Abstract
Energy consumption is rising around the world and most of the activities in the developed countries are dependent on electric energy. Thermal energy storages are one the ways to reduce energy consumption and balance the electrical power usage during the day. With thermal energy storages, or to be precise, with ice storage systems, energy usage peak can the shifted from the afternoon or early night to low-load hours of morning or midnights. In this study, a 3D unsteady numerical analysis was done in order to examine an ice storage system equipped with serpentine tube heat exchanger. The influence of two geometrical parameters, namely, tube diameter and serpentine tube row distance were investigated. The examined range for diameter and the serpentine tube row spacing were 15–21 and 30–100 mm, respectively. The results showed that placing the serpentine tube rows more distant to each other increases the ice formation so that the ice formation rate for the highest serpentine tube row distance compared to when the serpentine tube row distance is the lowest is higher by 24.68%. On the contrary, with a larger tube diameter, the rate of ice formation was decreased so that the smallest diameter had almost 5.9% more ice formation rate in comparison with the case with the largest tube diameter.
Similar content being viewed by others
1 Introduction
Energy consumption in the world is rapidly increasing, and developed countries have a high dependence on electrical energy in all their activities. Nowadays, it’s obvious that electrical energy has a lot of applications and saving electrical energy solves a lot of energy demand problems all over the world [1].
The energy consumption is expected to increase by 48% in 2040, on the other hand, the excessive use of fossil fuels has led the countries towards sustainable energy sources [2].
Thermal energy storage is a solution for shifting peak demand and making the electrical energy demand during day and night more balanced [3]. Cold storage systems are one of the technologies that can shift the load from the peak-load hours of the afternoon or early night to low-load hours in the morning or midnight. Producing and storing cold in low-load periods of time can reduce the size of cooling equipment and reduces the amount of electrical energy demand. Also, by shifting the peak-loads to low-load hours in these systems, the cost of energy can be decreased, because the energy cost is lower in low-load hours [4]. Adding a TES to the Heating, ventilation, and air conditioning (HVAC) systems can easily reduce the cooling or heating system energy costs and it also can have an important role in reducing carbon emissions [5]. Using TES systems can also make the opportunity to store energy when the energy source is intermittent. For example, in solar systems, the energy can be absorbed during the daylight and this stored energy can be used in the night for heating purposes. This way the intensity changes in the solar radiation which can be created by the clouds can also be damped and the usable energy can be more balanced in the time [6]. Rahdar et al. [7] studied two strategies to reduce the power consumption of ventilation systems at peak hours. They added an ice storage system and a phase-change material (PCM) storage to the HVAC system. The results showed that energy consumption for cooling purposes in systems equipped with an ice storage system and PCM were 4.9 and 7.58% lower than conventional air conditioning systems, respectively. In addition, the production of CO2 was 17.8% and 27.2% lower than conventional air conditioning systems, respectively. Kang et al. [8] studied the ice storage air conditioning system. Their examinations showed that the use of ice storage not only lowers costs but also significantly reduces the electricity demand during peak periods time.
Cold storage technologies have different types and they consist of a combination of storage media, charge, and discharging mechanisms. The most common storage materials are water, ice, and eutectic salts. Harvesting, Ice Slurry, and Encapsulated Ice are some of ice storage types. These storages utilize a one-phase refrigerant like ethylene glycol and water solution for charge and discharge purposes. Ice-on-coil ice storage systems are either melting from the inside (internal melt) or outside (external melt) [8].
