Abstract
This work aimed at treating OMW by developing an environmentally friendly and cost-effective media capable of removing phenolic compounds upon the interaction between magnetic nanoparticles and sorbent material such as zeolite. Magnetite (Fe3O4) and goethite (FeO(OH)) nanoparticles were prepared using the microemulsion method by using dimethylene-1,2-bis (dodecyl dimethyl ammonium bromide) (CTAB) as a surfactant. The prepared nanoparticles had magnetic properties. X-Ray Diffraction (XRD), and Transmission Electron Microscope (TEM) were used for the structural and morphological characterization. The average crystalline sizes for magnetite and goethite obtained from TEM were 7–16 and 5–14 nm, respectively. The nanocomposite media of magnetite and goethite with zeolite were prepared via coupling natural Jordanian zeolite with magnetic nanoparticles using two approaches; mixing and coating. The adsorption process of phenolic compounds was analyzed by the direct photometric method after treating real samples with nanocomposite media at different conditions. In the batch experiment, the optimum conditions were percent of media to OMW 3%, contact time 3 days, pH of 5, and room temperature. Results revealed that the percent removal of phenolic compounds was 79% and 80% for magnetite/zeolite and goethite/zeolite, respectively using the mixing approach, while with coating approach of magnetite/zeolite, the percentage removal was 75%. A significant improvement of % removal from 61 to 93% was observed with the new composite media of granular activated carbon (GAC) with various percentages to the (Fe3O4, FeO(OH)/zeolite) even at pH of 3.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Olive cultivation is one of the most exclusive cultivations in the Mediterranean basin. Jordan is one of the leading countries in olive oil production and it is considered an important cash crop representing an important source of income. In Jordan, olive trees cover around 131,000 hectares of its area, with about 17 million trees planted mainly in the northern region of Jordan, representing around 50% of the total number of trees in Jordan. Around 73% of olive trees are used in producing olive oil [1]. Different methods are used for the extraction of olive oil, such as pressing (traditional), centrifugation (continuous), three-phase decanter, and two-phase decanter [2, 3].
Since Jordan is among the top ten olive-producing countries in the world, it has more than 20 million olive trees and 130 olive mills located throughout the Kingdom. More than 90% of them are supplied with modern and full-automatic production lines with 272 lines and 378 tons/h production capacity [4, 5]. Seventy percent of the mills are located in Northern Jordan, whereas the rest are in the middle (22%) and the south (8%). However, olive oil production process usually yields next to olive oil (20%), semisolid waste (30%), and aqueous liquor (50%), which is olive mill wastewater (OMW) or zibar as named locally in Jordan [6].
OMW is characterized as acidic (pH in the range 3.5–5.5), dark-colored with strong olive smell, high fatty oil, high values of COD, BOD, COD/BOD ratio, phenolic compounds (0.5–24 g/L), and good electrical conductivity. OMW consists of a high amount of sugars, inorganic compounds, organic acids, and high hazardous organic compounds (polyphenols) [7,8,9,10]. These polyphenolic compounds and their derivatives have an impact on humans, plants, and animals, usually through direct exposure causing impairment of the nervous system, damaging the liver and kidney, and causing diarrhea [11]. Moreover, polyphenols impair the biodegradable activity of microorganisms, release phytotoxic compounds that affect the aquatic organisms, physiological processes in plants, and suppress soil microorganisms [2, 12, 13]
Hence, olive oil-producing countries face a crucial problem in finding environmental and economical solutions for handling OMW and preventing its pouring into valleys and water reservoirs. A huge amount of highly toxic liquid waste (ranging from 3 to 70 million m3) is produced seasonally (from October to December) worldwide [2] and 200 000 m3 are produced in Jordan [4, 5]. Therefore, it is important to treat this contaminated water before its discharge into public waterway systems.
Several technologies have been developed to improve the quality of OMW and sequester phenolic and organic compounds [14], including biological methods like the addition of anaerobic [15,16,17] or aerobic cultures [18,19,20], physicochemical [14, 21,22,23,24,25,26] and ozonation with electrocoagulation [27] in addition to chemical methods like using Fenton reagent [28, 29], surfactants [21, 23], and adsorption processes [6, 11, 30, 31]. Some techniques showed high effectiveness for wastewater treatment application due to their simplicity, no odor emissions, and ease of design but have some limitation such as high cost and toxicity [32]. Al-Bawab et al. have developed and characterized different cost-effective and eco-friendly media; for instance one of them consisting of oxidized granular activated carbon (GAC-OX) and non-oxidized granular activated carbon (GAC) impregnated with nonionic surfactants to adsorb phenolic compounds from OMW [24], others were devolped by using several smart media [25, 26].
Recently, nanotechnology has emerged as a fascinating technology for removing and purifying wastewater from various contaminations such as heavy metals, color, biological toxins, inorganic, organic solvents, and pathogens that cause diseases such as typhoid fever and cholera [33]. Moreover, the unique physicochemical properties of nanomaterials, in addition to their large surface-to-volume ratio, have made them powerful adsorbents for pollutants’ removal from wastewater [33,34,35,36]. Several researches have investigated the effect of different nanomaterials for its use in wastewater treatment, including activated carbon, carbon nanotubes, zinc oxide, titanium oxide, and several iron oxides such as magnetite (Fe3O4), maghemite (Ƴ-Fe2O3), hematite (ᾳ-Fe2O3), ferrihydrite, goethite, and lepidocrocite [37, 38] [21, 39, 40].
