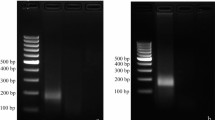
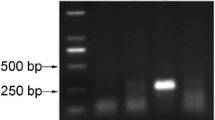
Abstract
Onion yellow dwarf virus (OYDV) is an important virus affecting Allium species like onion and garlic worldwide. Enzyme-linked immunosorbent assays (ELISA) and reverse transcription polymerase chain reaction (RT-PCR) assays are two most commonly used assays for the detection of OYDV but the sensitivity of ELISA is less than that of RT-PCR. PCR based assays are often considered for their sensitivity, specificity but time consuming and require specific technical expertise and costly equipments. To counter these difficulties, a simple and rapid RT-RPA assay for the detection of OYDV was developed. A common set of primer was designed to amplify the coat protein region of OYDV to perform RT-PCR and RT-RPA. Specificity as well as sensitivity test using tenfold serial dilution series of the purified RNA was performed for both the assays. Both symptomatic and asymptomatic samples of different varieties of onion were collected from Delhi NCR region and evaluated for OYDV infection using RT-PCR along with RT-RPA assay. The comparative results have shown that sensitivity of RT-RPA was similar to RT-PCR. However, RT-RPA is simple, and rapid assay for the large scale virus indexing of onion.


Similar content being viewed by others
References
Abd El-Wahab AS (2009) Aphid-transmission efficiency of two main viruses on garlic in Egypt, Onion yellow dwarf virus (OYDV-G) and Leek yellow stripe virus (LYSV-G). Acad J Entomol 2(1):40–42
Abeer Abd El-Wahab AS, Elnagar S, El-Sheikh MAK (2009) Incidence of aphid-borne Onion yellow dwarf virus (OYDV) in Alliaceae crops and associated weeds in Egypt. In: 4th conference on recent technologies in agriculture, pp 21–33
Amer HM, Abd El Wahed A, Shalaby MA, Almajhdi FN, Hufert FT, Weidmann M (2013) A new approach for diagnosis of bovine coronavirus using a reverse transcription recombinase polymerase amplification assay. J Virol Methods 193:337–340
Arya M, Baranwal VK, Ahlawat YS, Singh L (2006) RT-PCR detection and molecular characterization of onion yellow dwarf virus associated with garlic and onion. Curr Sci 91:1230–1234
Babu B, Washburn BK, Miller SH, Poduch K, Sarigul T, Knox GW, Ochoa-Corona FM, Paret ML (2017) A rapid assay for detection of Rose rosette virus using reverse transcription-recombinase polymerase amplification using multiple gene targets. J Virol Methods 240:78–84
Boyle DS, Lehman DA, Lillis L, Peterson D, Singhal M, Armes N, Parker M, Piepenburg O, Overbaugh J (2013) Rapid detection of HIV-1 proviral DNA for early infant diagnosis using recombinase polymerase amplification. mBio 4:1–8
Celli MG, Torrico AK, Kiehr M (2013) Striking differences in the biological and molecular properties of onion and garlic isolates of onion yellow dwarf virus. Arch Virol 158:1377–1382
Chandu D, Paul S, Parker M, Dudin Y, King-Sitzes J, Perez T, Mittanck DW, Shah M, Glenn KC, Piepenburg O (2016) Development of a rapid point-of-use DNA test for the screening of genuity® roundup ready 2 yield® soybean in seed samples. Biomed Res Int 2016:1–12
Chen J (2010) Molecular detection of Cucumber Mosaic Virus and other RNA viruses based on new techniques. In: Experimental plant virology. Advanced topics in science and technology in China. Springer, Berlin. https://doi.org/10.1007/978-3-642-14119-5_2
Chodorska M, Paduch-Cichal E, Kalinowska E, Szyndel MS (2014) First report of Onion yellow dwarf virus, Garlic common latent virus and Shallot latent virus on garlic in Poland. Plant Dis 98:858
