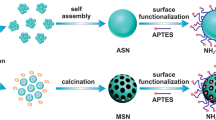
Abstract
Chelating and ion-exchange N-functionalized mesoporous silicas (SBA-15) as selective adsorbents for removal of heavy metals were synthesized using template method. Fourier transform infrared spectroscopy, TEM analysis, N2 adsorption/desorption isotherms and titration analysis confirmed successful functionalisation of the tri-sodium salt of N-(triethoxysilylpropyl)ethylenediaminetriacetic acid (EDTA), protonated primary amine (NH3+Cl−) and its combinations onto the mesoporous silica (SBA-EDTA/NH2). The obtained materials featured beneficial properties of mesoporous silica SBA-15 with its high surface area and were successfully fictionalized with N-containing groups. The synthesized series of silicas were investigated for removal of Cr(III), Mn(III), Pb(II), Cd(II) and Cu(II) from model water solutions. The adsorption of target ions increased with the increase pH and its concentration in solution. The adsorption equilibrium data were well fitted to Langmuir isotherm model and maximum monolayer adsorption capacities for cations Pb(II), Cd(II), Cr(III) and Mn(II) were 185.6 mg g−1, 111.2 mg g−1, 57.7 mg g−1 and 49.4 mg g−1, respectively. The chelating interaction was considered as the main adsorption mechanism for metal ions (Cr(III), Mn(II), Pb(II), Cd(II), and Cu(II)). The adsorption capacities of SBA-EDTA and SBA-EDTA/NH2 samples toward studied metal ions were consistent with the Lewis ‘hard and soft acids and bases’ theory. The metal removal efficiency of adsorbents was near 96–92% during three regeneration cycles. All these results indicated that the produced N-functionalized silica were promising for applications in environmental and analytical separation fields.







Similar content being viewed by others
References
Pradel M, Aissani L, Villot J, Baudez JC, Laforest V (2016) From waste to added value product: towards a paradigm shift in life cycle assessment applied to wastewater sludge—a review. J Clean Prod 131:60–75. https://doi.org/10.1016/j.jclepro.2016.05.07675
Zhou C, Ge S, Yu H, Zhang T, Cheng H, Sun Q, Xiao R (2018) Environmental risk assessment of pyrometallurgical residues derived from electroplating and pickling sludges. J Clean Prod 177:699–707. https://doi.org/10.1016/j.jclepro.2017.12.285
Islam S, Ahmed K, Raknuzzaman M, Mamun H-A, Kamrul M (2015) Heavy metal pollution in surface water and sediment: a preliminary assessment of an urban river in a developing country. Islam Ecol Indicators 48:282–291. https://doi.org/10.1016/j.ecolind.2014.08.016
Cegłowskia M, Gierczyka B, Frankowskia M, Popendab L (2018) A new low-cost polymeric adsorbents with polyamine chelating groups for efficient removal of heavy metal ions from water solutions. React Funct Polym 131:64–74. https://doi.org/10.1016/j.reactfunctpolym.2018.07.006
Jaishankar M, Tseten T, Anbalagan N (2014) Toxicity, mechanism and health effects of some heavy metals. Interdiscip Toxicol 7(2):60–72. https://doi.org/10.2478/intox-2014-0009
Ayangbenro AS, Babalola OO (2017) A new strategy for heavy metal polluted environments: a review of microbial biosorbents. Int J Environ Res Public Health 14(1):94. https://doi.org/10.3390/ijerph14010094
Chételat J, Ackerman JT, Eagles-Smith CA, Hebert CE (2019) Methylmercury exposure in wildlife: a review of the ecological and physiological processes affecting contaminant concentrations and their interpretation. Sci Total Environ 711:135117. https://doi.org/10.1016/j.scitotenv.2019.135117
Aaseth J, Wallace DR, Vejrup K, Alexander J (2020) Methylmercury and developmental neurotoxicity: a global concern. Curr Opin Toxicol 19:80–87. https://doi.org/10.1016/j.cotox.2020.01.005
Engwa AG, Ferdinand PU, Nwalo FN, Unachukwu NM (2019) Mechanism and health effects of heavy metal toxicity in humans. In: Poisoning in the Modern World - New Tricks for an Old Dog. https://doi.org/10.5772/intechopen.82511
