Abstract
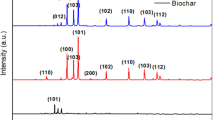
The waste recycling is one of the smart methods to control the increasing demand for raw materials and environmental pollution. Photocatalytic biochars were prepared by carbonization of waste textiles that would not be reused in the industry. Virgin and FeCl2, H2SO4, H3PO4 impregnated waste samples were carbonized at 300–400 °C for varying times. The functional groups and crystal structure of biochars were investigated by FT-IR and XRD respectively. SEM and BET analyzes were used to examine the morphology and surface properties of the biochars. FeCl2 and H3PO4 impregnated samples presented well-crystalline and porous structure. Formation of functional groups increased at lower temperatures due to the catalytic effect. The highest dye removal by adsorption and photo catalytic effect were recorded as 25.65 mg/g and 97.8%, respectively. The order of efficiency for dye removal according to pretreatment was determined as FeCl2 > H3PO4 > H2SO4 > raw400 > raw350. Adsorbent-photocatalytic biochars were prepared by carbonization of chemically pretreated waste materials with a simple method. Environmental protection and economic production were achieved.
Graphical Abstract





Similar content being viewed by others
Data availability
The data of these study are available to download from [https://data.mendeley.com/datasets/h6k6k37m9r].
References
Yaashikaa PR, Kumar PS, Varjani S, Saravanan A (2020) A critical review on the biochar production techniques, characterization, stability and applications for circular bioeconomy. Biotechnol Rep 28:e00570. https://doi.org/10.1016/j.btre.2020.e00570
Yang C, Liu J, Lu S (2021) Pyrolysis temperature affects pore characteristics of rice straw and canola stalk biochars and biochar-amended soils. Geoderma 397:115097. https://doi.org/10.1016/j.geoderma.2021.115097
Zakaria R, Jamalluddin NA, Abu Bakar MZ (2021) Effect of impregnation ratio and activation temperature on the yield and adsorption performance of mangrove based activated carbon for methylene blue removal. Res Mater 10:100183. https://doi.org/10.1016/j.rinma.2021.100183
Khuong DA, Nguyen HN, Tsubota T (2021) Activated carbon produced from bamboo and solid residue by CO2 activation utilized as CO2 adsorbents. Biomass Bioenergy 148:106039. https://doi.org/10.1016/j.biombioe.2021.106039
Guclu C, Alper K, Erdem M, et al (2021) Activated carbons from co-carbonization of waste truck tires and spent tea leaves. Sustain Chem Pharm 21:100410. https://doi.org/10.1016/j.scp.2021.100410
Hwang B, Yi SH, Chun SE (2021) Dual-role of ZnO as a templating and activating agent to derive porous carbon from polyvinylidene chloride (PVDC) resin. Chem Eng J 422:130047. https://doi.org/10.1016/j.cej.2021.130047
Naganathan KK, Faizal ANM, Zaini MAA, Ali A (2021) Adsorptive removal of Bisphenol a from aqueous solution using activated carbon from coffee residue. Mater Today Proc 47:1307–1312. https://doi.org/10.1016/j.matpr.2021.02.802
Sime T, Fito J, Nkambule TTI et al (2023) Adsorption of congo red from textile wastewater using activated carbon developed from corn cobs: the studies of isotherms and kinetics. Chem Afr. https://doi.org/10.1007/s42250-022-00583-2
Lokteva ES, Golubina EV, Antonova MV et al (2015) Chlorobenzene hydrodechlorination catalyst prepared via the pyrolysis of sawdust impregnated with palladium nitrate. Kinet Catal 56:764–773. https://doi.org/10.1134/S0023158415060099
Müller BR (2021) Preparation and characterization of K2CO3-doped powdered activated carbon for effective in-vitro adsorption of deoxynivalenol. Bioresour Technol Rep 15:100703. https://doi.org/10.1016/j.biteb.2021.100703
Pazira R, Lashanizadegan A, Sharififard H, Darvishi P (2018) Xylene removal from dilute solution by palm kernel activated charcoal : Kinetics and equilibrium analysis Advances in Environmental Technology Xylene removal from dilute solution by palm kernel activated charcoal : Kinetics and equilibrium analysis. Adv Environ Technol 2:83–92. https://doi.org/10.22104/AET.2018.2989.1145
Yuan Z, Xu Z, Zhang D et al (2018) Mesoporous activated carbons synthesized by pyrolysis of waste polyester textiles mixed with Mg-containing compounds and their Cr(VI) adsorption. Colloids Surf, A 549:86–93. https://doi.org/10.1016/j.colsurfa.2018.04.008