Several studies have investigated the effectiveness of iron oxides nanoparticles in wastewater treatment. For instance, Lakshmanan studied the application of magnetic nanoparticles with reactive filter materials to treat sewage wastewater. Magnetic nanoparticles were synthesized by microemulsion and conventional co-precipitation methods [41]. Nassar et al. used iron oxide nanoparticles as an adsorbent to treat OMW rapidly due to the high surface area of the nanoparticles, which provides more active sites for the adsorption process. The results indicated successful removal of the COD and phenolic compounds [34]. Also, Sharma et al. have used iron oxide nanoparticles as an adsorbent for arsenic removal from wastewater [42]. At the same time, Elfeky et al. used Fe3O4 nanoparticles composite to remove the Cr(VI) from wastewater at strongly pH-dependent. Overall, it can be concluded that the Fe3O4 has high efficiency in the remediation of wastewater with the advantage of low-cost and high applicability easy collection from the Cr (VI) contaminated wastewater [43].
In another study, Das et al. used green syntheses of two natural products coated by magnetite, which effectively removes the content of wastewater such as the organic materials and toxic metal ions. These magnetite nanoparticles can work as an antioxidant and antibacterial agent [44].
Iron oxides, including oxides (magnetite) and oxy-hydroxides (goethite), in nanoscale have exhibited great potential for many technological applications due to their crystalline nanostructure, morphological and magnetic properties [39, 45]. Goethite (ᾳ-FeO(OH)) is a red-brownish color that exhibits an orthorhombic symmetry with a specific surface area of 8–200 m2/g. Goethite is a candidate for applications in water purification to remove toxic ions [39, 46]. Magnetite (Fe3O4) is a black ferromagnetic mineral that has two iron cations; Fe(II) and Fe(III). The crystalline structure forms include octahedron and rhombodecahedron and have a specific surface area of 4–100 m2/g [39, 46]. Utilization of magnetite and goethite nanoparticles to wastewater treatment design can lead to easiness in separation the waste from the medium using simple magnetic process, resulting in efficient, economical, scalable, and nontoxic techniques [45, 47].
Different routes and methods have been used to prepare nanoparticles, these include hydrothermal [48, 49], microwave, laser ablation, sol–gel, electro-deposition, co-precipitation, and the microemulsion methods [39, 50,51,52]. Among these, microemulsion method is considered a recent method for nanoparticles’ preparation. A microemulsion system is a heterogeneous mixture containing surfactant, oil, and water mixed to form small enough droplets of an immiscible phase (non-polar or polar) dispersed in another phase (continuous phase). Surfactants and co-surfactants are added to lower interfacial tension between the two phases (immiscible and continuous phases) to stabilize these droplets leading to microemulsions being thermodynamically stable [50, 53, 54].
According to the nature of dispersed phases “Bancroft rule,” two types of microemulsions are (O/W) oil-in-water (micelle) and (W/O) water-in-oil (inverse micelle). Inverse micelle can be employed to control the shape and size of iron oxides nanoparticles. This also regulates the particle size distribution by controlling the amount of oil/water/surfactant mixture [50]. Geng et al. reported the synthesis of goethite nanoparticles by microemulsion method using copolymer [55]. Chin and Yacob synthesized magnetic iron oxide nanoparticles by inverse micelle microemulsion. Magnetite nanoparticles were prepared by the W/O microemulsion method using several surfactants (SDS, Brij 30, DTAB, and CTAB) [56]. Results showed that the structure of the surfactant plays a role in regulating the nanoparticles’ microstructure [50]. Asab et al. prepared Fe3O4 nanoparticles via microemulsion method using Tween-80 and SDS surfactants at varying temperatures and precursor concentrations. Thus, the microemulsion method was an effective method for getting controllable size nanoparticles [57]. However, this method suffers from two disadvantages: the use of large amounts of water in the washing step to get rid of excess surfactant and the low yield of nanoparticles [39].
Magnetite nanoparticles can be coupled with activated carbon, clay, or zeolite to enhance their adsorption capacity. For instance, Fe3O4/bentonite nanocomposite was prepared by the chemical co-precipitation method and was used for the adsorption of methylene blue from aqueous solutions. The adsorption capacity of the nanocomposite increased by increasing the contact time and the initial solution pH; however, it decreased by increasing the mass of the adsorbent [58]. Synthetic or natural zeolites are widely used in wastewater treatment, waste gas treatment, and in agriculture. It is a promising candidate for nanoparticles support and hosting. Jahangirian et al. and Nyankson et al. synthesized zeolite/Fe3O4 nanocomposite directly by precipitation method in a one-step reaction [59, 60].
The goal of this research is to create a green and cost-effective media that can remove organic and phenolic compounds through the interaction of magnetic nanoparticles (magnetite and goethite) with different ratios of sorbent materials (zeolite) using mixing and coating approaches, and using (GAC) with various percentages of (Fe3O4, FeO(OH)/zeolite) that will provide highly effective, reusable, and environmentally friendly OMW treatment in a laboratory and controlled industrial scale. The percent removal of phenols was determined at various contact times between the media and OMW, different media to OMW percentages, different temperatures, and different pH levels.
2 Experimental
2.1 Reagents and materials
The following chemicals were used: iron(II) chloride tetrahydrate (BBC chemicals, Ghana), iron(II) sulfate heptahydrate (DaeJung Chemicals & Metals, Korea), iron(III) chloride hexahydrate (Fisher, Japan), hexanol (Scharlau, Spain), heptane (DaeJung Chemicals & Metals, Korea), methanol (MERCK, Germany), absolute ethanol (S&C UK), sodium hydroxide (Jinhuada, China), ammonia (25% NH3) (LABCHEM, USA), Natural zeolite (Al-Mafraq, Jordan), dimethylene-1,2-bis(dodecyl dimethyl ammonium bromide) (CTAB) (Scharlau, European Union), phenol (Acros, USA), potassium dihydrogen phosphate (Purum, Switzerland), potassium ferricyanide (Peking’s, China), di-potassium hydrogen phosphate (Riedel–de Haen, Germany), 4-amino antipyrine (Purum, Switzerland), ammonium hydroxide (Konover, USA), Calgon MRX-P granular activated carbon (GAC) was obtained from Calgon Carbon Corporation. In all experiments, distilled water was used.
