Abstract
In this work, the Ceria (CeO2) was synthesized by co-precipitation method. The thick films of synthesized CeO2 were prepared by screen printing technique. Films were fired at 350 °C, 400 °C, 450 °C for 2 h in muffle furnace. These films were characterized by XRD to investigate the structural properties of prepared material. The XRD confirmed the single phase cerium oxide nanoparticles. Scanning electron microscopy was utilized for the morphological parameters of prepared cerium oxide nanoparticles. The cubic shaped cerium nanoparticles with various diameters were observed from SEM images. The elemental composition of ceria was studied by EDS, where the perfect elemental composition of ceria was confirmed. The Fourier transform infra red spectroscopy was employed to find out metal- oxide vibrational bands in the cerium oxide nanoparticles. The effect of firing temperature was studied for cerium oxide calcined at 350 °C, 400 °C and 450 °C nanoparticles to study the various parameters such as Grain Size, Temperature Coefficient (TC), Stacking Fault Probability (α), Dislocation Density (ρ), Surface area, Activation Energy and RMS Microstrain (e).
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The nanotechnology now a day at the top edge of research due to its remarkable application in various fields of catalysis. Due to versatile application of nanotechnology, it attracted the attention of many researchers. The material science covers various applications such as homogeneous and heterogeneous catalysis, solar cells, gas sensors, SOFC (Solid oxide fuel cell), high density memory devices, biosensors, drug delivery mechanism, photocatalysis, green synthesis using plant extract, chemotherapy, organic conversions, waste water treatment or effluent treatment etc. [1, 2].
A wide range of materials have been synthesized for various applications of nanoscience. Generally the transition metals oxides, very efficient catalyst have been used for various applications. But now a day’s use of inner transition metal oxide is a new trend in the field of catalysis, particularly lanthanum, cerium and europium oxide are extensively used materials for sensing mechanism [3, 4] The pure oxide in the form of M2O3 and pervoskite materials are frequently used materials. The most widely used material among lanthanide series ceria nanoparticles. Cerium possess some exceptional properties such as SOFC, oxygen ion conductor [5], solid state electrolytes for electrochemical devices, oxygen storage capacitor (OSC), sunscreen for UV absorbent, grating for chemical and mechanical planarization, hybrid solar cells [4].Cerium oxide has a cubic fluorite structure with band gap 3.26 eV, hence cerium is can be utilized as effective semiconductor due to sufficient distance between valance band and conduction band. It has lattice constant found to be 5.411 with \({\text{Fm}}\bar{3}{\text{m,}}\# 225\) as space group.
The common methods used for the synthesis of ceria nanoparticles are co-precipitation method, sol–gel method, forced hydrolysis, hydrothermal method, surfactant templating method, combustion method, synthesis using plant extract, Synthesis using ball grinding method. All the above listed methods are recognized method for the synthesis of various nanoparticles [6,7,8,9].
Most of the researchers have been reported the applications of synthesized nanoparticles in various filed, particularly the inner transition metal oxides are found to be excellent catalyst in the field of sensing mechanism as sensors, transducers, electronic and magnetic applications. Especially lanthanum and cerium oxides, doped with minute concentration of inner transition metals, transition metals and some selected P-block metals enhanced the catalytical reactivity of cerium and lanthanum oxides. Researchers also reported that the lanthanum and cerium oxides are potent catalyst for sensor mechanism [10,11,12].
In the present research authors are highlighting the structural based properties of cerium oxide which are studied with the help of X-ray diffractometer, Scanning electron microscope (SEM), Fourier transform infra red (FT-IR) spectroscopy etc. The method of material synthesis and microstructural defects leads to change the various properties of material such as optical, physical, electrical and sometimes magnetic properties of the material. These minute changes in the material may be influence on the catalytical and desired substantial properties. Literature also reports that these minute defects leads to decrease the sensing and adsorption related properties of solid materials [13,14,15].
Ceria nanomaterials prepared by co-precipitation method were studied for different structural properties. These nanoparticles were extremely utilized as sensors for most of the oxidizing and reducing gases, but the microstructural investigation of ceria nanoparticles has not been reported. Thus authors are reporting this research for finding any structural defects, etymology produced in the ceria nanoparticles synthesized by co-precipitation method.