Jan AT, Azam M, Siddiqui K, Ali A, Choi I, Haq QM (2015) Heavy metals and human health: mechanistic insight into toxicity and counter defense system of antioxidants. Int J Mol Sci 16(12):29592–29630. https://doi.org/10.3390/ijms161226183
Islam A, Ahmad H, Zaidi N, Kumar S (2016) A graphene oxide decorated with triethylene-tetramine-modified magnetite for separation of chromium species prior to their sequential speciation and determination via FAAS. Microchim Acta 183:289–296. https://doi.org/10.1007/s00604-015-1641-2
Ipeaiyeda AR, Ayoade AR (2017) Flame atomic absorption spectrometric determination of heavy metals in aqueous solution and surface water preceded by co-precipitation procedure with copper(II) 8-hydroxyquinoline. Appl Water Sci 7:4449–4459. https://doi.org/10.1007/s13201-017-0590-9
Martinis EM, Olsina RA, Altamirano JC, Wuilloud RG (2008) Sensitive determination of cadmium in water samples by room temperature ionic liquid based preconcentration and electrothermal atomic absorption spectrometry. Anal Chim Acta 628:41–48. https://doi.org/10.1016/j.aca.2008.09.001
Wu P, Gao Y, Cheng G, Yang W, Lv Y, Hou X (2008) Selective determination of trace amounts of silver in complicated matrices by displacement-cloud point extraction coupled with thermospray flame furnace atomic absorption spectrometry. J Anal At Spectrom 23:752–757. https://doi.org/10.1039/B719579F
Khulbe KC, Matsuura T (2018) Removal of heavy metals and pollutants by membrane adsorption techniques. Appl Water Sci 8:19. https://doi.org/10.1007/s13201-018-0661-6
Vardhan KH, Kumar PS, Panda RC (2019) A review on heavy metal pollution, toxicity and remedial measures: current trends and future perspectives. J Mol Liq 290:111197. https://doi.org/10.1016/j.molliq.2019.111197
Renu AM, Singh K (2017) Heavy metal removal from wastewater using various adsorbents: a review. J Water Reuse Desalin 07(4):387–419. https://doi.org/10.2166/wrd.2016.104
Diagboya PNE, Dikio ED (2018) Silica-based mesoporous materials; emerging designer adsorbents for aqueous pollutants removal and water treatment. Micropor Mesopor Mater 266:252–267. https://doi.org/10.1016/j.micromeso.2018.03.008
Da’na E (2017) Adsorption of heavy metals on functionalized-mesoporous silica: a review. Microporous Mesoporous Mater 247:145–157. https://doi.org/10.1016/j.micromeso.2017.03.050
Lim J, Goh SS, Liow SS, Xue K, Loh XJ (2019) Molecular gel sorbent materials for environmental remediation and wastewater treatment. J Mater Chem A 7:18759–18791. https://doi.org/10.1039/C9TA05782J
Huang W, Zhang Y, Li D (2017) Adsorptive removal of phosphate from water using mesoporous materials: a review. J Environ Manage 193:470–482. https://doi.org/10.1016/j.jenvman.2017.02.030
Miller PJ, Shantz DF (2020) Amine-functionalized ordered mesoporous silicas as model materials for liquid phase acid capture. AIChE J. https://doi.org/10.1002/aic.16918
Wamba AGN, Kofa GP, Koungou SN, Thue PS, Lima EC, Reis GS, Kayem JG (2018) Grafting of Amine functional group on silicate based material as adsorbent for water purification: a short review. J Environ Chem Eng 6:3192–3203. https://doi.org/10.1016/j.jece.2018.04.062
Hao Sh, Verlotta A, Aprea P, Pepe F, Caputo D, Zhu W (2016) Optimal synthesis of amino-functionalized mesoporous silicas for the adsorption of heavy metal ions. Microporous Mesoporous Mater 236:250–259. https://doi.org/10.1016/j.micromeso.2016.09.008
Wei J, Chen S, Li Y, He Z, Geng L, Liao L (2020) Aqueous Cu(II) ion adsorption by amino-functionalized mesoporous silica KIT-6. RSC Adv 10:20504–20514. https://doi.org/10.1039/D0RA03051A
Saad R, Hamoudi S, Belkacemi K (2008) Adsorption of phosphate and nitrate anions on ammonium-functionnalized mesoporous silicas. J Porous Mater 15:315–323. https://doi.org/10.1007/s10934-006-9095-x
Gupta R, Gupta SK, Pathak DD (2019) Selective adsorption of toxic heavy metal ions using guanine-functionalized mesoporous silica [SBA-16-g] from aqueous solution. Microporous Mesoporous Mater 288:109577. https://doi.org/10.1016/j.micromeso.2019.109577