2.1.1 OMW samples
OMW used in this study was collected from the sink of wastewater olive mill (Bilal plant-Jordan). It is a modern automated mill located in northwestern Jordan, in Balqa governorates (Mahis–Balqa). Upon reception, the OMW samples were filtrated to get rid of the total suspended solids (TSS) materials. The pH was adjusted to be in the range 2–3 by the addition of hydrochloric acid (2 M) to avoid phenolic compounds decomposition. The samples were stored in dark aspirators at 22 °C to avoid photodecomposition. The general characterizations of olive mill wastewater are summarized in Table 1 [61].
3 Preparation of nanoparticles
3.1 Preparation of nanoparticles and nanocomposites
3.1.1 Preparation of goethite nanoparticles
The synthesis process was performed by mixing two microemulsion systems with identical reagents but different in the aqueous phase-type; one containing metal ions and another containing precipitating agent. The first microemulsion solution contained metal salts of FeCl2.4H2O and Fe3Cl3.6H2O with an adjusted ratio of Fe3+/Fe2+ (2:1), 30% of water, 30% of CTAB, 40% hexanol. The second microemulsion contained 45% ammonia as precipitating agent dispersed in the CTAB/hexanol. The two microemulsions were mixed under stirring for 3:30 h at 27 °C to obtain the nanoparticles, then separated and dried in an oven at 40–50 °C; (the reaction was done under normal atmospheric pressure) [50].
3.1.2 Preparation of magnetite nanoparticles
Two grams of CTAB were dissolved in 20.0 mL of heptane and 12.0 mL of hexanol. The microemulsion was prepared by shaking the mixture for 20 min at 40 °C under argon atmosphere. Then 0.5 mL of 0.5 M of iron (II) sulfate was added to the microemulsion. After 2 min, 1.0 mL of 0.5 M of aqueous iron (III) chloride was also added when the color changed to brown. Another microemulsion containing the same oil and surfactant phase, but differs in containing 2.0 mL of ammonia (25% NH3) solution, was added dropwise with vigorous stirring at 70 °C. The magnetite was formed, and the color quickly turned black. The mixture was heated at 70 °C for 3 h. The product was left to standstill for 2 h before being washed with ethanol and water. Finally separated and dried in an oven at 50 °C [50].
3.1.3 Preparation of magnetic nanocomposites
Magnetic nanocomposites were prepared by addition of nanoparticles (goethite or magnetite) to natural zeolites. Two approaches were used as follows: mixing and coating.
3.1.4 Preparation of nanocomposite media (Fe3O4 or FeO(OH)/zeolite) using mixing
A mixture of 0.1 g natural zeolite, 100 mL distilled water, and 0.1 g of the nanoparticles (magnetite or goethite) was placed in a flask and stirred vigorously for 30 min. During stirring, 20 mL of 2.5 M of NaOH were added dropwise. The mixture was stirred for 30 min. Finally, the synthesized nanocomposite was filtered, washed with distilled water, and air-dried at room temperature.
3.1.5 Preparation of nanocomposite Fe3O4/zeolite media using coating
The coating method is considered environmentally friendly due to using non-hazardous solvents and low-cost raw materials. Four grams of zeolite were added to 40 mL of distilled water followed by stirring to make a homogenous suspension. After that, a solution of (FeCl3 and FeCl2) was added with a molar ratio of 2:1 then stirred for 30 min. The mixture was stirred vigorously and 20 mL of 2.5 M NaOH was slowly added and stirred for another 30 min. The synthesized product was filtered, washed with distilled water, and finally air-dried at room temperature [59].
3.1.6 Preparation of nanocomposite media (Fe3O4, FeO(OH)/zeolite via mixing) with GAC
Nanocomposite (Fe3O4, or FeO(OH)/zeolite) prepared via mixing was mixed with GAC at different ratios to study its influence on adsorption capacity towards phenolic compounds.
3.2 Adsorption of phenolic compounds on nanocomposite media
At room temperature (25 ± 2 °C), batch adsorption experiments were performed by soaking media of (Fe3O4 or FeO(OH)/zeolite) with OMW in a test tube. Sample mixtures were vigorously shaken on vortex for 2 min then left overnight with shaking. The suspension mixtures were separated by centrifugation. The clear supernatant was analyzed using UV/Visible Spectrophotometer (Direct photometric method).
In order to optimize the adsorption process, several factors were studied such as contact time, nanoparticles ratio in media, pH, and temperature using real OMW samples. The effect of contact time between OMW and the sorbent media was evaluated by analyzing phenolic compounds’ removal for up to four days. The phenolic compunds’ removal was also evaluated at two adsorbent to OMW ratios (3% and 5%) at optimum contact time. In addition, the method of adsorbent preparation (mixing or coating) was also evaluated. The effect of nanoparticles’ content in the adsorbent was evaluated by varying the nanoparticles’ ratio from 1.0 to 10%. Three temperatures using water bath (4, 25, and 37 °C) were also tested to determine the effect of temperature variation on phenols’ removal, whereas the pH effect on the removal was evaluated in the range 3 to 11 (noting that the pH of fresh OMW is 5, but it is usually adjusted to 3 by the addition of HCl to prevent degradation of OMW real samples upon storage). The effect of GAC ratio was also taken into consideration; several GAC to magnetite and goethite ratios were tested ranging from 20 to 50%.
