Vajravelu M, Martin Y, Ayyappan S, Mayakrishnan M (2018) Seasonal influence of physico-chemical parameters on phytoplankton diversity, community structure and abundance at Parangipettai coastal waters, Bay of Bengal, South East Coast of India. Oceanologia 60:114–127. https://doi.org/10.1016/j.oceano.2017.08.003
Murugesan P, Muniasamy M, Muthuvelu S, Vijayalakshmi S, Balasubramanian T (2011) Utility of benthic diversity in assessing the health of an ecosystem. Indian J GeoMar Sci 40:783–793
Silambarasan A, Sivaraj S, Muthuvelu S, Bharathidasan V, Murugesan P (2016) Influence of environmental parameters on abundance and diversity of phytoplankton in Pichavaram mangroves, southeast coast of India. Indian J GeoMar Sci 45:591–602
Kinne O (1971) Salinity: animals–invertebrates. In: Kinne O (ed) Marine ecology, environmental factors, vol 1. Wiley, New York, pp 821–996
Sridhar R, Thangaradjou T, Kumar SS, Kannan L (2006) Water quality and phytoplankton characteristics in the Palk Bay, southeast coast of India. J Environ Biol 27:561–566
Mostofa KM, Liu CQ, Mottaleb MA, Wan G, Ogawa H, Vione D, Yoshioka T, Wu F (2013) Dissolved organic matter in natural waters. In: Mostofa K, Yoshioka T, Mottaleb A, Vione D (eds) Photobiogeochemistry of organic matter. Environ Sci Engineer (Environmental Engineering. Springer, Berlin, pp 1–137
Morgan AM, Royer TV, David MB, Gentry LE (2006) Relationships among nutrients, chlorophyll-, and dissolved oxygen in agricultural streams in Illinois. J Environ Qual 35:1110. https://doi.org/10.2134/jeq2005.0433
Sigamani S, Perumal M, Arumugam S, Jose HP, Veeraiyan B (2015) AMBI indices and multivariate approach to assess the ecological health of Vellar–Coleroon estuarine system undergoing various human activities. Mar Pollut Bull 100:334–343. https://doi.org/10.1016/j.marpolbul.2015.08.028
Abdo MH (2005) Physico-chemical characteristics of Abu Za’baal ponds, Egypt. Egypt J Aquat Res 31:1–15
Toma JJ, Assad ZS, Baez DR (2013) Water quality assessment of some well water in Erbil city by quality index, Kurdistan region-Iraq. J Adv Lab Res Biol 4(4):125–130
Thasneem TA, Nandan SB, Geetha PN (2018) Water quality status of Cochin Estuary, India. Indian J GeoMar Sci 47:978–989
Kumar PS, Nuncio M, Ramaiah N, Sardesai S, Narvekar J, Veronica F, Jane TP (2007) Eddy-mediated biological productivity in the Bay of Bengal during fall and spring inter-monsoons. Deep Sea Res 54:1619–1640. https://doi.org/10.1016/j.dsr.2007.06.002
Mujumdar M, Salunke K, Rao SA, Ravichandran M, Goswami BN (2010) Diurnal cycle induced amplification of sea surface temperature intraseasonal oscillations over the Bay of Bengal in summer monsoon season. IEEE Geosci Remote Sens Lett 8:206–210. https://doi.org/10.1109/LGRS.2010.2060183
Shanthi R, Poornima D, Raja K, Sarangi RK, Saravanakumar A, Thangaradjou T (2015) Inter-annual and seasonal variations in hydrological parameters and its implications on chlorophyll a distribution along the southwest coast of Bay of Bengal. Acta Oceanol Sin 34:94–100. https://doi.org/10.1007/s13131-015-0689-5
Wu Y, Zhang J, Liu SM, Zhang ZF, Yao QZ, Hong GH, Cooper I (2007) Sources and distribution of carbon within the Yangtze River system. Estuar Coast Shelf Sci 71:13–25. https://doi.org/10.1016/J.ECSS.2006.08.016
Marescaux A, Thieu V, Borges AV, Garnier J (2018) Seasonal and spatial variability of the partial pressure of carbon dioxide in the human-impacted Seine River in France. Sci Rep 8:13961. https://doi.org/10.1038/s41598-018-32332-2
Hutchins RHS, Prairie YT, del Giorgio PA (2019) Large-scale landscape drivers of CO2, CH4, DOC, and DIC in Boreal River networks. Global Biogeochem Cycles 33:125–142. https://doi.org/10.1029/2018GB006106
Nagelkerken IS, Blaber SJ, Bouillon S, Green P, Haywood M, Kirton LG, Meynecke JO, Pawlik J, Penrose HM (2008) The habitat function of mangroves for terrestrial and marine fauna: a review. Aquat Bot 89:155–185. https://doi.org/10.1016/j.aquabot.2007.12.007
Martin D (1970) Marine chemistry. In: Kieffer WF (ed) Marine chemistry. Marcel Dekker, New York