In this research paper we are reporting structural and morphological investigation of ceria nanoparticles under the influence of firing temperature effect ranging from 350 to 450 °C. The structural and morphological study involves effect on the parameters such as Grain size, Stacking fault Probability (α), Dislocation density (ρ) RMS micro-strain, surface area and activation energy [16,17,18].
2 Materials and methods
All the in chemicals used in synthesis are of AR grade purchased from Loba chemie, Mumbai such as [Ce(NO3)3]·6H2O, propylene glycol, ammonia, Double purified water. The chemicals were used without further purification.
2.1 Synthesis of ceria nanoparticles by co-precipitation method
The refinement of ceria nanoparticles conjoin with; [Ce(NO3)3]·6H2O, propylene glycol and ammonia. Preparation of desired solutions are uniformed with double purified water, disorganized Nano-crystalline CeO2 powder was prepared by controlled addition of aqueous ammonia to a mixture of 0.1 M aqueous solution of cerium nitrate and propylene glycol; the ratio of concentration of propylene glycol to cerium nitrate solution was kept 1:1. The special arrangement was made to add drop wise aqueous ammonia into the solution with constant stirring. After complete swiftness, the precipitated hydroxide was washed with purified water, and then pure hydroxide in a crucible was placed in a kiln for fireside (at 400 °C) about 120 min. The Phase purity and the degree of crystallinity of the resulting CeO2 sample were monitored by XRD analysis [19, 20].
2.2 Fabrication of ceria thick films by screen printing method
The thick films of pure Ceria (CeO2) were prepared by screen printing technique, in which the inorganic and organic ratio was kept constant as 70:30 respectively. The inorganic part comprises by pure ceria nanoparticles. Whereas organic parts reckon in with 8% BCA (Butyl Carbitol Acetate) and 92% ethyl cellulose; these compounds were assembled in mortar and pestle which has lavement with acetone and dried [21]. BCA added drop wise in accumulated mixtures to achieve the proper pseudo-plastic phase (Thixotropic), which glutinous in nature; then applied on previously shaped glass substrate (2 × 1 cm) by screen printing method. The screen of nylon (40 s, mesh number 355) was used for screen printing. The standard mask size was developed on the screen by photolithography technique, after that the films were kept as it is for 15 min, then films were dried under IR lamp for 30 min [22, 23]. Finally the films were calcined under muffle furnace at 600 °C for 4 h. The pure ceria thick films are ready for characterization and further use.
3 Characterization methods
3.1 X-ray diffraction
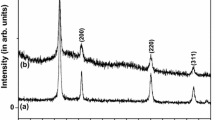
The Structural properties of ceria thick film were analyzed by using X-ray diffraction method. The material ceria was calcined at three different temperatures 350 °C, 400 °C and 450 °C. The typical XRD spectrum of cerium oxide is as depicted in Fig. 1 the spectrum shows 2θ values from 28.302, 32.851, 47.270, 56.089, 58.889, 69.178, 76.528, 78.838, which corresponds to hkl planes as 111, 200, 220,311, 222, 400, 331, and 420 respectively. Data obtained from XRD confirms the single phase cubic cerium oxide formation. The average particle size calculated from Scherrer formula (Eq. 1) fired at 350 °C, 400 °C, 450 °C was 13.63 nm, 12.63 nm, 13.7 nm respectively.
where D is average particle size, K is constant (0.9 to 1), λ = wavelength of X-rays (1.54 Å) β is full width half maxima (FWHM) of diffracted peak, θ is the angle of diffraction. The XRD pattern of cerium oxide nanoparticles matches with the JCPDS standard number 750390.
3.2 Texture coefficient: (TC)
Values which are obtained using Eq. (2) it has seen that TC advice oneness for conjecturally distributed specimen; whereas TC is larger than oneness of preferentially oriented (hkl) plane. The inferior values of TC irradiates that, the films have poor crystallinity and this may be improved at higher firing temperature Fig. 2 shows the variation of the texture coefficient for the firing temperature ranging from 300 to 450 °C for (111), (200), (220), (311), planes. It is observed that the preferred orientation is (111) plane for all firing temperatures. The enhancement in preferred orientation is assigned to maximum number of grains belongs to this orientation [24, 25].