4 Characterization methods and instruments
4.1 Characterization of nanoparticles
The morphological analysis of nanoparticles (magnetite and goethite) was performed by X-Ray Diffraction (XRD) with MoKà1 radiation to confirm their formation. Transmission electron microscopy (TEM) was used to study the morphology and determine particle size of the synthesized nanoparticles.
4.2 Characterization of treated OMW
A series of phenol standards (0.5, 1.0, 1.5, 2.0, and 2.5 ppm) were prepared to construct a calibration curve. The blank, standards, and samples were all treated the same way by the direct photometric method: ammonium hydroxide (0.5 M, 2.5 mL) was added, and the pH was immediately adjusted to 7.9 ± 0.1 using phosphate buffer; then, 4-amino antipyrine solution (1.0 mL) and potassium ferricyanide solution (1.0 mL) were added and mixed. After 15 min, the absorbance at 500 nm was determined.
5 Results and discussion
5.1 Preparation of nanoparticles by microemulsion method
Surfactants occupy an important role in nanoparticles’ synthesis by adsorbing to the surface of formed nanoparticles and lowering it is surface energy [60]. Magnetic nanoparticles (magnetite and goethite) were synthesized using water in oil microemulsion. For Fe3O4, the microemulsion solution contained heptane as the oil phase, hexanol as the co-surfactant, and N,N,N-Trimethylhexadecan-1-aminium bromide (CTAB) as the surfactant in the surfactant phase. FeO(OH) nanoparticles were also prepared using the same surfactant (CTAB) by mixing two microemulsion solutions where the aqueous phase contained a metal salt and the reducing phase contained a reducing agent. The surfactant structure affects the regulation of the microstructure of nanoparticles crystal such as headgroup charge, hydrophobic chain length, and head group size. Figure 1 shows the structure of CTAB, which is a cationic surfactant that has a long hydrophobic chain (16 carbon atoms). According to critical packing parameter theory, an increase of hydrophobic chain length leads to a bigger critical packing parameter, which favors the formation of reverse micelles, while the ammonium headgroup size of CTAB is more favorable for the formation of reverse micelles due to the small size [50]. Furthermore, CTAB is easy to handle and inexpensive. These magnetic particles were easily separated by a magnet, and the synthesis was efficient, and non-toxic materials were used.
5.2 Characterization
5.2.1 XRD and TEM characterization of magnetic materials
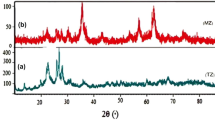
The XRD patterns of the two magnetic materials (magnetite Fe3O4, goethite FeO(OH)) are shown in Fig. 2. The diffractograms indicate the formation of goethite and cubic crystal iron oxide Fe3O4, with the main (hkl) Miller indices of goethite like; 311, 111, (220), (511), (422), (400), and (222) are clearly present in the pattern. Moreover, the (hkl) values for magnetite are indexed as (220), (311), and (400). Small amounts of impurity phases are observed in the goethite pattern which could not be indexed due to detection limits of the diffractometer. Confirmed by standard data, the intensity of samples’ peaks is relatively high which is an indication of high crystallinity [62].
Transmission Electron Microscope (TEM) images were used to characterize the prepared nanoparticles in relation to size and morphology. The TEM images showed dimensions in the nanoscale with an average crystalline size of magnetite and goethite around 7–16 nm and 5–14 nm, respectively as shown in Fig. 3. The TEM images for goethite showed acicular nanoparticles (Fig. 3A1) which are similar to standard goethite TEM image (Fig. 3A2). In addition, the shape of prepared magnetite nanoparticles (Fig. 3B1) was very similar to standard magnetite nanoparticles (Fig. 3B2). These results are comparable with that reported by in literature [62, 63].
5.2.2 Magnetization measurements of magnetic materials
The magnetic hysteresis loops for MZ mag mixing, MZ mag coating, and MZG mixing roasted at room temperature are shown in Fig. 4. It is obvious that the saturation magnetization of MZG Mixing is about three times larger compared with the saturation magnetization of both MZ mag samples. The magnetic loops of MZ mag samples have quite ferromagnetic characteristics, and the saturation value of the magnetization goes up to 0.6 emu/g at room temperature when the applied magnetic field is up to about 2000 mT. However, the goethite sample shows a trend of ferromagnetic characteristics up to about 2000 mT with a saturation trend starting at 2000 mT with a magnetization of 1.6 emu/g. We believe that, for all samples, further increasing the applied magnetic field will slightly increase the value of magnetization and it will be kept constant with a quite negligible hysteresis. Therefore, we could conclude that due to the relatively low magnetization of the sample, higher applied magnetic fields are needed in such experiments to see clearly the magnetization hysteresis curves.
5.2.3 Characterization of zeolitic tuff
The chemical composition of natural Jordanian zeolitic tuff that was collected from Tell Geis was determined using X-ray fluorescence spectroscopy (XRF) and the data are presented in Table 2.
6 Phenol determination (direct photometric method)
Since phenol is colorless, calibration curve construction based on the absorbance of phenol in the UV region (at λmax of 270 nm) is possible. However, it is hard to quanitatively determine phenol’s concentrations below 0.5 ppm. Alternatively, the calibration curve was constructed for the immediate determination of phenols, through the preparation of a series of phenol standard samples that reacted with 4-aminoantipyrine in presence of potassium ferricyanide K3Fe(CN)6, at pH (7.9 ± 0.1). The resultant mixture gave a reddish-brown color due to the antipyrine dye of the formed complex, and phenol was measured at λmax 500 nm; Fig. 5 shows the calibration curve; the concentration was determined photometrically using this calibration curve [64].