Jannesari and Abdollahi [9] used two methods of thin ring and fins for improving the ice-on-coil storage system. They revealed that the ice formation was improved up to 34% while using rings and it was increased by 21% when fins were used. Shih and Chou [10] have numerically examined the process of freezing process in a tank with a specific volume around several cylinders with different arrangements using commercial CFD software. In their study, the number and arrangements of cylinders have been investigated. The investigated number of tubes varied between 2 and 8 cylinders. The results show that the heat transfer between the walls of the cylinders and the surrounding in the 4-cylinder configuration was the most significant. Yang et al. [11] examined the effect of the refrigerant inlet temperature on the ice formation process of an ice storage from ice-on-coil type. Simulation results showed that the lower inlet temperature of the refrigerant increases the thickness of the ice and additionally increases the heat exchange efficiency so that a shorter time is needed for ice formation. Erek and Ezan [12] have experimentally and numerically investigated the effects of ethylene glycol refrigerant inlet conditions, such as the temperature and mass flow rate on the ice formation in an ice storage system, the results showed that the effect of the refrigerant temperature on ice formation is much greater than its mass flow rate. Ezan et al. [13] have investigated the energy and exergy analysis of an ice-on-coil thermal energy storage system. In their study, they examined the effects of refrigerant inlet temperature and flow rate on the storage charge time. They revealed that reducing the refrigerant inlet temperature from − 5 to − 15° C, reduces the storage charge time by almost 50%. In a numerical simulation conducted by Sang et al. [14] a vertical, ice-on-coil latent heat storage was studied.
Bi et al. [15] experimentally examined the simultaneous external and internal discharging process in open and closed ice storage systems. Their results indicated that the performance and the stability of the cold release rate in closed ice storage were better. They stated that increasing the flowrate can only affect the outlet discharge temperature until a certain point, and after that, raising the flowrate cannot influence the discharge temperature anymore.
Song et al. [16] introduced the combination of chilled-water and ice storage systems and they examined the effects of the ice storage volume ratio on the economical aspects of the cold storage system. Their study showed that with higher ice storage volume ratio, the operating daily cost was drastically reduced, however it raised the initial investment required for the system.
Li et al. [17] used numerical simulations in order to examined temperature distributions during the charge and discharge processes in a coiled heat exchanger. They found out that using agitators can make the phase change process faster and enhance the heat transfer coefficient. It was also shown that the discharging process starts from the lower parts of the heat exchanger.
The utilization of staggered heat exchangers in latent heat thermal energy storage system was both experimentally and numerically examined in the work of Koukou et al. [18]. The impacts of heat transfer fluid temperature and buoyancy forces on the discharging process were tested. The results indicated that the existence of natural convection reduced the discharging process time.
Heat exchangers are means that can be employed for transferring heat between two or more fluids with different temperatures. Heat exchangers are used in a wide range of applications, including power plants, industries, and air conditioning systems. Tubular heat exchangers, such as double pipe, shell-and-tube, plate heat exchangers, which include the serpentine tube, gasketed plate-and-frame, and plate-fin heat exchangers are some of these heat exchangers [19, 20].
Among these heat exchangers, serpentine tubes have been studied in a few literates. Zhao et al. [21] numerically examined the performance of a membrane helically coiled heat exchanger and a membrane serpentine heat exchanger. Their study showed although the heat transfer rate in the helically coiled heat exchanger was better, the overall performance for both of these two heat exchangers was good. Michael et al. [22] have carried out a 3D simulation on the shell and serpentine tube heat exchanger. The tube geometry was serpentine and the simulations were done for different materials of the serpentine tubes.
In the present article, the charging process (solidification) in an ice-on-coil ice storage system is numerically investigated. The examined ice storage system is equipped with a heat exchanger consisted of two rows serpentine tubes which is the novelty of this article. Three-dimensional transient numerical simulations have been performed to evaluate the influences of some geometrical parameters including the tube diameter and the distance of serpentine tube rows on the solidification process.
Novelty of Present Paper According to the literature review in the above paragraphs, there are not many studies on the solidification process in the ice-on-coil ice storage systems by numerical simulation. Also, the tube configuration for the coolant in the present ice storage system is innovative, and for the first time, the effects of some influential geometrical parameters in this type tube configuration have been evaluated through a comprehensive numerical study.
2 Physical model and assumptions
2.1 Physical model
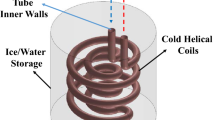
In this investigation, ice storage with a volume of 15 L was simulated. The ice storage had a cuboid shape with the length of 240 mm, the width of 180 mm and the height of 347 mm, and it was filled with water. In the ice storage, a cold serpentine tube heat exchanger was present. Figure 1a shows the schematics of the problem and Fig. 1b presents geometrical parameters that could be changed in the serpentine tube heat exchanger inside the storage.