3.3 Stacking fault probability (α)
Stacking fault probability arises when one atomic plane sets out of series than others to start piling up; on the other hand lattice remains surpassing. Due to stacking fault only the position of peak shows variation from JCPDS for its corresponding sample. The stacking fault is most of the time considered as a planar defect. As the defect is associated with shifting of the planes two theta values of XRD data. When the particular solid material is calcined at different temperatures these types of are more pronounced and can be identified by the XRD analysis. Whenever the order of stacking fault is higher in a particular material then the exact cross slip dislocation can be found. Whereas at lower temperature the perfect disorder is cannot be observed but small dislocation can be seen. The diagram for preferred plane orientation for stacking fault probability is as shown in Fig. 3.
3.4 Micro strain
Broaden X-ray diffracted line frame work mainly observed by non-prototype of instrument, wavelength dispersion and microstructural flaw in the crystal. Out of these three the microstructural flaw is sectioned into size broadening and strain broadening. Size broadening results due to confined size of domain occupy by stacking faults or by other flaws which are irrelevant with each other on the other hand strain broadening is conveyed by altered position of the atom with respect to their cited lattice parameter. An identical compressive or tensile strain (microstrain) is outcome of peak shift of the X-ray diffraction lines, whereas an identical stastical separation of tensile and compressive strain together outcomes into broaden diffraction lines (microstrain). The detailed method of for determining microstrain by X-ray diffraction lines are depicted by Williamson and hall plot. It is reputed scheme which explains qualitative knowledge of anisotropy broadening. Here, Slope of a plot of (βcos θ/λ) versus (2sin θ/λ) gives the value of the RMS Microstrain Fig. 3 depicts the Williamson-Hall plot for CeO2 thick films with different deformation from planes (111) to (420) at different calcined temperature, Here in Fig. 3 the points are scattered then microstrain is RMS that is if (βcos θ/λ) is nonmonotonic function of (2sin θ/λ), the broadening is termed anisotropic. The RMS microstrain calculated at different firing temperatures indicate a decreasing trend with firing temperature is given in Table 1 (Figs. 4, 5).
3.5 Scanning electron microscopy (SEM)
The SEM images of prepared CeO2 material is as depicted in Fig. 6. The material was calcined at three different temperatures at 350 °C, 400 °C, 450 °C. From SEM images it is found that the crystal size of ceria nanoparticles is increased with rise in temperature. The various size ceria nanoparticles can be seen from the SEM images as shown in Fig. 6. The calculated particle size of crystalline ceria particles is as shown in Table 1. Due to effect of calcination temperature on the nanoparticles, the atomic mobility if increase. This leads to movement of atoms to more comfortable sites on the surface such voids, interstitial positions, grain boundaries etc. The ceria nanoparticles found to be very effectively adhered on the film surface. As well as the surface area and homogeneous surface of ceria nanoparticles is found to be very effective in electrical conductivity and sensing mechanism (Table 2).
3.6 Energy dispersive spectroscopy (EDS)
The cerium oxide nanoparticles were characterized by EDS to know the elemental composition of prepared material. The EDS spectrum of prepared CeO2 calcined at 350 °C, 400 °C, 450 °C is as depicted in Fig. 7 from which the perfect elemental composition of cerium oxide is can be seen. The mass percentage of cerium is found to be decrease with increase in temperature as the oxygen mass percentage was increased with increase in temperature. This reverse trend is observed in case of Ceria nanoparticles is due to the inherent SOFC (Solid oxide fuel cell), OSC (oxygen storage capacitor) properties of ceria. The effect of firing temperature on the elemental composition is clearly can be seen from the following data represented in Table 3.
