Abstract
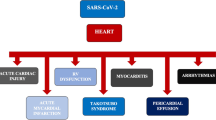
Takotsubo syndrome (TTS) is caused by catecholamine surge, which is also observed in COVID-19 disease due to the cytokine storm. We performed a systematic literature search using PubMed/Medline, SCOPUS, Web of Science, and Google Scholar databases to identify COVID-19-associated TTS case reports and evaluated patient-level demographics, clinical attributes, and outcomes. There are 12 cases reported of TTS associated with COVID-19 infection with mean age of 70.8 ± 15.2 years (range 43–87 years) with elderly (66.6% > 60 years) female (66.6%) majority. The time interval from the first symptom to TTS was 8.3 ± 3.6 days (range 3–14 days). Out of 12 cases, 7 reported apical ballooning, 4 reported basal segment hypo/akinesia, and 1 reported median TTS. Out of 12 cases, during hospitalization, data on left ventricular ejection fraction (LVEF) was reported in only 9 of the cases. The mean LVEF was 40.6 ± 9.9% (male, 46.7 ± 5.7%, and female, 37.7 ± 10.6%). Troponin was measured in all 12 cases and was elevated in 11 (91.6%) without stenosis on coronary angiography except one. Out of 11 cases, 6 developed cardiac complications with 1 case each of cardiac tamponade, heart failure, myocarditis, hypertensive crisis, and cardiogenic shock in 2. Five patients required intubation, 1 patient required continuous positive airway pressure, and 1 patient required venovenous extracorporeal membrane oxygenation. The outcome was reported in terms of recovery in 11 (91.6%) out of 12 cases, and a successful recovery was noted in 10 (90.9%) cases. COVID-19-related TTS has a higher prevalence in older women. Despite a lower prevalence of cardiac comorbidities in COVID-19 patients, direct myocardial injury, inflammation, and stress may contribute to TTS with a high complication rate.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Takotsubo syndrome (TTS, takotsubo cardiomyopathy, stress cardiomyopathy, or “broken heart syndrome”) is characterized by acute left ventricular dysfunction usually in the setting of physical or emotional stress [1]. Conditions of acute stress leading to catecholamine surge have been suggested as pathophysiological mechanisms to date [2]. According to the World Health Organization, the current ongoing COVID-19 pandemic has infected over 10 million people and has led to approximately 500,000 deaths worldwide. A high burden of acute cardiac injury (19.7–27.8%), leading to significantly high mortality, has been reported in these patients [3, 4]. COVID-19 patients with cardiovascular injury have been reported to have a high burden of underlying cardiovascular comorbidities [3, 4]. Furthermore, accumulating evidence suggests a picture of severe systemic inflammation and cytokine storm in COVID-19 patients [5]. Hyperinflammatory states could lead to acute stress and injury, evident from elevated markers of myocardial injury such as C-reactive protein, pro-calcitonin, creatine kinase, myoglobin, and N-terminal pro b-type natriuretic peptide (NT-proBNP) in these patients [6]. Emerging evidence suggests a picture of cytokine storm syndrome, resembling cytokine release syndrome, in COVID-19 patients [7]. It has been observed that cytokine release syndrome is accompanied by catecholamine surge [8], which can predispose to TTS in COVID-19 patients. However, limited data on TTS in COVID-19 patients with only a handful of case reports promoted us to systematically review the published cases and pertinent outcomes.
Methods
We searched PubMed/Medline, Web of Science, SCOPUS, and Google Scholar until June 15, 2020 for case reports and case series using these keywords: COVID-19, SARS-CoV-2, takotsubo syndrome/takotsubo cardiomyopathy, stress-induced cardiomyopathy, and broken heart syndrome. All the published case reports included in the final analysis were in English except one in Italian. Since the number of case reports is few, we translated 1 case report in Italian [9] using Google translator. Data from the article were curated and summarized in the form of country of origin, age, and gender of the patients, their presenting complaint, any coexisting comorbidities, medical interventions during hospitalization, and their outcome. Continuous variables were presented as means ± standard deviations and categorical data as absolute values and percentages. All data extraction and descriptive analysis were performed using Microsoft Excel.