7 Application of media on real OMW samples
7.1 Nanocomposite media of (Fe 3 O 4 , FeO(OH)/zeolite) preparation
Natural zeolite is a cheap and abundant material in Jordan, whereas the prepared magnetic nanoparticles are chemically synthesized and on a small scale. For that, it is advantageous to couple the magnetic nanoparticles with natural zeolite as an adsorbent to enhance the efficiency of phenolic compounds’ adsorption and reduce the amount used of magnetic materials. Removal percentage of phenol was determined using Eqs. 1 and 2.
where [COMW]Pre is the phenolic concentration in OMW pre-treatment in ppm.
[COMW]Post is the phenolic concentration in OMW post-treatment in ppm.
The concentrations of phenolic compounds were determined using the calibration curve by spectrophotometric method.
7.2 Media evaluation
Zeolite was used as adsorption media coupled with the magnetic nanoparticles. The % removal of phenol was studied under various conditions. Tables 3 and 4 show that the % removal of zeolite coupled with magnetic nanoparticles is larger compared with zeolite alone as a control. The influence of pH on % removal was also evaluated (Table 4). Even though it has no considerable effect on zeolite alone (% removal was 48% and 50% at pH values of 3 and 5 respectively), it impacted the % removal when zeolite was coupled with magnetic particles. Higher pH values improved % removal by more than 20%. The pH of fresh OMW is around 5, which is suitable to obtain improved % removal of phenolic compounds using coupled media. However, when OMW is stored in the lab, the pH was adjusted to a range of 2–3 by using HCl to avoid phenolic compounds decomposition.
This study investigated the effect of % between media and raw OMW; 3% and 5% were tested. The results obtained that % removal of phenolic compounds was little increase with increase % of magnetite (Fe3O4) and goethite (FeO(OH))/Ziolite/Olive Mill Wastewater (media/OMW), recommended soaked is 3% to less consume materials.
The % removal of phenols was determined at different contact times between the media and OMW (Table 3). The media were soaked with OMW from (overnight to 1 week) with mixing at room temperature. The results showed % removal was increased within 3 days of soaking media with OMW. After more than 3 days, % removal was reduced, which could be attributed to phenolic compounds decomposition.
Different % of Nps in zeolite media were evaluated for % removal of phenols. The results are showed in Table 3. The removal % of phenolic compounds was not affected significantly with the increase of nanoparticles’ dosage in the sorbent media. An increase from 1 to 10% of nanoparticles’ ratio improved removal of phenols by less than 5%.
Results reveal that there is a small difference in % removal between the prepared media magnetite (Fe3O4) and goethite (FeO(OH))/zeolite, using mixing and coating approaches. Table 4 shows the % removal of phenol for each approach. The results indicate that the more basic the system is, the more favorable the adsorption on these media regardless of the desgin approaches used.
In order to study the pH effect on % removal, several experiments were conducted for zeolite coupled with magnetic nanoparticles media, the results are summarized in Table 5. The results showed that the optimum pH is 11. However, pH 5 (which is the pH of fresh OMW) gave good % removal of phenolic compounds; however, the pH is usually adjusted to 3.0 for OMW real samples in the lab prior to storage to reduce degration of these samples. These results show that immediate treatment of OMW with these nanocomposites media will give satisfactory results for % removal of phenolic compounds.
Temperature has an impact on adsorption processes in general, and usually higher temperatures lead to less adsorption which might have an impact on % removal of phenols. The temperature variations experienced during olive harvest season in Jordan (October–December) vary from rarely below 5 °C at night and rarely above 30 °C during day. It is essential to study the impact of such temperature variation on the removal process. The % removal of phenolic compounds was determined at three temperatures (4, 25, and 37 °C). The results (Table 6) indicate that the % removal of phenols using the nanocompsoites media used was not significantly affected with varying of temperature.
Several studies showed a favorable impact of GAC on adsorption. New media were prepared of (Fe3O4, FeO(OH)/zeolite) and various percentages of GAC (20, 30, 40, and 50%) and studied for their potential to enhance % removal capacity of phenolic compounds. The results are summarized in Table 7. The media used were MZG and MZ mag, 2.5% of (Nps/zeolite). A significant improvement of % removal was observed with these new composite media even at pH 3.
8 Conclusion
-
It can be concluded that there was no impact of mixing and coating approaches on % removal. In addition, the % removal was not altered significantly with increasing ratio of nanoparticles in the nanocomposite media (Nps/zeolite) and percent of (media/OMW). As for the equilibrium time, 3-day contact time was adequate. When more contact time was applied (more than 3 days), the adsorption process was reversible. While the % removal of phenolic compounds was increased with more basic OMW and vice versa, it was found that a pH of 11 was the optimum. In addition, adding GAC to the nanocomposite media enhanced the adsorption capacity of phenolic compounds. A temperature variation in the range 4–30 °C, which is the common temperature variation, had no significant effect on the effectiveness of phenolic compounds’ removal using the nanocomposite and new nanocomposite media that were under investigation in this study.