The influence of two of these parameters on the ice formation rate in ice storage was evaluated. These two parameters were the serpentine tube diameter (d) and the serpentine tube row distance (h).
In order to examine these effects, eight models of this heat exchanger were simulated. Cases A, B, C, and E were created to study the effects of serpentine tube row distance while cases F, G, I and J were used in order to reveal the influence of tube diameter. All of these models have been shown in Fig. 2. It should be noted that for these comparisons the heat transfer areas were kept identical. In other words, for each of these groups, the heat transfer area was fixed. Table 1 lists the details for the geometrical parameters. As stated before, the fluid in the ice storage was water, also the tubes were selected with the copper material. The physical properties for these two materials are available in Table 2.
2.2 Assumptions
In order to simulate the process of phase change in the problem, the enthalpy porosity method was utilized [24, 25].
The simulation was simplified using these assumptions:
-
1.
The fluid flow is unsteady, laminar and incompressible.
-
2.
The density variation with the phase change was neglected and the density was selected to be constant. The value for the density was selected to be the average of the density of solid and liquid phases. It should be noted that the effects of temperature on the density variations were also ignored.
-
3.
The effect of natural convection was included by the Boussinesq approximation.
-
4.
The heat transfer through the outer walls of the storage was neglected and they were assumed as insulated boundaries.
-
5.
In order to reduce the calculation time and reduce the number of grids, the refrigerant flow was ignored and a fixed temperature boundary with the temperature of − 10° was applied in the tubes. This assumption was made by considering the refrigerant flow rate to be very intense.
-
6.
Different thermal properties for thermal conductivity and thermal capacity of solid and liquid phases were applied to the solution, but the effects of temperature on these properties were neglected.
3 Mathematical formulation and simulation procedure
In this segment, the governing equations will be presented and the characteristics and aspects of the computational simulation will be discussed.
3.1 Governing equations
The Boussinesq approximation equation for including the influence of buoyancy and natural convection [26]:
The continuity equation [27]:
The momentum equation [27]:
And the equation for energy [27]:
The total enthalpy can be obtained by [28, 29]:
where:
hlat can be in the range of zero and hsf (respectively for the solid and liquid phases). λ indicates the liquid fraction. Besides, the term Σ is used for summation of the latent heat for every time step and for every cell to calculate the total latent heat. The sensible heat can be written as:
And the equation for the liquid fraction is [30]:
The term S in Eq. 3 is Darcy’s law damping source term, which affects the momentum equation:
In which Cmush is the constant of the mushy zone which was selected to be 105 kg/m3 s during these simulations [24].
3.2 Initial and boundary conditions
The initial temperature of the whole computational domain was 274.15 K, and the velocities for all of the directions were set to zero. As stated, before the outer walls of the storage were considered insulated. Also, a fixed temperature of 263.15 K was applied inside the tube walls. For the contact area between the tube and the water in the storage, the coupled boundary condition was selected.
3.3 Numerical and solution method
The numerical method of finite volume was utilized in order to simulate the unsteady process of phase change. For this purpose, ANSYS Fluent 19.2 commercial CFD software was used.
The process of phases change was simulated by the enthalpy-porosity method. Also, the double-precision option was activated in order to improve the accuracy of the simulation. The continuity, velocity, and energy residuals were set to 10−3, 10−3, and 10−6, respectively. Values of under-relaxation factors were selected to be 0.3 for pressure, 1 for density and body forces and energy, 0.7 for the momentum, and finally 0.9 for the liquid fraction update. In the solution method, for pressure–velocity coupling the SIMPLE algorithm was utilized, the least square cell-based was applied for gradient spatial discretization and for transient formulation first-order implicit was used. Also, the QUICK scheme was used for both momentum and energy equations discretization.
3.4 Model validation
For validation of this simulation, the experimental study of Sasaguchi et al. [31] was employed and the process of ice formation around two cylinders with fixed temperatures were simulated. The result has been presented in Fig. 3. It can be seen that the results are in good agreement and the deviation between these two has a maximum value of 8.13%.