Results
Our search identified 25 articles; 9 were excluded due to duplication, 5 were excluded because they were review articles on COVID-19, and did not report any cases with TTS. Finally, 10 articles describing 12 patients for the analysis were selected [9,10,11,12,13,14,15,16,17,18] (Tables 1 and 2). The mean age of the reported patients was 70.8 ± 15.2 years (range 43–87 years). Of all the reported cases, 66.6% (n = 8) were women and mostly elderly (n = 8; > 60 years, 66.6%) patients. Most of the reported cases were from Italy (50%) and the USA (25%), while Belgium, Spain, and Switzerland contributed 1 case each. Only 3 reports (25%) identified the triggering/stress event in these cases. Among 12 cases, only 2 cases had positive family contact history, 1 had no contact history, and 9 did not report any contact history.
Out of 12 cases, cardiovascular comorbidities were reported (hypertension was reported in 8 (66.66%), diabetes in 5 (41.6%), and dyslipidemia in 2 (16.6%)). The most common presenting symptoms noticed were shortness of breath/dyspnea in 12 (100%), fever in 8 (66.6%), and chest pain in 7 (58.3%) of the cases. The time interval within the first symptom to the development of TTS was 8.3 ± 3.6 days (range 3–14 days). Out of 12 cases, chest imaging in the form of a chest x-ray or CT chest was present in 11 cases. The most common chest imaging findings were bilateral opacities in 8 (72.7%) and ground-glass opacity in 6 (54.5%) cases. Overall, 9 of the reported cases had heart failure with reduced ejection fraction on echocardiography with myocardial injury noted by elevated troponin I but no significant stenosis on coronary angiography. Only 1 patient had angiographically significant proximal LAD disease requiring two drug-eluting stents. Only 9 (75%) had abnormalities on ECG, with ST segment elevation in 6 (50%), T-wave inversion in 6 (50%), prolonged QT-interval in 6 cases (50%), and low voltage complex in 1 (11.1%) case.
Out of 12 cases, during hospitalization, data on left ventricular ejection fraction (LVEF) was reported in only 9 of the cases. The mean LVEF was 40.6 ± 9.9% (male, 46.7 ± 5.7%, and female, 37.7 ± 10.6%). Brain natriuretic peptide (BNP) was reported in 6 out of 12 cases and was found to be elevated in all the 6 cases. Out of 12 cases, in 8 cases, C-reactive protein was measured and found to be elevated in all the 8 cases (168.6 ± 71.9 mg/l). Ferritin was measured in only 2 of the cases out of 12 and was raised in both the cases (1010 ± 589.7 ng/ml). In 2 of the cases, the interleukin-6 level was measured and was found to be raised (37.5 ± 41.7 pg/ml). Troponin was measured in all the reported 12 cases and was high in 11 (91.6%). Out of 12 cases, 7 reported apical ballooning, 4 reported basal segment hypo/akinesia, and 1 reported median TTS.
Out of 12 cases, 11 (91.6%) cases reported at least one complication. Out of 11 cases, 6 developed cardiac complications with 1 case each of cardiac tamponade, heart failure, myocarditis, hypertensive crisis, and cardiogenic shock in 2. Because of respiratory failure, 5 patients were intubated and 1 patient was kept on CPAP. Furthermore, 1 patient developed septic shock. Of 12 cases, 10 (90.9%) cases showed successful recovery, while 1 did not report the outcome.
Discussion
To our knowledge, this is the first systematic review of COVID-19 patients developing TTS. Existing literature suggests that postmenopausal females are more prone to developing TTS [19] as was observed in our report. Loss of sympatholytic effect of estrogen and increased myocardial and vascular response to beta-adrenergic receptors in post-menopausal women has been suggested as one of the several reasons for increased risk of TTS [20]. However, it remains unexplored if this mechanism also poses a greater risk of TTS among elderly women with concomitant COVID-19 infection as compared with men.