3.7 Infra-red spectroscopy
The infra-red spectroscopy is normally enables the chemical bonding formation in the compounds. These bonding can be detected in terms of stretching and bending mode of frequencies. The metal oxide linkages have a typical stretch in IR radiations and hence these unique frequencies can be detected by using vibrational stretching bands. Cerium oxide is fluorite type material with cubic arrangement. The typical IR spectrum of prepared ceria nanoparticles is as shown in Fig. 8, where the different frequency bands can be observed. The typical Ce–O stretching vibration of the material calcined at 350 °C, 400 °C, 450 °C found at 794.30 cm−1, 764 cm−1 and 763.6 Cm−1 respectively.Where as deformed water stretching is found at 1627.50 cm−1, 1596.9 cm−1 and 1591.5 cm−1 respectively for cerium oxide calcined at 350 °C, 400 °C, 450 °C.The broad peaks in each cerium oxide calcined at different temperatures is typically found at 3437.3, 3453.3, 3361.2.
4 Conclusions
This research work has been attributed to the synthesis of Ceria nanoparticles by a cost effective co-precipitation method. Subsequently the screen printed thick films of ceria nanoparticles were applied for the investigation of some structural parameters such as Microstrain, dislocation density, RMS, texture coefficient and stacking fault probability. The crystallographic parameters as mention above found to be influenced due to increase temperature effect. The texture coefficient and stacking fault probability was studied and discussed for the major diffraction line such as (111). The effect of temperature on various microstructural properties studied in the present research, (represented in Table 1). The particle size reported from SEM found to be increased due to increase in grain size at higher temperature. The specific surface area is observed to be decrease due to increase in grain size, as there is an inverse relation between grain size and surface area. The planar defect stacking fault probability is increased with the rise in temperature probably due to enhancement of randomness at higher temperature. As all these parameters are closely related to each other regarding the structural aspects of the materials. The study of such parameters can be useful while working with the structural and morphological investigations of the materials.
References
Tang M-H, Zhou Y-C, Zheng X-J, Wei Q-P, Cheng C-P, Ye Z, Hu Z-S (2007) Microstructure and electrical properties of CeO2 ultra-thin films for MFIS feram Applications. Trans Nonferr Met Soc 17:S741–S746
Zhang L, Fang Q, Huang Y, Xu K, Chu PK, Ma F (2018) Oxygen vacancy enhanced gas sensing performance of CeO2 graphene heterostructure at room temperature. Anal Chem. https://doi.org/10.1021/acs.analchem.8b01768
Dhall A, Self W (2018) Cerium oxide nanoparticles: a brief review of their synthesis methods and biomedical applications. Antioxidants. https://doi.org/10.3390/antiox7080097
Farahmandjou M, Zarinkamar M, Firoozabadi TP (2016) Synthesis of cerium oxide (CeO2) nanoparticles using simple co-precipitation method. Revista Mexicana de Física 62:496–499
Acharya SA, Gaikwad VM, Sathe V, Kulkarni SK (2014) Influence of gadolinium doping on the structure and defects of ceria under fuel cell operating temperature. Appl Phys Lett 104:113508. https://doi.org/10.1063/1.4869116
De Marzi L, Monaco A, De Lapuente J, Ramos D, Borras M, Di Gioacchino M, Santucci S, Poma A (2013) Cytotoxicity and genotoxicity of ceria nanoparticles on different cell lines in vitro. Mol Sci 14:3065–3077. https://doi.org/10.3390/ijms14023065
Hu C, Zhang Z, Liu H, Gao P, Wang ZL (2006) Direct synthesis and structure characterization of ultrafine CeO2 Nanoparticles. Nanotechnology 17:5983–5987. https://doi.org/10.1088/0957-4484/17/24/013
Chen MY, Zu XT, Xiang X, Zhang HL (2007) Effects of ion irradiation and annealing on optical and structural properties of CeO2 films on sapphire. Phys B 389:263–268. https://doi.org/10.1016/j.physb.2006.06.162