References
M. El-Hiary, B. Dhehibi, and S .Kassam. Market study and marketing strategy for olive and olive oil sector in the southern arid part of Jordan. Journal of agricultural science and technology: JAST. 21 (5) 1065–1077 (2019) .https://hdl.handle.net/20.500.11766/10665
S. Ayoub, K. Al-Absi, S. Al-Shdiefat, D. Al-Majali, D. Hijazean, Effect of olive mill wastewater land-spreading on soil properties, olive tree performance and oil quality. Sci. Hortic. 175, 160–166 (2014). https://doi.org/10.1016/j.scienta.2014.06.013
C. Scopetani et al., Olive oil-based method for the extraction, quantification and identification of microplastics in soil and compost samples. Sci. Total Environ. 733, 1–12 (2020). https://doi.org/10.1016/j.scitotenv.2020.139338
S. Ayoub, Management of olive by-products in Jordan. Ensuring sustainability of Jordanian olive sector conference. Amman, Jordan. 24–25 April (2017)
M. Halalsheh, G. Kassab, K. Shatanawi, Impact of legislation on olive mill wastewater management: Jordan as a case study. Water Policy. 23, 343–357 (2021). https://doi.org/10.2166/wp.2021.171
M. Niaounakis, and C.P. Halvadakis, Olive processing waste management literature review and patent survey, 2nd ed., UK: Elsevier, pp. 139–169 (2006).https://www.elsevier.com/books/olive-processing-waste management/niaounakis/978–0–08–044851–0
N. Adhoum, L. Monser, Decolourization and removal of phenolic compounds from olive mill wastewater by electrocoagulation. Chem. Eng. Process. 43, 1281–1287 (2004). https://doi.org/10.1016/j.cep.2003.12.001
I. Calabrese, G. Gelardi, M. Merli, G. Rytwo, L. Sciascia, and M. L. T. Liveri, New tailor-made bio-organoclays for the remediation of olive mill waste water, IOP Conference Series: Materials Science and Engineering. 47 (2013), doi: https://doi.org/10.1088/1757-899x/47/1/012040.
F. Hanafi, M. Mountadar, and O. Assobhei, Combined electrocoagulation and fungal processes for the treatment of olive mill wastewater. Fourteenth International Water Technology Conference (IWTC 14 2010). Cairo, Egypt, 269–281 (2010).
F.Z. El Hassani, A. Fadile, M. Faouzi, A. Zinedine, M. Merzouki, M. Benlemlih, The long term effect of Olive Mill Wastewater (OMW) on organic matter humification in a semi-arid soil. Heliyon. 6, e03181 (2020). https://doi.org/10.1016/j.heliyon.2020.e03181
I.A. Bello, M.A. Oladipo, A.A. Giwa, and D.O. Adeoye, Adsorptive removal of phenolics from wastewater—a review, International Journal of Basic & Applied Science. 2 79–90 (2013). https://www.ijobas.pelnus.ac.id/index.php/ijobas/hoj
J. Torrecilla, Phenolic compounds in olive oil mill wastewater, In Olives and Olive Oil in Health and Disease Prevention. V. R. Preedy, and R. R. Watson, Eds.; Academic Press, pp. 357–365 (2010), ISBN 9780123744203
G. Enaime, A. Bacaoui, A. Yaacoubi, M. Belaqziz, M. Wichern, and M. Lübken, Phytotoxicity assessment of olive mill wastewater treated by different technologies: effect on seed germination of maize and tomato, Environmental Science and Pollution Research. 27 (2020), doi: https://doi.org/10.1007/s11356-019-06672-z.
A. Al Bawab, N. Ghannam, S. Abu-Mallouh, A. Bozeya, R. A. Abu-Zurayk, Y. A. Al-Ajlouni, F. Odeh, and M. A. Abu-Dalo, Olive mill wastewater treatment in Jordan: a review. In IOP Conference Series: Materials Science and Engineering. 305 012002 (2018). https://iopscience.iop.org/article/https://doi.org/10.1088/1757-899X/305/1/012002
I. Arvanitoyannis, A. Kassaveti, S. Stefanatos, Olive oil waste treatment: a comparative and critical presentation of methods, advantages & disadvantages. Crit. Rev. Food Sci. Nutr. 47, 187–229 (2007). https://doi.org/10.1080/10408390600695300
T.H. Ergüder, E. Güven, G.N. Demirer, Anaerobic treatment of olive mill wastes in batch reactors. Process Biochem. 36, 243–248 (2000). https://doi.org/10.1016/S0032-9592(00)00205-3
A.I. Vavouraki, M.A. Dareioti, M. Kornaros, Olive mill wastewater (OMW) polyphenols adsorption onto polymeric resins: part I—batch anaerobic digestion of OMW. Waste and Biomass Valorization. 12, 2271–2281 (2021). https://doi.org/10.1007/s12649-020-01168-1
M.I. Massadeh, N. Modallal, Ethanol production from olive mill wastewater (OMW) pretreated with Pleurotus sajor-caju. Energy Fuels. 22, 150–154 (2008). https://doi.org/10.1021/ef7004145
A. Fraij, M. Massadeh, Use of pleurotus sajor-caju for the biotreatment of olive mill wastewater. Romanian Biotechnological Letters. 20, 10611–10617 (2015)
Y. Jaouad, M. Villain-Gambier, L. Mandi, B. Marrot, N. Ouazzani, Comparison of aerobic processes for olive mill wastewater treatment. Water Sci. Technol. 81, 1914–1926 (2020). https://doi.org/10.2166/wst.2020.247
A. Al-Bawab, F. Alshawawreh, M. A. Abu-Dalo, N. A. Al-Rawashdeh, and A. Bozeya, Separation of soluble phenolic compounds from olive mill wastewater (OMW) using modified surfactant. Fresenius Environmental Bulletin, 26 1949–1958 (2017).https://www.prt-parlar.de/download_feb_2017/
G. Chen, A.S. Mujumdar, Application of electrical fields in dewatering and drying. Dev. Chem. Eng. Miner. Process. 10, 429–441 (2002). https://doi.org/10.1002/apj.5500100413
F. Odeh, A. Al Bawab, M. Fayyad, and A. Bozeya, Surfactant enhanced olive oil mill wastewater remediation, APCBEE Procedia. 5 96–101 (2013), doi: https://doi.org/10.1016/j.apcbee.2013.05.017.