Rajasegar M, Srinivasan M, Khan SA (2002) Distribution of sediment nutrients of Vellar estuary in relation to shrimp farming. Indian J Mar Sci 31:153–156
Pravinkumar M, Murugesan P, Prakash RK, Elumalai V, Viswanathan C, Raffi SM (2013) Benthic biodiversity in the Pichavaram mangroves, southeast coast of India. J Oceanogr Mar Sci 4:1–11. https://doi.org/10.5897/joms12.004
Khan SA, Raffi SM, Lyla PS (2005) Brachyuran crab diversity in natural (Pichavaram) and artificially developed mangroves (Vellar estuary). Curr Sci 88:1316–1324
Dean HK (2008) The use of polychaetes (Annelida) as indicator species of marine pollution: a review. Revis de Biol Trop 56:11–38
Sigamani S, Samikannu M, Alagiri TG (2019) Assessment of effluent stressed ecosystem of Cuddalore coastal waters—a bio-indicator approach. Thalass Int J Mar Sci. https://doi.org/10.1007/s41208-019-00128-4
Zhang Y, Guo F, Meng W, Wang X-Q (2009) Water quality assessment and source identification of Daliao river basin using multivariate statistical methods. Environ Monit Assess 152:105–121. https://doi.org/10.1007/s10661-008-0300-z
Medeiros AS, Biastoch RG, Luszczek CE, Wang XA, Muir DC, Quinlan R (2012) Patterns in the limnology of lakes and ponds across multiple local and regional environmental gradients in the eastern Canadian arctic. Inland Waters 2:59–76. https://doi.org/10.5268/IW-2.2.427
Mukherjee J, Banerjee M, Banerjee A, Roy M, Ghosh PB, Ray S (2014) Impact of environmental factors on the carbon dynamics at hooghly estuarine region. J Ecosyst 2014:1–10. https://doi.org/10.1155/2014/607528
Lamptey E, Armah AK (2008) Factors affecting macrobenthic fauna in a tropical hypersaline coastal lagoon in Ghana, West Africa. Estuar Coast 31:1006–1019. https://doi.org/10.1007/s12237-008-9079-y
Sivaraj S, Murugesan P, Muthuvelu S, Vivekanandan KE, Vijayalakshmi S (2014) AMBI and M-AMBI indices as a robust tool for assessing the effluent stressed ecosystem in Nandgaon coastal waters, Maharashtra, India. Estuar Coast Shelf Sci 146:60–67. https://doi.org/10.1016/j.ecss.2014.05.024
Andem AB, Okorafor KA, Eyo VO, Ekpo PB (2014) Ecological impact assessment and limnological characterization in the intertidal region of Calabar River using benthic macroinvertebrates as bioindicator organisms. Int J Fish Aquat Stud 1:8–14
Zabbey N, Arimoro FO (2017) Environmental forcing of intertidal benthic macrofauna of Bodo Creek, Nigeria: preliminary index to evaluate cleanup of Ogoniland. Reg Stud Mar Sci 16:89–97. https://doi.org/10.1016/J.RSMA.2017.08.004
Redding JM, Cory RL (1975) Macroscopic benthic fauna of three tidal creeks adjoining the Rhode River, Maryland. US Geol Surv Water Resour Investig 39:1–28. https://doi.org/10.3133/wri7539
Gambi MC, Musco L, Giangrande A, Badalamenti F, Micheli F, Kroeker KJ (2016) Distribution and functional traits of polychaetes in a CO2 vent system: winners and losers among closely related species. Mar Ecol Prog Ser 550:121–134. https://doi.org/10.3354/meps11727
Molari M, Guilini K, Lins L, Ramette A, Vanreusel A (2019) CO2 leakage can cause loss of benthic biodiversity in submarine sands. Mar Environ Res 144:213–229. https://doi.org/10.1016/J.MARENVRES.2019.01.006
Khan SA, Manokaran S, Lyla PS (2014) Assessment of ecological quality of Vellar and Uppanar estuaries, southeast coast of India, using benthos. Indian J GeoMar Sci 43:1–7
Acknowledgements
The authors are thankful to the Director and Dean, CAS in Marine Biology, Annamalai University, for encouragement and facilities provided. We gratefully acknowledge the UGC-SAP (G4 (1)41045/2015) Programme for financial support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Human and animal rights statement
This article does not contain any studies with animals, while sampling, care and experimental use of animals were followed with proper guidelines.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Palanivel, P.S., Veeraiyan, B., Palingam, G. et al. Influence of physico-chemical parameters and pCO2 concentration on mangroves-associated polychaetes at Pichavaram, southeast coast of India. SN Appl. Sci. 1, 1550 (2019). https://doi.org/10.1007/s42452-019-1581-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-019-1581-2