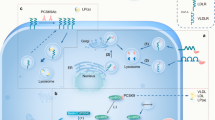
Abstract
Cardiovascular disease is the leading cause of mortality in chronic kidney disease (CKD) and dialysis patients. This association may be attributed to the highly atherogenic dyslipidemia, which is characteristically present in these patients. Despite their proven benefit on cardiovascular outcomes in the general population, statin-based therapies have proven less effective in patients with CKD. Recent discoveries in the physiology of lipid metabolism, regarding the role of proprotein convertase subtilisin/kexin type 9 (PCSK9) and lipoprotein (a) [Lp(a)] in atherosclerosis have provided new perspectives. Data from randomized trials in the general population testing the efficacy and safety of novel drug therapies, specifically targeting these molecules, indicate significant reductions in cardiovascular events and mortality. However, patients with reduced renal function, who may benefit the most from such treatments, were excluded from these studies, making these therapeutic approaches inapplicable to this complicated population. There is an urgent call for further studies to examine whether new treatments such as those targeting PCSK9 would manage to decrease the high cardiovascular mortality rates in CKD and dialysis patients.


Similar content being viewed by others
Data Availability
This is a review manuscript, and we have not obtained any patients’ data.
Code Availability
This is a review manuscript, and a software code was not used.
References
Lindner A, Charra B, Sherrard DJ, et al. Accelerated atherosclerosis in prolonged maintenance hemodialysis. N Engl J Med. 1974;290:697–701.
Foley RN, Parfrey PS, Sarnak MJ. Clinical epidemiology of cardiovascular disease in chronic renal disease. Am J Kidney Dis. 1998;32:S112–9.
Go AS, Chertow GM, Fan D, et al. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–305.
Matsushita K, van der Velde M, Astor BC, et al. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet. 2010;375:2073–81.
McCullough PA, Li S, Jurkovitz CT, et al. Chronic kidney disease, prevalence of premature cardiovascular disease, and relationship to short-term mortality. Am Heart J. 2008;156:277–83.
Vogel J. The pathological anatomy of the human body. Philadelphia: Lea and Blanchard; 1847.
Kannel WB, Dawber TR, Kagan A, et al. Factors of risk in the development of coronary heart disease–six year follow-up experience The Framingham Study. Ann Intern Med. 1961;55:33–50.
Kannel WB, Castelli WP, Gordon T, et al. Serum cholesterol, lipoproteins, and the risk of coronary heart disease. The Framingham study. Ann Intern Med. 1971;74:1–12.
Ferro CJ, Mark PB, Kanbay M, et al. Lipid management in patients with chronic kidney disease. Nat Rev Nephrol. 2018;14:727–49.
Wadhera RK, Steen DL, Khan I, et al. A review of low-density lipoprotein cholesterol, treatment strategies, and its impact on cardiovascular disease morbidity and mortality. J Clin Lipidol. 2016;10:472–89.
Bermudez-Lopez M, Forne C, Amigo N, et al. An in-depth analysis shows a hidden atherogenic lipoprotein profile in non-diabetic chronic kidney disease patients. Expert Opin Ther Targets. 2019;23:619–30.
Wanner C, Krane V, Marz W, et al. Atorvastatin in patients with type 2 diabetes mellitus undergoing hemodialysis. N Engl J Med. 2005;353:238–48.
Fellstrom BC, Jardine AG, Schmieder RE, et al. Rosuvastatin and cardiovascular events in patients undergoing hemodialysis. N Engl J Med. 2009;360:1395–407.
Baigent C, Landray MJ, Reith C, et al. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomised placebo-controlled trial. Lancet. 2011;377:2181–92.
Palmer SC, Navaneethan SD, Craig JC, et al. HMG CoA reductase inhibitors (statins) for dialysis patients. Cochrane Database Syst Rev. 2013;11:CD004289. https://doi.org/10.1002/14651858.CD004289.pub5
Herrington WG, Emberson J, Mihaylova B, et al. Impact of renal function on the effects of LDL cholesterol lowering with statin-based regimens: a meta-analysis of individual participant data from 28 randomised trials. Lancet Diabetes Endocrinol. 2016;4:829–39.
Fernandez-Prado R, Perez-Gomez MV, Ortiz A. Pelacarsen for lowering lipoprotein(a): implications for patients with chronic kidney disease. Clin Kidney J. 2020;13:753–7.
Abifadel M, Varret M, Rabes JP, et al. Mutations in PCSK9 cause autosomal dominant hypercholesterolemia. Nat Genet. 2003;34:154–6.