In this study, only a handful of cases reported classic triggering events like the development of TTS after physical stress like intubation and/or emotional stress pointing towards the physical or emotional stress towards the development of TTS in this COVID-19 patients [10, 11, 14]. However, a large number of cases did not report any particular triggering event. In addition to the physical impact, an emotional impact in the form of social isolation leading to anxiety and stress during this pandemic could also trigger TTS [21]. We found that more than half of the patients had a current or past history of underlying cardiovascular comorbidities including non-ischemic cardiomyopathy in 1, hypertension in 8, diabetes in 5, and dyslipidemia in 1 case [9,10,11,12,13, 17, 18]. Patients with these comorbidities have a high burden of pro-inflammatory cytokines like interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α) [22]. Although the exact mechanism of TTS in COVID-19 is not fully explained, a cytokine storm syndrome-like picture has been seen in COVID-19 patients [7], with a high burden of pro-inflammatory cytokines like IL-6, which was reported in 2 cases [10, 18]. The cytokine storm syndrome has been noted to be accompanied by a surge in catecholamines [8]. This surge could lead to direct catecholamine toxicity and myocardial damage leading to TTS [23]. We noticed that COVID-19 patients had raised inflammatory markers in terms of raised CRP and ferritin. Furthermore, a high burden of pro-inflammatory state in these patients could lead to coagulation abnormalities and related complications. Bernardi et al., in their case report, descried left ventricular thrombus formation in a COVID-19 patient with TTS [24].
More than half of the cases presented with the classical symptoms seen in COVID-19 including chest pain and dyspnea [9, 10, 15,16,17,18], which is also frequently seen in patients with TTS [25]. Furthermore, a modest increase in cardiac troponin and ECG changes suggestive of myocardial injury was noted in all the cases, which is commonly seen in TTS [26], and could mimic acute coronary syndrome (ACS). Furthermore, SARS-CoV-2 infection could lead to myocarditis which could be misdiagnosed as TTS [15]. However, the absence of any significant coronary lesions, in most the patients who underwent coronary angiography, makes ACS an unlikely culprit.
Besides, ventriculography demonstrated the apical ballooning in most of the cases [9, 11, 14, 17, 18], apical hypo/akinesia with or without basal hyperkinesia [9, 10, 12], basal or mid and basal segment hypo/akinesia [12, 14,15,16], and median TTS [13] characteristic of TTS variants. Even though most of the cases recovered and discharged successfully, more than 80% of the patients developed complications such as cardiac tamponade, heart failure, cardiogenic shock, myocarditis, hypertensive crisis, or respiratory failure. A marked decrease in the systolic left ventricular function has been reported in the acute phase of TTS [1] with a prognosis depending on the nature of triggering factors. TTS secondary to emotional factors has shown a good prognosis while TTS secondary to medical conditions or procedure has shown unfavorable short and long-term prognosis [27]. All patients with QT interval prolongation except one were on hydroxychloroquine for COVID-19 treatment. Hydroxychloroquine has arrhythmogenic potential and should be used cautiously in COVID-19 patients with a high burden of myocardial injury as it could further contribute to the risk of dysrhythmias and worse outcomes in TTS.
Some limitations warrant attention while inferring these results: first, small sample size with the only case reports with TTS triggered by COVID-19 related stress was included. Second, there is a chance for publication bias, as the more challenging cases are more likely to be reported and published; and third, the lack of generalizability as the demographics and baseline information cannot be used for outcomes of a larger population without a control group.
Conclusions
COVID-19-associated direct myocardial injury, inflammation, and stress may account for TTS despite low cardiac comorbidities. COVID-19-related TTS is predominant in older women with a high complication rate with the majority of cases recovering successfully.
References
Templin C, Ghadri JR, Diekmann J, Napp LC, Bataiosu DR, Jaguszewski M, et al. Clinical features and outcomes of takotsubo (stress) cardiomyopathy. N Engl J Med. 2015;373:929–38.
Wittstein IS, Thiemann DR, Lima JAC, Baughman KL, Schulman SP, Gerstenblith G, et al. Neurohumoral features of myocardial stunning due to sudden emotional stress. N Engl J Med. 2005;352:539–48.
Shi S, Qin M, Shen B, Cai Y, Liu T, Yang F, et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020;5:802–10.
Guo T, Fan Y, Chen M, Wu X, Zhang L, He T, et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19). JAMA Cardiol. 2020;5:811–8.
Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506.
Shi S, Qin M, Cai Y, Liu T, Shen B, Yang F, et al. Characteristics and clinical significance of myocardial injury in patients with severe coronavirus disease 2019. Eur Heart J. 2020;41:2070–9.
Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–4.
Staedtke V, Bai R-Y, Kim K, Darvas M, Davila ML, Riggins GJ, et al. Disruption of a self-amplifying catecholamine loop reduces cytokine release syndrome. Nature. 2018;564:273–7.
Moderato L, Monello A, Lazzeroni D, Binno S, Giacalone R, Ferraro S, et al. Takotsubo syndrome during SARS-CoV-2 pneumonia: a possible cardiovascular complication. G Ital Cardiol. 2020;21:417–20.