3.5 Grid and time-step size study
A series of tests were done for examining the grid independence for Model E with three different number of grid numbers including 1.2, 1.7 and 2.2 million of mesh numbers, the results for liquid have been presented in Fig. 4a. Based on these results, since the liquid fraction values for 1.7 and 2.2 million grids were almost the same, thus this number of cells was selected for all of the models.
The generated mesh for Case E has been shown in Fig. 5. It was tried to reduce the size of mesh in the areas near the tubes, because of higher gradients in those areas.
Also, some tests were done in order to evaluate the appropriate time-step size. Tests were done for time-step sizes of 2, 1 and 0.5 s. The results have been presented in Fig. 4b. Again, the liquid fraction values for simulations with 1 and 0.5 time step-sizes were in close agreement, so the time-step size for all the models was set to 1 s. It should be noted that for acquiring an accurate result 100 number of iterations was performed for each time step of the simulation.
4 Results and discussions
4.1 The effects of serpentine tube row distance (H)
In this segment, the influence of serpentine tube row distance (H) on the liquid fraction and ice formation will be discussed. Figure 6 presents the effects of serpentine tube row distance on the reduction rate of the liquid fraction. It should be noted that a lower liquid fraction means a higher amount of ice in the storage. By comparing plots of liquid fraction for Models A, B, C, and E, with the serpentine tube row distances of 30, 50, 70 and 100 mm, respectively, it can be concluded that the model with highest serpentine tube row distance (h = 100 mm) has a better ice formation performance. The rate of ice formation for the model with h = 100 mm is 24.68% higher in comparison to the model with h = 30 mm. Thus, it can be concluded a higher value for the serpentine tube row distance leads to a better ice formation rate in the ice storage system, but this difference loses its significance when the row distance is higher than 70 mm, so that the difference between the ice formation rate of models with row distances of 70 and 100 mm are not considerable.
To investigate the distribution of formed ice in the storage the liquid fraction contours can be employed.
Figure 7 shows liquid fraction contours for models with serpentine tube row distance varying from 30 to 100 mm. According to these liquid fraction contours it can be observed that with higher serpentine tube row distances, a better distribution of ice can be achieved in the storage. Also, the formation of an ice block (which can cause block the flow of water in the external discharge process) can be retarded with using a higher serpentine tube row distance, this makes a high serpentine tube row distance a great choice for both internal and external discharge processes.
The temperature contours for the areas around the serpentine tubes for four different times and four different distances between serpentine tube rows have been illustrated in Fig. 8. According to Fig. 8, it can be seen that in all of the cases, over time a larger area around serpentine tubes reaches to solidification temperature, solidifies and the temperatures in these areas even get lower than solidification temperature. From these contours, it can be demonstrated that with increasing the distance between two rows of serpentine tubes, a larger area gets cold and the temperature reduction happens in a wider region. It means that with increasing the distance between serpentine tubes rows wider areas get to solidification temperature and even lower than that, so the volume of formed ice increases. These results are in very good agreement with contours of the liquid fraction which was presented in Fig. 6.
4.2 The effects of tube diameter
In this segment, the influence of tube diameter (d) on the ice formation and liquid fraction will be analyzed. Figure 9 shows the influence of the serpentine tube diameter on the liquid fraction reduction rate. By comparing liquid fractions of Models F, G, I and J with the tube diameter of 15, 17, 19 and 21 mm, it can be observed that the model with smallest tube diameter (d = 15 mm) has a better ice formation performance. At t = 240 min or the end of the simulation, the model with d = 15 mm has 5.9% better ice formation rate compared to the model with d = 21.
Figure 10 shows liquid fraction contours for the models with serpentine tube diameters varying from 15 to 21 mm. Based on these liquid fraction contours it can be observed that even though with the smaller tube diameter ice formation rate is higher, but since the formed ice reaches the storage wall, the external discharge process for this case can be more difficult. On the other hand, if the ice storage design is based on the internal discharge process, the smaller diameter is preferred since it can create more amount of ice.