Conci VC, Canavelli A, Lunello P (2003) Yield losses associated with virus-infected garlic plants during five successive years. Plant Dis 87:1411–1415
Daher RK, Stewart G, Boissinot M, Bergeron MG (2016) Recombinase polymerase amplification for diagnostic applications. Clin Chem 62(7):947–958
Dovas CI, Hatziloucas E, Salomon R, Barg E, Shiboleth Y, Katis NI (2001a) Comparison of methods for virus detection in Allium spp. J Phytopathol 149:731–737
Dovas CI, Hatziloucas E, Salomon R, Barg E, Shiboleth Y, Katis NI (2001b) Incidence of viruses infecting Allium spp. in Greece. Eur J Plant Pathol 107:677–684
Drake CJ, Tate HD, Harris HM (1933) The relationship of aphids to the transmission of yellow dwarf of onion. J Econ Entomol 26(4):841–846
Elnagar S, Abdel-Kader El-Sheikh M, Salah El-Deen Abd El-Wahab A (2011) Effect of natural infection with Onion yellow dwarf virus (OYDV) on yield of onion and garlic crops in Egypt. J Life Sci 5(8):634–638
Hoa NV, Ahlawat YS, Pant RP (2003) Partial characterization of onion yellow dwarf virus from onion in India. Indian Phytopathol 56(3):276–282
Ibrahim LM, Awad MAE, Abou-Zeid AA, Gamal-Elin AS (1996) Isolation and identification of onion yellow dwarf virus in Egypt. J Appl Sci 11(4):184–196
Jiao Y, Xu C, Li J, Gu Y, Xia C, Xie Q, Xie Y, An M, Xia Z, Wu Y (2020) Characterization and a RT-RPA assay for rapid detection of chilli veinal mottle virus (ChiVMV) in tobacco. Virol J 17:33
Kapoor R, Srivastava N, Kumar S, Saritha RK, Sharma SK, Jain RK, Baranwal VK (2017) Development of a recombinase polymerase amplification assay for the diagnosis of Banana bunchy top virus in different banana cultivars. Arch Virol 162:2791–2796
Katis NI, Maliogka VI, Dovas CI (2012) Viruses of the genus Allium in the Mediterranean region. Adv Virus Res 84:163–208. https://doi.org/10.1016/B978-0-12-394314-9.00005-1
Kumar S, Baranwal VK, Joshi S, Arya M, Majumder S (2010) Simultaneous detection of mixed infection of Onion yellow dwarf virus and an Allexivirus in RT-PCR for ensuring virus free onion bulbs. Indian J Virol 21(1):64–68
Kumar P, Dhawan P, Mehra R (2011) Characterization, transmission and host range of onion yellow dwarf virus. Plant Dis Res 26(2):176
Kumar P, Dhawan P, Mehra R (2012) Symptoms and losses caused by Onion yellow dwarf virus and Iris yellow spot virus diseases of onion crop in Northern India. J Mycol Plant Pathol 42(1):153–160
Leisova-Svobodova L, Karlova-Smekalova K (2011) Detection of garlic viruses using SYBR green real-time reverse transcription-polymerase chain reaction. J Phytopathol 159(6):429–434
Liljander A, Yu M, O’Brien E, Heller M, Nepper JF, Weibel DB, Gluecks I, Younan M, Frey J, Falquet L, Jores J (2015) Field-applicable recombinase polymerase amplification assay for rapid detection of Mycoplasma capricolum sub sp. capripneumoniae. J Clin Microbiol 53:2810–2815
Lillis L, Lehman D, Singhal MC, Cantera J, Singleton J, Labarre P, Toyama A, Piepenburg O, Parker M, Wood R, Overbaugh J, Boyle DS (2014) Non-instrumented incubation of a recombinase polymerase amplification assay for the rapid and sensitive detection of proviral hiv-1 dna. PLoS ONE 9:6–9
Lobato IM, O’Sullivan-Trac CK (2018) Recombinase polymerase amplification: basics, applications and recent advances. Trends Anal Chem 98:19–35
Majumder S, Johari S (2014) First report of onion yellow dwarf virus and garlic common latent virus infection in garlic from Nepal. J Plant Pathol 96:4
