Abstract
Herein, a new polymeric nanocomposite of 2,5-diphenyloxazole (PPO) and polystyrene (PS) doped with cerium fluoride (CeF3) nanoparticles (PS/PPO/CeF3) was prepared, characterized and its photoluminescence property was reported for the first time. The PS as a base matrix and PPO as a fluor were chose for a scintillator solution substrate and CeF3 nanoparticles dispersed into it to construct a new nanocomposite scintillator. The PS liquid polymeric matrix with 0.5 wt% of PPO was loaded with different percentage of CeF3 nanoparticles. Structural characterizations showed successful preparation of nanocomposites and a mean size of CeF3 nanoparticles as (13 ± 3) nm in the base polymer matrix. The measured photoluminescence showed an enhancement about 3 times in comparison to PS/PPO composite when 10% of CeF3 nanoparticles doped into the polymeric composite matrix under UV excitation. This effect is due to fluorescence resonance energy transfer in Ce3+ ions (5d → 4f transition energy) to the PPO within the PS/PPO/CeF3 nanocomposite. Observed results were presented and discussed.
Similar content being viewed by others
1 Introduction
Convectional organic scintillators due to fast time response, easy fabrication and low cost are widely used in some sector of fundamental and applied field. Because of their low stopping power, they are unsuitable for high energy X and gamma radiation [1, 2]. In the last two decades new composites of polymer base organic scintillators doped by inorganic nanoparticles were suggested [3,4,5,6,7,8,9]. The transfer energy referred as forester mechanism could lead to the enhancement of composites luminescence.
Early organic–inorganic scintillator composites were made used a grinding method, but their transparency was low [3]. This problem can be solve by using small size of nanoparticles as a filler via increasing the index matching of the components [4]. For example, Kang et al. [5] matched index of nanoparticles and the polymer matrix and reported some nanocomposites with high transparency. Cai et al. [6] reported on luminescence quenching of the polymer due to the charge transfer between the polymer and BiI3 in a conjugated polymer composite with BiI3. Oleic acid as a matrix was loaded with high nanoparticle doping while keeping the transparency up to 70% [7]. Feller et al. [8] incorporated non-fluorescent Gd2O3 nanocrystals in polyvinyl toluene (PVT) matrix and reported an enhancement in its luminescence due to fluorescence resonance energy transfer (FRET) from PVT to Gd2O3 was reported.
CeF3 nanoparticles due to the allowed 5d–4f transition in Ce3+ ion have significant fluorescence properties, high density, short decay time, fast response and high resistance to radiation. Due to 5d–4f transition in Ce3+ ions, the doped Ce3+ ions are good candidate materials for fast and efficient scintillators. However, most of the Ce3+ions emission is within the UV range which could not directly detect by photodetecors. Therefore to transfer the emission energy from CeF3 to higher wavelengths, a wavelength shifter is needed. Sahi et al. [9] used CeF3 nanoparticles dispersed into PVT matrix with small amount of 2,5 diphenyloxazol (PPO) as a shifter wavelength. A luminescence enhancing is observed under UV excitation to 3 time due to FRET from CeF3 to PPO.
Due to weak fluorescence efficiency and short optical attenuation length of polymers, they cannot be an effective scintillator. But their role as transfer energy of incident energy to flour is important [10], and the most of plastic scintillators is PVT, PPO and polystyrene (PS) polymers matrix. In this research, due to low cost and availability of PS we chosen it as a scintillator solution substrate. The CeF3 nanoparticles synthesized and dispersed into the PS matrix in conjunction with PPO to construct a new nanocomposite scintillator. The luminescence of PPO was enhanced under UV excitation.
2 Experimental
2.1 Materials
Polystyrene (PS), 2,5-diphenyloxazole (PPO), sodium fluoride (NaF) and cerium (III) nitrate hexahydrate (Ce(NO3)3·6H2O) with high purity were purchased from Sigma-Aldrich.
2.2 Preparation of CeF3 nanoparticles
Precipitation reaction method was used to synthesize the CeF3 nanoparticles [9]. Briefly, 80 ml of NaF, 30 mmol, purged with argon gas and heated at 80 °C under vigorous stirring. After that 60 ml of Ce(NO3)3·6H2O, 10 mmol, added into the above solution dropwise and the temperature was kept at 80 °C for 4 h. The resulting CeF3 precipitates were centrifuged and separated from the solution and then washed with deionized water, three times, and finally dried at 50 °C.
2.3 Preparation of PS/PPO/CeF3 nanocomposite
Firstly the PS/PPO with 0.5 wt% of PPO, 1.99 g of PS and 0.01 g of PPO were dissolved in 10 ml cyclohexane as solvent. Then solution was stirred for 24 h at room temperature. Then different amount of prepared CeF3 nanoparticles, were added into the above mixture and vigorous stirred for 24 h at room temperature. Finally, the PS/PPO/CeF3 nanocomposite scintillator with 0.5 wt% of PPO and different amount (wt%) of CeF3 nanoparticles were prepared.