Shahbazi A, Younesi H, Badiei A (2011) Functionalized SBA-15 mesoporous silica by melamine-based dendrimer amines for adsorptive characteristics of Pb(II), Cu(II) and Cd(II) heavy metal ions in batch and fixed bed column. Chem Eng J (Lausanne) 168:505–518. https://doi.org/10.1016/j.cej.2010.11.053
Kołodyńska D (2013) Application of a new generation of complexing agents in removal of heavy metal ions from different wastes. Environ Sci Pollut Res 20:5939–5949. https://doi.org/10.1007/s11356-013-1576-2
Huang J, Ye M, Qu YQ, Chu LF, Chen R, He QZ, Xu DF (2012) Pb(II) removal from aqueous media by EDTA-modified mesoporous silica SBA-15. J Colloid Interface Sci 385:137–146. https://doi.org/10.1016/j.jcis.2012.06.054
Zhao D, Huo Q, Feng J, Chmelka BF, Stucky GD (1998) Nonionic triblock and star diblock copolymer and oligomeric surfactant syntheses of highly ordered, hydrothermally stable, mesoporous silica structures. J Am Chem Soc 7863:6024–6036. https://doi.org/10.1021/ja974025i
Brunauer JS, Emmet PH, Teller E (1938) Adsorption of gases in multimolecular layers. J Am Chem Soc 60:309–319. https://doi.org/10.1021/ja01269a023
Barrett EP, Joyner LG, Halenda PP (1951) The determination of pore volume and area distributions in porous substances. I. Computations from nitrogen isotherms. J Am Chem Soc 73:373–380. https://doi.org/10.1021/ja01145a126
Lin-Vien D, Colthup NB, Fateley WG, Grasselli JG (1991) In the handbook of infrared and raman characteristic frequencies of organic molecules. Academic Press, London
Han L, Ruan J, Li Y, Terasaki O, Che Sh (2007) Synthesis and characterization of the amphoteric amino acid bifunctional mesoporous silica. Chem Mater 19(11):2860–2867. https://doi.org/10.1021/cm0705845
Zaitsev VN, Kobylinskaya NG (2005) Properties of silicas chemically modified by monodentate amines studied by conductometry. Russ Chem Bull 54:1842–1846. https://doi.org/10.1007/s11172-006-0046-0
Zhao D, Jing S, Xu J, Yang H, Zheng W, Song T, Zhang P (2013) Recycle adsorption of Cu2+ on amine-functionalized mesoporous silica monolithic. Chem Res Chin Univ 29:793–797. https://doi.org/10.1007/s40242-013-2442-y
Lever ABP (1974) Charge transfer spectra of transition metal complexes. J Chem Educ 5:612–616. https://doi.org/10.1021/ed051p612
Smith RM, Martell AE (1987) Critical stability constants, enthalpies and entropies for the formation of metal complexes of aminopolycarboxylic acids and carboxylic acids. Sci Total Envir 64(1–2):125–147. https://doi.org/10.1016/0048-9697(87)90127-6
Faghihian H, Naghavi M (2014) Synthesis of amine-functionalized MCM-41 and MCM-48 for removal of heavy metal ions from aqueous solutions. Sep Sci Technol 49:214–220
Pearson RG (1963) Hard and soft acids and bases. J Am Chem Soc 85:3533–3539. https://doi.org/10.1021/ja00905a001
Langmuir I (1918) The adsorption of gases on plane surfaces of glass, mica and platinum. J Am Chem Soc 40(9):1361–1403. https://doi.org/10.1021/ja02242a004
Freundlich HMF (1906) Uber die adsorption in lasungen. Z Phys Chem 57:385–490. https://doi.org/10.1515/zpch-1907-5723
Dubinin MM (1960) The potential theory of adsorption of gases and vapors for adsorbents with energetically non-uniform surface. Chem Rev 60:235–266. https://doi.org/10.1021/cr60204a006
Acknowledgements
Authors are grateful to the MES of Ukraine (project M/10-2020) and project “Multifunctional hybrid adsorbents for water purification” supported by the Swedish Research Council (Vetenskapsrådet) Swedish Research links Program, Dnr. 2018-2021.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Rights and permissions
About this article
Cite this article
Kobylinska, N., Dudarko, O., Kessler, V. et al. Enhanced Removal of Cr(III), Mn(II), Cd(II), Pb(II) and Cu(II) from Aqueous Solution by N-functionalized Ordered Silica. Chemistry Africa 4, 451–461 (2021). https://doi.org/10.1007/s42250-021-00230-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42250-021-00230-2