Minceva M, Markovska L, Meshko V (2007) Removal of Zn2+, Cd2+ and Pb 2+ from binary aqueous solution by natural zeolite and granulated activated carbon. Maced J Chem Chem Eng 26:125–134
Cuong DV, Matsagar BM, Lee M et al (2021) A critical review on biochar-based engineered hierarchical porous carbon for capacitive charge storage. Renew Sustain Energy Rev 145:111029. https://doi.org/10.1016/j.rser.2021.111029
Miao Q, Li G (2021) Potassium phosphate/magnesium oxide modified biochars: Interfacial chemical behaviours and Pb binding performance. Sci Total Environ 759:143452. https://doi.org/10.1016/j.scitotenv.2020.143452
Trakal L, Šigut R, Šillerová H et al (2014) Copper removal from aqueous solution using biochar: effect of chemical activation. Arab J Chem 7:43–52. https://doi.org/10.1016/j.arabjc.2013.08.001
Xu Z, Sun Z, Zhou Y et al (2019) Insights into the pyrolysis behavior and adsorption properties of activated carbon from waste cotton textiles by FeCl3-activation. Colloids Surf, A 582:1–10. https://doi.org/10.1016/j.colsurfa.2019.123934
Rustamov YI, Mammadova SG, Rustamova GY, Nasirova YAG (2019) The synthesis of cellulose derivatives with special properties from cellulose- containing natural materials and priority fields of their use. 39–43
Herath A, Navarathna C, Warren S et al (2022) Iron/titanium oxide-biochar (Fe2TiO5/BC): a versatile adsorbent/photocatalyst for aqueous Cr(VI), Pb2+, F- and methylene blue. J Colloid Interface Sci 614:603–616. https://doi.org/10.1016/j.jcis.2022.01.067
Welter N, Leichtweis J, Silvestri S et al (2022) Preparation of a new green composite based on chitin biochar and ZnFe2O4 for photo-Fenton degradation of Rhodamine B. J Alloys Compounds 901:163758. https://doi.org/10.1016/j.jallcom.2022.163758
Wu Y, Liu B (2022) Mg(NO3)2·6H2O-modified porous carbon derived from peanut shell: formation mechanism and efficient removal of p-nitrophenol. React Kinet Mech Catal 135:2085–2098. https://doi.org/10.1007/s11144-022-02212-y
Zahedifar M, Seyedi N, Salajeghe M, Shafiei S (2020) Nanomagnetic biochar dots coated silver NPs (BCDs-Ag/MNPs): A highly efficient catalyst for reduction of organic dyes. Mater Chem Phys 246:122789. https://doi.org/10.1016/j.matchemphys.2020.122789
Lin Y, Wu S, Yang C et al (2019) Preparation of size-controlled silver phosphate catalysts and their enhanced photocatalysis performance via synergetic effect with MWCNTs and PANI. Appl Catal B Environ 245:71–86. https://doi.org/10.1016/j.apcatb.2018.12.048
Chen T, Luo L, Deng S et al (2018) Sorption of tetracycline on H3PO4 modified biochar derived from rice straw and swine manure. Biores Technol 267:431–437. https://doi.org/10.1016/j.biortech.2018.07.074
Sadeghi Rad T, Yazici ES, Khataee A et al (2023) Ultrasound-assisted photocatalytic decomposition of rifadin with biochar and CNT-based NiCr layered double hydroxides. Surf Interfaces 36:102628. https://doi.org/10.1016/j.surfin.2022.102628
Mohammed Dakhil R, Sumer Gaaz T, Al-Amiery A et al (2019) Synthesis and characterization of erbium trioxide nanoparticles as photocatalyzers for degradation of methyl orange dye. Drink Water Eng Sci 12:15–21. https://doi.org/10.5194/dwes-12-15-2019
Li Z, Hanafy H, Zhang L et al (2020) Adsorption of congo red and methylene blue dyes on an ashitaba waste and a walnut shell-based activated carbon from aqueous solutions: Experiments, characterization and physical interpretations. Chem Eng J 388:124263. https://doi.org/10.1016/j.cej.2020.124263
Scharte B (2010) Phosphorus-based flame retardancy mechanisms-old hat or a starting point for future development? Materials 3:4710–4745. https://doi.org/10.3390/ma3104710
Wei R, Cang D, Bai Y et al (2016) Reduction characteristics and kinetics of iron oxide by carbon in biomass. Ironmaking Steelmaking 43:144–152. https://doi.org/10.1179/1743281215Y.0000000061
Sawant SY, Munusamy K, Somani RS et al (2017) Precursor suitability and pilot scale production of super activated carbon for greenhouse gas adsorption and fuel gas storage. Chem Eng J 315:415–425. https://doi.org/10.1016/j.cej.2017.01.037
Alagha O, Manzar MS, Zubair M et al (2020) Magnetic Mg-Fe/LDH intercalated activated carbon composites for nitrate and phosphate removal from wastewater: insight into behavior and mechanisms. Nanomaterials 10:1–22. https://doi.org/10.3390/nano10071361
Mishra A, Sardar M (2015) Isolation of genomic DNA by silane-modified iron oxide nanoparticles. nanotechnology: novel perspectives and prospects. McGraw Hill Education, New York, pp 309–315