The contours of temperature for four different times and four different values of serpentine tube diameters have been illustrated in Fig. 11. According to Fig. 11, it can be observed that for all of the cases, over time a larger area reaches the solidification temperature, freezes and the temperatures in these regions even get lower than solidification temperature. From these contours, it can be understood that with smaller serpentine tube diameters, the temperature drop can be observed in a larger area. In other words, with reducing the serpentine tube diameter, wider areas get to solidification temperature and even lower than that, so the amount of ice formation rises. Again, these results are totally compatible with the contours of the liquid fraction which was presented in Fig. 10.
5 Conclusion
In this study, a 3D numerical simulation was carried out to examine the effects of two geometrical parameters including serpentine tube diameter and serpentine tube row distance on the phase change process in an ice storage system. The result indicates that:
-
With smaller tube diameters, the rate of ice formation increases. But the created ice can reach the storage wall and cause ice block formation. Thus, in the external discharge process in which the water flow should pass through the space between the tubes and the walls, the discharging process is done with a slower rate, in other words, with increasing tube diameters the external discharging process could be done more efficiently.
-
Larger serpentine tube rows distances can be used for increasing the ice formation rate. It also can reduce the chance of ice block formation. So, for both internal and external discharging processes, a higher serpentine tube row distance is preferable.
-
In the conventional ice-on-coil ice storage system, usually simple or u-type tubes are used. Present innovative geometric of the coolant tube probably has better performance compared to these conventional systems because it covers more regions in the water tank, which leads to a higher rate of ice production.
Abbreviations
- \(A\) :
-
Heat transfer area (mm2)
- \(C_{mush}\) :
-
Mushy zone constant (kg/m3 s)
- \(C_{P}\) :
-
Specific heat (J/kg K)
- \(D\) :
-
Tube outer diameter (mm)
- \(\vec{g}\) :
-
Gravity (m/s2)
- \(H\) :
-
Serpentine tube row distance (mm)
- \(h\) :
-
Specific enthalpy (J/kg)
- \(h_{sf}\) :
-
Latent heat of fusion (J/kg)
- \(k\) :
-
Thermal Conductivity (W/m k)
- \(L\) :
-
Tube length (mm)
- \(P\) :
-
Serpentine tube pitch (mm)
- \(\vec{s}\) :
-
Source term (N/m3)
- \(T\) :
-
Temperature (K)
- \(\vec{V}\) :
-
Velocity vector (m/s)
- \(\beta\) :
-
Expansion coefficient (1/K)
- \(\uplambda\) :
-
Liquid fraction
- \(\upmu\) :
-
Dynamic viscosity (Pa s)
- \(\uprho\) :
-
Density (kg/m3)
- ref:
-
Reference
- 0:
-
Reference
- sens:
-
Sensible
- lat :
-
Latent
- tot :
-
Total
- S :
-
Solid
- liq :
-
Liquid
References
Knebel DE (1990) Off-peak cooling with thermal storage. ASHRAE J 32(4):40–44
Pocketbook S (2016) EU transport in figures. Publications Office of the European Union, Luxembourg
Basecq V et al (2013) Short-term storage systems of thermal energy for buildings: a review. Adv Build Energy Res 7(1):66–119
Thumann A (1987) Optimizing HVAC systems. Fairmont Pr, pp 1–400
Yi W, Dong W (2015) Modeling and simulation of discharging characteristics of external melt ice-on coil storage system. Int J Smart Home 9(2):179–192
Korti AIN (2016) Numerical heat flux simulations on double-pass solar collector with PCM spheres media. Int J Air Cond Refrig 24(02):1650010
Rahdar MH, Emamzadeh A, Ataei A (2016) A comparative study on PCM and ice thermal energy storage tank for air-conditioning systems in office buildings. Appl Therm Eng 96:391–399
Kang Z et al (2017) Research status of ice-storage air-conditioning system. Procedia Eng 205:1741–1747
Jannesari H, Abdollahi N (2017) Experimental and numerical study of thin ring and annular fin effects on improving the ice formation in ice-on-coil thermal storage systems. Appl Energy 189:369–384