A. Al-Bawab, N. Ghannam, R. A. Abu-Zurayk, F. Odeh, A. Bozeya, S. Abu-Mallouh, N. A. Al-Rawashdeh, and M. A. Abu-Dalo, Olive mill wastewater remediation by granular activated carbon impregnated with active materials. Fresenius Environmental Bulletin. 27 2118–2126 (2018). https://www.prt-parlar.de/download_feb_2017/
M. Abu Dalo, J. Abdelnabi, and A. Al Bawab, Preparation of activated carbon derived from Jordanian olive cake then functionalization with copper oxide for adsorption of phenolic compounds from olive mill wastewater” Materials. 14. 21, 6636–6655. (2021) DOI: https://doi.org/10.3390/ma14216636
M. Abu Dalo , J. Abd elnabi, N. Al Rawashdeh , B. Albiss , and A. Al Bawab, “Coupling coagulation-flocculation to volcanic tuff-magnetite nanoparticles adsorption for olive mill wastewater treatment, Environmental Nanotechnology, Monitoring & Management. 17. (2021) https://doi.org/10.1016/j.enmm.2021.100626
M. Khani et al., Olive mill wastewater (OMW) Treatment by hybrid processes of electrocoagulation/catalytic ozonation and biodegradation. Environ. Eng. Manag. J. 19, 1401–1410 (2020)
F. Doosti, R. Ghanbari, H. A. Jamali, and H. Karyab, Optimizing fenton process for olive mill wastewater treatment using response surface methodology, Fresenius Environmental Bulletin. 26 5942–5953 (2017). https://www.prt-parlar.de/download_feb_2017/
E. Domingues, E. Fernandes, J. Gomes, S. Castro-Silva, R.C. Martins, Olive oil extraction industry wastewater treatment by coagulation and Fenton’s process. Journal of Water Process Engineering. 39, 101818 (2021). https://doi.org/10.1016/j.jwpe.2020.101818
M.O. Azzam, S. Al-Gharabli, M. Al-Harahsheh, Olive mills wastewater treatment using local natural Jordanian clay. Desalin. Water Treat. 53, 627–636 (2013). https://doi.org/10.1080/19443994.2013.846232
N. Solomakou, A.M. Goula, Treatment of olive mill wastewater by adsorption of phenolic compounds. Reviews in Environmental Science and Bio/Technology. 20, 839–863 (2021). https://doi.org/10.1007/s11157-021-09585-x
Q. Xie, J. Xie, Z. Wang, D. Wu, Z. Zhang, H. Kong, Adsorption of organic pollutants by surfactant modified zeolite as controlled by surfactant chain length. Microporous Mesoporous Mater. 179, 144–150 (2013). https://doi.org/10.1016/j.micromeso.2013.05.027
K. Jain, A.S. Patel, V.P. Pardhi, S.J.S. Flora, Nanotechnology in wastewater management: a new paradigm towards wastewater treatment. Molecules 26, 1797 (2021). https://doi.org/10.3390/molecules26061797
N. N. Nassar, L. A. Arar, N. N. Marei, M. M. Abu Ghanim, M. S. Dwekat, and S. H. Sawalha, Treatment of olive mill based wastewater by means of magnetic nanoparticles: decolourization, dephenolization and COD removal, Environmental Nanotechnology, Monitoring & Management. 1–2 14–23 (2014), doi: https://doi.org/10.1016/j.enmm.2014.09.001.
P. Hariani, M. Faizal, R. Syarofi, M. Marsi, D. Setiabudidaya, synthesis and properties of Fe3O4 nanoparticles by co-precipitation method to removal procion dye. International Journal of Environmental Science and Development. 4, 336–340 (2013). https://doi.org/10.7763/IJESD.2013.V4.366
A. Al-Bsoul et al., Optimal conditions for olive mill wastewater treatment using ultrasound and advanced oxidation processes. Sci. Total Environ. 700, 134576 (2020). https://doi.org/10.1016/j.scitotenv.2019.134576
M. Anjum, R. Miandad, M. Waqas, F. Gehany, M.A. Barakat, Remediation of wastewater using various nano-materials. Arab. J. Chem. 12, 4897–4919 (2019). https://doi.org/10.1016/j.arabjc.2016.10.004
A. Al Bsoul, M. Hailat, A. Abdelhay, M. Tawalbeh, I. Jum’h, and K. Bani-Melhem, Treatment of olive mill effluent by adsorption on titanium oxide nanoparticles, Science of The Total Environment. 688 1327–1334 (2019), doi: https://doi.org/10.1016/j.scitotenv.2019.06.381.
M. Mohapatra and S. Anand, Synthesis and applications of nano-structured iron oxides/hydroxides - A review, International Journal of Engineering, Science and Technology. 2 127–146 (2010). eISSN: 2141–2839
M. Al-Shaweesh, M. Mohammed, D. Al-Kabariti, D. Khamash, S. Al-Zawaidah, M. Hindiyeh, W. Omar, Olive mill wastewater (OMW) treatment by using ferric oxide dephenolization and chemical oxygen demand removal, Global NEST Journal. 20 558–563 (2018) https://journal.gnest.org/publication/gnest_02650
R. Lakshmanan, Application of magnetic nanoparticles and reactive filter materials for wastewater treatment. PhD Thesis, Royal Institute of Technology, Albanova university center, Stockholm, (2013). ISBN: 978–91–7501–948–2 TRITA-BIO REPORT 2013:20 ISSN: 1654–2312
A. Vilela, F. Cosme, and T. Pinto, Emulsions, foams, and suspensions: the microscience of the beverage industry, Beverages. 4 (2018), doi: https://doi.org/10.3390/beverages4020025.