Gumus H, Buyükkidan B (2022) Pollution removal performance of chemically functionalized textile waste biochar anchored poly (vinylidene fluoride) adsorbent. J Turk Chem Soc Sect A Chem 9:777–792
Zhang L, Tu LY, Liang Y et al (2018) Coconut-based activated carbon fibers for efficient adsorption of various organic dyes. RSC Adv 8:42280–42291. https://doi.org/10.1039/c8ra08990f
Sartova K, Omurzak E, Kambarova G et al (2019) Activated carbon obtained from the cotton processing wastes. Diam Relat Mater 91:90–97. https://doi.org/10.1016/j.diamond.2018.11.011
Akinhanmi TF, Ofudje EA, Adeogun AI et al (2020) Orange peel as low-cost adsorbent in the elimination of Cd(II) ion: kinetics, isotherm, thermodynamic and optimization evaluations. Bioresour Bioprocess 7:1. https://doi.org/10.1186/s40643-020-00320-y
Ouedrhiri A, Ait Himi M, Youbi B et al (2022) Biochar material derived from natural waste with superior dye adsorption performance. Mater Today Proc 66:259–267. https://doi.org/10.1016/j.matpr.2022.04.928
Khan A, Qamar M, Muneer M (2012) Synthesis of highly active visible-light-driven colloidal silver orthophosphate. Chem Phys Lett 519–520:54–58. https://doi.org/10.1016/j.cplett.2011.11.015
Behrouzeh M, Mehdi Parivazh M, Danesh E et al (2022) Application of Photo-Fenton, Electro-Fenton, and Photo-Electro-Fenton processes for the treatment of DMSO and DMAC wastewaters. Arab J Chem 15:1. https://doi.org/10.1016/j.arabjc.2022.104229
Rauf MA, Ashraf SS (2009) Fundamental principles and application of heterogeneous photocatalytic degradation of dyes in solution. Chem Eng J 151:10–18. https://doi.org/10.1016/j.cej.2009.02.026
Xie G, Liu Z, Zhu Z et al (2004) Simultaneous removal of SO2 and NOx from flue gas using a CuO/Al2O3 catalyst sorbent: II Promotion of SCR activity by SO2 at high temperatures. J Catal 224:42–49. https://doi.org/10.1016/j.jcat.2004.02.016
Zhang L, Zou W, Ma K et al (2015) Sulfated temperature effects on the catalytic activity of CeO2 in NH3-selective catalytic reduction conditions. J Phys Chem C 119:1155–1163. https://doi.org/10.1021/jp511282c
Aichour A, Zaghouane-Boudiaf H, Djafer Khodja H (2022) Highly removal of anionic dye from aqueous medium using a promising biochar derived from date palm petioles: Characterization, adsorption properties and reuse studies. Arab J Chem 15:103542. https://doi.org/10.1016/j.arabjc.2021.103542
Luo S, Li S, Zhang S et al (2022) Visible-light-driven Z-scheme protonated g-C3N4/wood flour biochar/BiVO4 photocatalyst with biochar as charge-transfer channel for enhanced RhB degradation and Cr(VI) reduction. Sci Total Environ 806:150662. https://doi.org/10.1016/j.scitotenv.2021.150662
Cheng S, Zhao S, Xing B et al (2022) Preparation of magnetic adsorbent-photocatalyst composites for dye removal by synergistic effect of adsorption and photocatalysis. J Clean Prod 348:131301. https://doi.org/10.1016/j.jclepro.2022.131301
Park JH, Wang JJ, Meng Y et al (2019) Adsorption/desorption behavior of cationic and anionic dyes by biochars prepared at normal and high pyrolysis temperatures. Colloids Surf, A 572:274–282. https://doi.org/10.1016/j.colsurfa.2019.04.029
Yu F, Tian F, Zou H et al (2021) ZnO / biochar nanocomposites via solvent free ball milling for enhanced adsorption and photocatalytic degradation of methylene blue. J Hazard Mater 415:125511. https://doi.org/10.1016/j.jhazmat.2021.125511
Lu L, Shan R, Shi Y et al (2019) A novel TiO2/biochar composite catalysts for photocatalytic degradation of methyl orange. Chemosphere 222:391–398. https://doi.org/10.1016/j.chemosphere.2019.01.132
Acknowledgements
We would like to thank the Chemistry Department of the University of Kutahya Dumlupinar for their support in providing the laboratory equipment.
Funding
This work was financially supported by the Department of Scientific Research Project at the University of Kutahya Dumlupinar (DPÜ-BAP No: 2020-08).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflicts of interest.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gumus, H., Buyukkidan, B. A Simple and Green Preparation Route of Waste Textile Based Photocatalytic Biochars for Pollution Removal. Chemistry Africa 6, 629–642 (2023). https://doi.org/10.1007/s42250-023-00625-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42250-023-00625-3