Shih Y-C, Chou H (2005) Numerical study of solidification around staggered cylinders in a fixed space. Numer Heat Transf Part A Appl 48(3):239–260
Yang T, Sun Q, Wennersten R (2018) The impact of refrigerant inlet temperature on the ice storage process in an ice-on-coil storage plate. Energy Procedia 145:82–87
Erek A, AkifEzan M (2007) Experimental and numerical study on charging processes of an ice-on-coil thermal energy storage system. Int J Energy Res 31(2):158–176
Ezan MA, Erek A, Dincer I (2011) Energy and exergy analyses of an ice-on-coil thermal energy storage system. Energy 36(11):6375–6386
Sang WH et al (2016) Efficient numerical approach for simulating a full scale vertical ice-on-coil type latent thermal storage tank. Int Commun Heat Mass Transf 78:29–38
Bi Y et al (2019) Experimental investigation of ice melting system with open and closed ice-storage tanks combined internal and external ice melting processes. Energy Build 194:12–20
Song X et al (2018) Study of economic feasibility of a compound cool thermal storage system combining chilled water storage and ice storage. Appl Therm Eng 133:613–621
Li Y et al (2017) Analysis of the icing and melting process in a coil heat exchanger. Energy Procedia 136:450–455
Koukou MK et al (2018) Experimental and computational investigation of a latent heat energy storage system with a staggered heat exchanger for various phase change materials. Therm Sci Eng Prog 7:87–98
Bergman TL et al (2011) Fundamentals of heat and mass transfer. Wiley, Hoboken
Afsharpanah F et al (2018) Numerical study of heat transfer enhancement using perforated dual twisted tape inserts in converging–diverging tubes. Heat Transf Asian Res 47(5):754–767
Zhao Z et al (2011) Numerical studies on flow and heat transfer in membrane helical-coil heat exchanger and membrane serpentine-tube heat exchanger. Int Commun Heat Mass Transf 38(9):1189–1194
Michael S, John KK, Krishnan A, Shanid KK, Mathew M (2017) Comparative CFD analysis of shell and serpentine tube heat exchanger. Int Res J Eng Technol 4:1171–1175. https://doi.org/10.5281/zenodo.1310808
Michalek T, Kowalewski TA, Sarler B (2005) Natural convection for anomalous density variation of water: numerical benchmark. Prog Comput Fluid Dyn Int J 5(3–5):158–170
Brent A, Voller V, Reid K (1988) Enthalpy-porosity technique for modeling convection-diffusion phase change: application to the melting of a pure metal. Numer Heat Transf Part A Appl 13(3):297–318
Gong Z-X, Devahastin S, Mujumdar AS (1999) Enhanced heat transfer in free convection-dominated melting in a rectangular cavity with an isothermal vertical wall. Appl Therm Eng 19(12):1237–1251
Faghri A, Zhang Y (2006) Transport phenomena in multiphase systems. Elsevier, Amsterdam
Bejan A (2013) Convection heat transfer. Wiley, Hoboken
Ajarostaghi M et al (2019) Numerical modeling of the melting process in a shell and coil tube ice storage system for air-conditioning application. Appl Sci 9(13):2726
Ajarostaghi SSM, Delavar MA, Dolati A (2017) Numerical investigation of melting process in phase change material (PCM) cylindrical storage considering different geometries. Heat Transf Res 9:1–21
Voller VR, Prakash C (1987) A fixed grid numerical modelling methodology for convection-diffusion mushy region phase-change problems. Int J Heat Mass Transf 30(8):1709–1719
Sasaguchi K, Kusano K, Viskanta R (1997) A numerical analysis of solid-liquid phase change heat transfer around a single and two horizontal, vertically spaced cylinders in a rectangular cavity. Int J Heat Mass Transf 40(6):1343–1354
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Pakzad, K., Mousavi Ajarostaghi, S.S. & Sedighi, K. Numerical simulation of solidification process in an ice-on-coil ice storage system with serpentine tubes. SN Appl. Sci. 1, 1258 (2019). https://doi.org/10.1007/s42452-019-1316-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-019-1316-4