2.4 Instrumentation
Crystalline structural, size and shape of the nanocomposites were studied by X-ray diffraction (XRD) using a Siemens D500 diffractometer with Cu Kα (operating at 40 kV and 30 mA), scanning electron microscope (SEM) and transmission electron microscopy (TEM) operating at 200 kV, respectively. Energy–dispersive X-ray analysis (EDAXA) coupled with SEM was used for the semi-quantities investigation of the nanocomposites. The absorption spectra (UV–Vis) of samples dispersed in methanol were measured by using a spectrophotometer (SHIMADZU UV-3450). A SHIMADZU RF-5301 PC spectrofluorometer was used for recording the photoluminescence emission (PL) spectrums. All the measurements were carried out at room temperature.
3 Results and discussion
3.1 Characterization of prepared nanocomposites
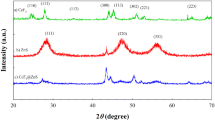
XRD patterns of the prepared CeF3 nanoparticles are shown in Fig. 1. In the range of 10°–70°, the observed peaks at 2θ positions of 24°, 28°, 35°, 44°, 45°, 51°, 53° and 65° are corresponding to space indexes as (110), (111), (112), (333), (113), (302), (221) and (223), respectively. This peak positions are agree with the data reported for CeF3 crystal (JCPDS No. 08-0045) and confirmed hexagonal phase of CeF3 crystals [9]. And also, it is demonstrated that no impurity peaks are detected and the high purity CeF3 is synthesized. With assumption spherical shape for nanoparticles, a particle diameter of about 10 nm was calculated according to Scherrer’s formula from the peak widths. This nanometer size of the prepared CeF3 particles allow us to uniform dispersion of them in the polymer matrixes.
The morphology and size of as-prepared CeF3 nanoparticles and PS/PPO/CeF3 nanocomposite were further examined by SEM and TEM (Figs. 2, 3). Figure 2 shows a typical SEM images of CeF3 nanoparticles (Fig. 2a) and PS/PPO/CeF3 nanocomposite (Fig. 2b). As can be seen from the SEM image of Fig. 2a, morphology of bulk CeF3 nanoparticles is irregular but their distribution is uniform. The strong peaks just for Ce and F atoms were observed in the corresponded EDXA spectrum (Fig. 2c) with atomic ratio as 1:2.8, which can match with CeF3 and showed there is not any impurity in the synthesized CeF3 nanoparticles. As can be seen from the SEM image of Fig. 2b, the CeF3 nanoparticles are well dispersed within the polymer matrix and it also confirms the correct way of making polymer nanocomposite in this study. In the corresponded EDXA spectrum of PS/PPO/CeF3 nanocomposite (Fig. 2d) the strong peaks of Ce and F atoms are still presented along with corresponded polymer elements, C, O and N atoms. Observed images and EDXA results successfully confirmed preparation of CeF3 nanoparticles with high purity and uniform dispersion of them into the polymer matrix.
The structural size of the prepared nanomaterials were calculated by TEM. Figure 3 shows the typical images of CeF3 nanoparticles (Fig. 3a), PS/PPO/CeF3 nanocomposite (Fig. 3b) and related distributions size of them (Fig. 3c, d). As displayed in Fig. 3a, the CeF3 nanoparticles exhibited spherical morphology and its agglomeration is small. Their distribution is shown in Fig. 3c that indicates the main average size of prepared CeF3 nanoparticles (13 ± 3 nm) is in good agreement with the result of the XRD pattern. The typical TEM image of PS/PPO/CeF3 nanocomposite is shown in Fig. 3b. As can be seen CeF3 nanoparticles uniformly dispersed in the polymer matrix with totally spherical shape. But, the size of the spherical CeF3 nanoparticles increased during dispersion in the polymer matrix and an agglomeration was occurred during synthesize of the PS/PPO/CeF3 nanocomposite. However, the observed uniform nanocomposite contains the CeF3 with the mean size as 100 ± 50 nm (Fig. 3d).
3.2 Photoluminescence study of the nanocomposites
The photoluminescence of the composition of components for construction of the PS/PPO/CeF3 nanocomposite is studied and compared together to show the observed enhancements. The photoluminescence (PL) emissions of PS and PPO, along with absorption spectrum of PPO measured at room temperature is shown in Fig. 4. As can be seen from Fig. 4, the existing overlap between the PPO absorption and PS emission spectrums is adequate to transfer energy between them. Consequently, more emission will be observed by PPO at longer wavelength.
The absorption and PL emission spectrum of PS/PPO, along with PL emission spectrum of CeF3 nanoparticles are presented in Fig. 5. As can be seen from Fig. 5, there is an overlaps between the emission of CeF3 and absorption of PS/PPO that in turn accomplish the condition for FRET. Thereupon energy can be transferred from CeF3 nanoparticles to PS/PPO. In addition, the lifetime of CeF3 (30 ns) [11, 12] is sufficiently longer that of PPO (1.6 ns) [13] satisfy FRET condition to occur.