Cohen J, Pertsemlidis A, Kotowski IK, et al. Low LDL cholesterol in individuals of African descent resulting from frequent nonsense mutations in PCSK9. Nat Genet. 2005;37:161–5.
Benn M, Nordestgaard BG, Grande P, et al. PCSK9 R46L, low-density lipoprotein cholesterol levels, and risk of ischemic heart disease: 3 independent studies and meta-analyses. J Am Coll Cardiol. 2010;55:2833–42.
Rogacev KS, Heine GH, Silbernagel G, et al. PCSK9 plasma concentrations are independent of GFR and do not predict cardiovascular events in patients with decreased GFR. PLoS ONE. 2016;11:e0146920.
Elewa U, Fernandez-Fernandez B, Mahillo-Fernandez I, et al. PCSK9 in diabetic kidney disease. Eur J Clin Invest. 2016;46:779–86.
Dong B, Wu M, Li H, et al. Strong induction of PCSK9 gene expression through HNF1alpha and SREBP2: mechanism for the resistance to LDL-cholesterol lowering effect of statins in dyslipidemic hamsters. J Lipid Res. 2010;51:1486–95.
Abujrad H, Mayne J, Ruzicka M, et al. Chronic kidney disease on hemodialysis is associated with decreased serum PCSK9 levels. Atherosclerosis. 2014;233:123–9.
Jin K, Park BS, Kim YW, et al. Plasma PCSK9 in nephrotic syndrome and in peritoneal dialysis: a cross-sectional study. Am J Kidney Dis. 2014;63:584–9.
Kostner GM, Gavish D, Leopold B, et al. HMG CoA reductase inhibitors lower LDL cholesterol without reducing Lp(a) levels. Circulation. 1989;80:1313–9.
Berthold HK, Seidah NG, Benjannet S, et al. Evidence from a randomized trial that simvastatin, but not ezetimibe, upregulates circulating PCSK9 levels. PLoS ONE. 2013;8:e60095.
Taylor BA, Thompson PD. Statins and their effect on PCSK9-impact and clinical relevance. Curr Atheroscler Rep. 2016;18:46.
Careskey HE, Davis RA, Alborn WE, et al. Atorvastatin increases human serum levels of proprotein convertase subtilisin/kexin type 9. J Lipid Res. 2008;49:394–8.
Seidah NG. PCSK9 as a therapeutic target of dyslipidemia. Expert Opin Ther Targets. 2009;13:19–28.
Awan Z, Seidah NG, MacFadyen JG, et al. Rosuvastatin, proprotein convertase subtilisin/kexin type 9 concentrations, and LDL cholesterol response: the JUPITER trial. Clin Chem. 2012;58:183–9.
Robinson JG, Farnier M, Krempf M, et al. Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372:1489–99.
Maki KC. The ODYSSEY Outcomes trial: clinical implications and exploration of the limits of what can be achieved through lipid lowering. J Clin Lipidol. 2018;12:1102–5.
Fitzgerald G, Kiernan T. PCSK9 inhibitors and LDL reduction: pharmacology, clinical implications, and future perspectives. Expert Rev Cardiovasc Ther. 2018;16:567–78.
Sabatine MS, Giugliano RP, Keech AC, et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376:1713–22.
Bergmark BA, O’Donoghue ML, Murphy SA, et al. An exploratory analysis of proprotein convertase subtilisin/kexin type 9 inhibition and aortic stenosis in the FOURIER trial. JAMA Cardiol. 2020;5:709–13.
Yeang C, Witztum JL, Tsimikas S. “LDL-C” = LDL-C + Lp(a)-C: implications of achieved ultra-low LDL-C levels in the proprotein convertase subtilisin/kexin type 9 era of potent LDL-C lowering. Curr Opin Lipidol. 2015;26:169–78.
Tsimikas S, Stroes ESG. The dedicated “Lp(a) clinic”: a concept whose time has arrived? Atherosclerosis. 2020;300:1–9.
Viney NJ, van Capelleveen JC, Geary RS, et al. Antisense oligonucleotides targeting apolipoprotein(a) in people with raised lipoprotein(a): two randomised, double-blind, placebo-controlled, dose-ranging trials. Lancet. 2016;388:2239–53.
Agrawal S, Zaritsky JJ, Fornoni A, et al. Dyslipidaemia in nephrotic syndrome: mechanisms and treatment. Nat Rev Nephrol. 2018;14:57–70.
Kronenberg F. Causes and consequences of lipoprotein(a) abnormalities in kidney disease. Clin Exp Nephrol. 2014;18:234–7.
Tsimikas S, Gordts P, Nora C, et al. Statin therapy increases lipoprotein(a) levels. Eur Heart J. 2020;41:2275–84.