Majumder S, Yadav V, Yakasai MA, Muhammad JY (2017) First report of Onion yellow dwarf virus in garlic from Nigeria. J Plant Path 99(1):299
Mekuria TA, Zhang S, Eastwel KC (2014) Rapid and sensitive detection of Little cherry virus 2 using isothermal reverse transcription-recombinase polymerase amplification. J Virol Methods 205:24–30
Pappu HR, Hellier BC, Dugan FM (2005) First report of Onion yellow dwarf virus, Leek yellow stripe virus, and Garlic common latent virus in garlic in Washington State. Plant Dis 89(2):205
Piepenburg O, Williams CH, Stemple DL, Armes NA (2006) DNA detection using recombination proteins. PLoS Biol 4(7):1115–1121
Sambrook J, Russell DE (2006) The condensed protocols from molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, New York
Schwartz HF, Mohan K (2008) Compendium of onion and garlic diseases and pests, 2nd edn. APS Press, Saint Paul, p 10
Shahraeen N, Lesemann DE, Ghotbi T (2008) Survey for viruses infecting onion, garlic and leek crops in Iran. Bull OEPP/EPPO Bull 38:131–135
Silva G, Bömer M, Nkere C, Kumar PL, Seal SE (2015) Rapid and specific detection of Yam mosaic virus by reverse-transcription recombinase polymerase amplification. J Virol Methods 222:138–144
Sivaprasad Y, Garrido P, Mendez K, Silvia P, Garrido A, Ramos L (2017) First report of Onion yellow dwarf virus infecting onion in Ecuador. J Plant Pathol 99(1):296
Srivastava N, Kapoor R, Kumar R, Kumar S, Saritha RK, Kumar S, Baranwal VK (2019) Rapid diagnosis of Cucumber mosaic virus in banana plants using a fluorescence-based real-time isothermal reverse transcription-recombinase polymerase amplification assay. J Virol Methods 270:52–58
Takaki F, Sano T, Yamashita K (2006) The complete nucleotide sequence of attenuated onion yellow dwarf virus: a natural potyvirus deletion mutant lacking the N-terminal 92 amino acids of HC-Pro. Arch Virol 151:1439–1445
Van Dijk P (1993) Survey and characterization of potyviruses and their strains of Allium species. Neth J Plant Pathol 2:1–48
Voncina D, Curic K, Fabek S, Toth N (2016) First report of Onion yellow dwarf virus, Leek yellow stripe virus, and Garlic common latent virus on garlic in Croatia. Plant Dis 100(3):656
Winiarczyk K, Solarska E, Sienkiewicz W (2014) Prevalence of infections with Onion yellow dwarf virus, Leek yellow stripe virus and Garlic common latent virus in plants from the genus Allium. Acta Sci Pol Hortorum Cultus 13(3):123–133
Yang Y, Qin X, Song Y, Zhang W, Hu G, Dou Y, Li Y, Zhang Z (2017) Development of real-time and lateral flow strip reverse transcription recombinase polymerase amplification assays for rapid detection of peste des petits ruminants virus. Virol J 14:24
Zhang S, Ravelonandro M, Russell P, McOwen N, Briard P, Bohannon S, Vrient A (2014) Rapid diagnostic detection of Plum pox virus in Prunus plants by isothermal AmplifyRP® using reverse transcription-recombinase polymerase amplification. J Virol Methods 207:114–120
Acknowledgements
Authors are thankful to the Head, Division of Plant Pathology, IARI, New Delhi for providing necessary facilities to conduct the experiments.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kumar, R., Pant, R.P., Kapoor, S. et al. Development of a reverse transcription-recombinase polymerase amplification (RT-RPA) assay for the detection of onion yellow dwarf virus (OYDV) in onion cultivars. Indian Phytopathology 74, 201–207 (2021). https://doi.org/10.1007/s42360-020-00311-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42360-020-00311-1