Elidrissi B, Addou M, Regragui M, Monty C, Bougrine A, Kachouane A (2000) Structural and optical properties of CeO2 thin films prepared by spray pyrolysis. Thin Solid Films 379:23–27
Guzman J, Carrettin S, Corma A (2005) Spectroscopic evidence for the supply of reactive oxygen during CO oxidation catalyzed by gold supported on nano crystalline CeO2. J Am Chem Soc 127:3286–3287
Kek-Merl D, Lappalainen J, Tuller HL (2006) Electrical properties of nanocrystalline CeO2 thin films deposited by in situ pulsed laser deposition. J Electrochem Soc 153(3):J15–J20
Liu YH, Zuo JC, Ren XF, Yong L (2014) Synthesis and characterization of cerium oxide (CeO2) nanoparticles by the precipitation method. Metalurgija 53:463–465
Qi G, Yang RT, Chang R (2004) MnOx-CeO2 mixed oxides prepared by co-precipitation for selective catalytic reduction of NO with NH3 at low temperatures. Appl Catal B 51:93–106
Si R, Flytzani-Stephanopoulos M (2008) Shape and crystal-plane effects of nanoscale ceria on the activity of Au-CeO2 catalysts for the water–gas shift reaction. Angew Chem 120:2926–2929
Akla AA, Mahmoud SA, Al-Shomar SM, Hassanien AS (2018) Improving microstructural properties and minimizing crystal imperfections of nanocrystalline Cu2O thin films of different solution molarities for solar cell applications. Mater Sci Semicond Process 74:183–192. https://doi.org/10.1016/j.mssp.2017.10.007
Trovarelli A (1996) Catalytic properties of ceria and CeO2-containing materials. Catal Rev 38(4):439–520. https://doi.org/10.1080/01614949608006464
Xiong Y-P, Kishimoto H, Yamaji K, Yoshinaga M, Horita T, Brito ME, Yokokawa H (2011) Electronic conductivity of pure ceria. Solid State Ionics 192:476–479. https://doi.org/10.1016/j.ssi.2010.07.017
Patil AV, Dighavkar CG, Sonawane SK, Shinde UP, Patil SJ, Borse RY (2010) Study of microstructural parameters of screen printed ZnO thick film sensors. Sens Transducers 117(6):62–70
Kanakaraju S, Mohana S, Sood AK (1997) Optical and structural properties of reactive ion beam sputter deposited CeO2 films. Thin Solid Films 305:191–195
Kasipar J, Fornasiero P, Graziani M (1999) Use of CeO2-based oxides In the three-way catalysis. Catal Today 50:285–298
Borhade AV, Tope DR, Agashe JA (2018) Synthesis, characterization and gas sensing performance of nanocrystalline ZrO2, 5%Y/ZrO2 and Ag–5%Y/ZrO2 catalyst. J Mater Sci Mater Electron 29: 7551–7561
Patil AV, Dighavkar CG, Sonawane SK, Patil SJ, Borse RY (2009) Effect of firing temperature on electrical and Structural characteristics of screen printed ZnO thick films. Optoelectr Adv Mater Rapid Commun 3(9):879–883
Patil AV, Dighavkar CG, Sonawane SK, Patil SJJ, Borse RY (2009) Formulation and characterization of Cr2O3 doped ZnO thick films as H2S gas sensor. Sensors & Transducers 108(9):189–197
Schuler T, Aegerter MA (1999) Optical, electrical and structural properties of sol gel ZnO: Al coatings. Thin Solid Films 351(1–2):125–131. https://doi.org/10.1016/S0040-6090(99)00211-4
Durrani SMA, Al-Kuhaili MF, Bakhtiari IA (2009) Carbon monoxide gas-sensing properties of electron-beam deposited cerium oxide thin films. Sens Actuators B 134(2008):934–939. https://doi.org/10.1016/j.snb.2008.06.049
Acknowledgements
Authors are gratefully acknowledged to the SAIF UDCT, Jalgaon (M.S) for XRD, SEM study. Authors are thankful to SAIF IIT Bombay for TEM and EDS analysis as well as SAIF Chandigarh (Punjab University) for FT-IR study. Authors are very thankful to Department of Chemistry, L.V.H. College, Panchavati, Nashik and Department of electronics L.V.H. College, Panchavati, Nashik.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Singh, R.D., Koli, P.B., Jagdale, B.S. et al. Effect of firing temperature on structural and electrical parameters of synthesized CeO2 thick films. SN Appl. Sci. 1, 315 (2019). https://doi.org/10.1007/s42452-019-0246-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-019-0246-5