Dabbagh MF, Aurora L, D’Souza P, Weinmann AJ, Bhargava P, Basir MB. Cardiac tamponade secondary to COVID-19. JACC Case Rep. 2020;2:1326–30.
Meyer P, Degrauwe S, Van Delden C, Ghadri JR, Templin C. Typical takotsubo syndrome triggered by SARS-CoV-2 infection. Eur Heart J. 2020;41:1860.
Minhas AS, Scheel P, Garibaldi B, Liu G, Horton M, Jennings M, et al. Takotsubo syndrome in the setting of COVID-19 infection. JACC Case Rep. 2020;2:1321–5.
Nguyen D, Nguyen T, De Bels D, Castro RJ. A case of takotsubo cardiomyopathy with COVID 19. Eur Heart J Cardiovasc Imaging. 2020;21:1052.
Roca E, Lombardi C, Campana M, Vivaldi O, Bigni B, Bertozzi B, et al. Takotsubo syndrome associated with COVID-19. Eur J Case Rep Intern Med. 2020;7:001665.
Sala S, Peretto G, Gramegna M, Palmisano A, Villatore A, Vignale D, et al. Acute myocarditis presenting as a reverse Tako-Tsubo syndrome in a patient with SARS-CoV-2 respiratory infection. Eur Heart J. 2020;41:1861–2.
Solano-López J, Sánchez-Recalde A, Zamorano JL. SARS-CoV-2, a novel virus with an unusual cardiac feature: inverted takotsubo syndrome. Eur Heart J. 2020;41:3106.
Pasqualetto MC, Secco E, Nizzetto M, Scevola M, Altafini L, Cester A, et al. Stress Cardiomyopathy in COVID-19 Disease. Eur J Case Rep Intern Med. 2020;7:001718.
Taza F, Zulty M, Kanwal A, Grove D. Takotsubo cardiomyopathy triggered by SARS-CoV-2 infection in a critically ill patient. BMJ Case Rep. 2020;13:e236561.
Kato K, Lyon AR, Ghadri J-R, Templin C. Takotsubo syndrome: aetiology, presentation and treatment. Heart. 2017;103:1461–9.
Ueyama T, Hano T, Kasamatsu K, Yamamoto K, Tsuruo Y, Nishio I. Estrogen attenuates the emotional stress-induced cardiac responses in the animal model of Tako-tsubo (ampulla) cardiomyopathy. J Cardiovasc Pharmacol. 2003;42(Suppl 1):S117–9.
Rivers J, Ihle JF. COVID-19 social isolation-induced takotsubo cardiomyopathy. Med J Aust. n/a.
Esposito K, Nappo F, Marfella R, Giugliano G, Giugliano F, Ciotola M, et al. Inflammatory cytokine concentrations are acutely increased by hyperglycemia in humans: role of oxidative stress. Circulation. 2002;106:2067–72.
Gianni M, Dentali F, Grandi AM, Sumner G, Hiralal R, Lonn E. Apical ballooning syndrome or takotsubo cardiomyopathy: a systematic review. Eur Heart J. 2006;27:1523–9.
Bernardi N, Calvi E, Cimino G, Pascariello G, Nardi M, Cani D, et al. COVID-19 pneumonia, takotsubo syndrome, and left ventricle thrombi. JACC Case Rep. 2020;2:1359–64.
Agewall S, Giannitsis E, Jernberg T, Katus H. Troponin elevation in coronary vs. non-coronary disease. Eur Heart J. 2010;32:404–11.
Ramaraj R, Sorrell VL, Movahed MR. Levels of troponin release can aid in the early exclusion of stress-induced (takotsubo) cardiomyopathy. Exp Clin Cardiol. 2009;14:6–8.
Ghadri JR, Kato K, Cammann VL, Gili S, Jurisic S, Di Vece D, et al. Long-term prognosis of patients with takotsubo syndrome. J Am Coll Cardiol. 2018;72:874–82.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Covid-19
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Singh, S., Desai, R., Gandhi, Z. et al. Takotsubo Syndrome in Patients with COVID-19: a Systematic Review of Published Cases. SN Compr. Clin. Med. 2, 2102–2108 (2020). https://doi.org/10.1007/s42399-020-00557-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42399-020-00557-w