Willeit P, Ridker PM, Nestel PJ, et al. Baseline and on-statin treatment lipoprotein(a) levels for prediction of cardiovascular events: individual patient-data meta-analysis of statin outcome trials. Lancet. 2018;392:1311–20.
Schwartz GG, Steg PG, Szarek M, et al. Alirocumab and cardiovascular outcomes after acute coronary syndrome. N Engl J Med. 2018;379:2097–107.
Bittner VA, Szarek M, Aylward PE, et al. Effect of alirocumab on lipoprotein(a) and cardiovascular risk after acute coronary syndrome. J Am Coll Cardiol. 2020;75:133–44.
Romagnuolo R, Scipione CA, Boffa MB, et al. Lipoprotein(a) catabolism is regulated by proprotein convertase subtilisin/kexin type 9 through the low density lipoprotein receptor. J Biol Chem. 2015;290:11649–62.
Tsimikas S, Karwatowska-Prokopczuk E, Gouni-Berthold I, et al. Lipoprotein(a) reduction in persons with cardiovascular disease. N Engl J Med. 2020;382:244–55.
Catapano AL, Graham I, De Backer G, et al. 2016 ESC/EAS guidelines for the management of dyslipidaemias. Eur Heart J. 2016;37:2999–3058.
Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139:e1082–143.
Dolgin E. Lp(a)-lowering drugs bolster cardiovascular pipeline. Nat Rev Drug Discov. 2020;19:154–5.
Charytan DM, Sabatine MS, Pedersen TR, et al. Efficacy and safety of evolocumab in chronic kidney disease in the FOURIER trial. J Am Coll Cardiol. 2019;73:2961–70.
Toth PP, Dwyer JP, Cannon CP, et al. Efficacy and safety of lipid lowering by alirocumab in chronic kidney disease. Kidney Int. 2018;93:1397–408.
Ishii T, Ogura M, Nakamori H, et al. Switching from lipoprotein apheresis to evolocumab in FH siblings on hemodialysis: case reports and discussion. CEN Case Rep. 2021;10:592–7.
González Sanchidrián S, Labrador Gómez PJ, Aguilar Aguilar JC, et al. Evolocumab for the treatment of heterozygous familial hypercholesterolemia in end-stage chronic kidney disease and dialysis. Nefrologia (Engl Ed). 2019;39:218–20.
Assessing the impact of lipoprotein (a) lowering with TQJ230 on major cardiovascular events in patients with CVD (Lp(a)HORIZON). In: ClinicalTrials.Gov. 2019. https://clinicaltrials.gov/ct2/show/NCT04023552. Accessed 25 Sept 2020
Lee E, Gibbs JP, Emery MG, et al. Influence of renal function on evolocumab exposure, pharmacodynamics, and safety. Clin Pharmacol Drug Dev. 2019;8:281–9.
NCT04510844: Evolocumab in advanced chronic kidney disease trial (EVO-CKD). In: ClinicalTrials.gov. 2020. https://clinicaltrials.gov/ct2/show/NCT04510844. Accessed 19/4/2022
Calò L. NCT04659525: Evolocumab plus ezetimibe in haemodialized statin-intolerant patients with hypercholesterolemia. In: ClinicalTrials.gov. 2021. https://clinicaltrials.gov/ct2/show/NCT04659525. Accessed 19/4/2022
Calò L. NCT04397653: Evolocumab plus ezetimibe in high risk haemodialized statin intolerant patients. In: ClinicalTrials.gov. 2020. https://clinicaltrials.gov/ct2/show/NCT04397653. Accessed 19/4/2022
Author information
Authors and Affiliations
Contributions
CL and CJF drafted the manuscript and performed the revisions, PAS updated the literature, AO performed critical analysis of the manuscript, and CJF provided supervision and mentorship.
Corresponding author
Ethics declarations
Ethics Approval
This is a review of the literature, and an ethics approval was not applicable.
Consent to Participate
This is a review manuscript, and we have included any study participants.
Consent for Publication
All authors reviewed this manuscript and consented to its publication.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Topical Collection on Medicine.
Rights and permissions
About this article
Cite this article
Loutradis, C., Sarafidis, P.A., Ortiz, A. et al. Is Our Increasing Understanding of PCSK9 and Lp(a) Metabolism the Key to Unlocking the Paradox of Statins Ineffectiveness in Reducing Cardiovascular Events in Advanced CKD?. SN Compr. Clin. Med. 4, 145 (2022). https://doi.org/10.1007/s42399-022-01242-w
Accepted:
Published:
DOI: https://doi.org/10.1007/s42399-022-01242-w