Different percentage of CeF3 nanoparticles were added into the PS matrix that contains constant percentage of PPO, 0.5 wt%, to proof enhancement effect of CeF3 nanoparticles on the emission of PS/PPO. Figure 6 shows the PL spectrum of excited PS/PPO/CeF3 nanocomposites at 290 nm. As can be seen the photoluminescence of PS/PPO composites notably enhanced by the CeF3 nanoparticles. As expected, due to the energy transfer from CeF3 nanoparticles to the PPO in the PS matrix as shown in Fig. 7, an enhancement in the emission intensity of nanocomposite are presented. Furthermore, absence of CeF3 nanoparticles emission in the spectrums, confirm the FRET phenomenon occurred between CeF3 nanoparticles and PS/PPO composite. Also, as can be seen from Fig. 6, an enhancement about 3 times was observed in the emission of nanocomposites up to 10 wt% of CeF3 doping, and then reduced to less than 1.5 times for 15 wt% of CeF3. It is clear that increasing in percentage of CeF3 in the nanocomposite, cause to a red shift in the emission of nanocomposites.
4 Conclusion
PS/PPO/CeF3 nanocomposite was prepared with 0.5 wt% PPO and various percentage of CeF3 nanoparticles. Its structure and luminescence properties of constituents were studied systematically. Our results showed an enhanced luminescence in PS/PPO when embedded with CeF3 nanoparticles. Enhancement in the luminescence of PS/PPO scintillator reaches about 3 times when doped with 10 wt% of CeF3 nanoparticles. This enhancement can be assigned to the energy transfer, the increase of the stopping power by doping CeF3 nanoparticles. However, our findings represent a new method to improve PS/PPO organic scintillators for radiation detection.
References
Knoll GF (2010) Radiation detection and measurement. Wiley, Hoboken
Milbrath BD, Peurrung AJ, Bliss M, Weber WJ (2008) Radiation detector materials: an overview. J Mater Res 23(10):2561–2581
Vasil’chenko V, Solov’Ev A (2003) Properties of composite scintillators in static and dynamic states. Instrum Exp Tech 46(6):758–764
McKigney EA, Del Sesto RE, Jacobsohn LG, Santi PA, Muenchausen RE, Ott KC, McCleskey TM, Bennett BL, Smith JF, Cooke DW (2007) Nanocomposite scintillators for radiation detection and nuclear spectroscopy. Nucl Instrum Methods Phys Res Sect A 579(1):15–18
Kang Z, Barta M, Nadler J, Wagner B, Rosson R, Kahn B (2011) Synthesis of BaF2: Ce nanophosphor and epoxy encapsulated transparent nanocomposite. J Lumin 131(10):2140–2143
Cai W, Chen Q, Cherepy N, Dooraghi A, Kishpaugh D, Chatziioannou A, Payne S, Xiang W, Pei Q (2013) Synthesis of bulk-size transparent gadolinium oxide–polymer nanocomposites for gamma ray spectroscopy. J Mater Chem C 1(10):1970–1976
Zhong H, Zhao Y, Li Y, Pei Q (2008) Photoluminescence quenching of conjugated polymer nanocomposites for gamma ray detection. Nanotechnology 19(50):505503
Feller RK, Purdy GM, Ortiz-Acosta D, Stange S, Li A, McKigney EA, Esch EI, Muenchausen RE, Gilbertson R, Bacrania M (2011) Large-scale synthesis of CexLa1−xF3 nanocomposite scintillator materials. J Mater Chem 21(15):5716–5722
Sahi S, Chen W, Jiang K (2015) Luminescence enhancement of PPO/PVT scintillators by CeF3 nanoparticles. J Lumin 159:105–109
Nurmukhametov R, Volkova L, Kabanov S (2006) Fluorescence and absorption of polystyrene exposed to UV laser radiation. J Appl Spectrosc 73(1):55–60
Moses W, Derenzo S, Weber M, Ray-Chaudhuri A, Cerrina F (1994) Scintillation mechanisms in cerium fluoride. J Lumin 59(1–2):89–100
Moses W, Derenzo SE (1989) Cerium fluoride, a new fast, heavy scintillator. IEEE Trans Nucl Sci 36(1):173–176
Luchowski R (2011) Two-photon excitation of 2,5-diphenyloxazole using a low power green solid state laser. Chem Phys Lett 501(4–6):572–574
Acknowledgements
The facilities of this work were supported by NSTRI and University of Shahrekord, and the authors are gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Karimi, M., Raeisi, M., Bagherzadeh, M. et al. Enhancement in photoluminescence properties of organic compound PS/PPO by cerium fluoride nanoparticles doping. SN Appl. Sci. 1, 635 (2019). https://doi.org/10.1007/s42452-019-0658-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-019-0658-2