S.A. Elfeky, S.E. Mahmoud, A.F. Youssef, Applications of CTAB modified magnetic nanoparticles for removal of chromium (VI) from contaminated water. J. Adv. Res. 8, 435–443 (2017). https://doi.org/10.1016/j.jare.2017.06.002
C. Das et al., Green Synthesis, Characterization and application of natural product coated magnetite nanoparticles for wastewater treatment. Nanomaterials 10, 1615 (2020). https://doi.org/10.3390/nano10081615
S. Cheriyamundath, S.L. Vavilala, Nanotechnology-based wastewater treatment. Water and Environment Journal. 35, 123–132 (2021). https://doi.org/10.1111/wej.12610
R.M. Cornell, and U. Schwertmann, The iron oxides: structure, properties reactions occurrence and uses. 2nd, completely revised and extended edition, Weinheim and New York (VCH Verlagsgeseiischaft mbH). 1996, xxxi 573 pp. ISBN 3–527–28576–8.
B. Nowack, T.D. Bucheli, Occurrence, behavior and effects of nanoparticles in the environment. Environ. Pollut. 150, 5–22 (2007). https://doi.org/10.1016/j.envpol.2007.06.006
R. Fan, X.H. Chen, Z. Gui, L. Liu, Z.Y. Chen, A new simple hydrothermal preparation of nanocrystalline magnetite Fe3O4. Mater. Res. Bull. 36, 497–502 (2001). https://doi.org/10.1016/S0025-5408(01)00527-X
A. Benhammada, D. Trache, M. Kesraoui, S. Chelouche, Hydrothermal synthesis of hematite nanoparticles decorated on carbon mesospheres and their synergetic action on the thermal decomposition of nitrocellulose. Nanomaterials 10(5), 968–988 (2020). https://doi.org/10.3390/nano10050968
T. Lu, J. Wang, J. Yin, A. Wang, X. Wang, T. Zhang, Surfactant effects on the microstructures of Fe3O4 nanoparticles synthesized by microemulsion method. Colloids Surf., A 436, 675–683 (2013). https://doi.org/10.1016/j.colsurfa.2013.08.004
Z. Zhou, X. Liu, H. Chan, Synthesis of Fe3O4 nanoparticles from emulsions. Journal of Materials Chemistry - J MATER CHEM. 11, 1704–1709 (2001). https://doi.org/10.1039/b100758k
E.Y. Shaba, J.O. Jacob, J.O. Tijani, M.A.T. Suleiman, A critical review of synthesis parameters affecting the properties of zinc oxide nanoparticle and its application in wastewater treatment. Appl Water Sci 11, 48–89 (2021). https://doi.org/10.1007/s13201-021-01370-z
R. Dhanapal, A review – Microemulsion. Asian J Pharm Res. 2, 23–29 (2012)
M.A. Yousuf, S. Jabeen, M.N. Shahi, M.A. Khan, I. Shakir, M.F. Warsi, Magnetic and electrical properties of yttrium substituted manganese ferrite nanoparticles prepared via micro-emulsion route. Results in Physics. 16, 102973–102979 (2020). https://doi.org/10.1016/j.rinp.2020.102973
F. Geng, Z. Zhao, H. Cong, J. Geng, H.-M. Cheng, An environment-friendly microemulsion approach to α-FeOOH nanorods at room temperature. Materials Research Bulletin - MATER RES BULL. 41, 2238–2243 (2006). https://doi.org/10.1016/j.materresbull.2006.04.030
A.B. Chin, I.I. Yaacob, Synthesis and characterization of magnetic iron oxide nanoparticles via w/o microemulsion and Massart’s procedure. J. Mater. Process. Technol. 191, 235–237 (2007). https://doi.org/10.1016/j.jmatprotec.2007.03.011
characterization and evaluation of antimicrobial activity, G. Asab, E. A. Zereffa, and T. Abdo Seghne, Synthesis of silica-coated Fe3O4 nanoparticles by microemulsion method. International Journal of Biomaterials. 2020, 1–12 (2020). https://doi.org/10.1155/2020/4783612
F. Hashem, Adsorption of methylene blue from aqueous solutions using Fe3O4 / bentonite nanocomposite. Hydrology Current Research. 3, 1–6 (2012). https://doi.org/10.4172/2157-7587.1000143
H. Jahangirian, M. H. S. Ismail, M. J. Haron, R. Rafiee-Moghaddam, K. Shameli, S. Hosseini, K. Kalantari, R. Khandanlou, E. Gharibshahi, and S. Soltaninejad, Synthesis and characterization of zeolite/Fe3O4 nanocomposite by green quick precipitation method. Digest Journal of Nanomaterials and Biostructures, 8 1405–1413 (2013). https://chalcogen.ro/index.php/journals/digest-journal-of-nanomaterials-and-biostructures/8-djnb/33-volume-8-number-4-october-december-2013
E. Nyankson et al., Characterization and Evaluation of Zeolite A/Fe3O4 Nanocomposite as a potential adsorbent for removal of organic molecules from wastewater. J. Chem. 2019, 1–13 (2019). https://doi.org/10.1155/2019/8090756
E.S. Aktas, S. Imre, L. Ersoy, Characterization and lime treatment of olive mill wastewater. Water Res. 35, 2336–2340 (2001). https://doi.org/10.1016/S0043-1354(00)00490-5
C. Blanco-Andujar, D. Ortega, Q.A. Pankhurst, N.T.K. Thanh, Elucidating the morphological and structural evolution of iron oxide nanoparticles formed by sodium carbonate in aqueous medium. J. Mater. Chem. 22, 12498–12506 (2012). https://doi.org/10.1039/C2JM31295F
G. Mustafa, B. Singh, and R. Kookana, Cadmium desorption from goethite in the presence of desferrioxamine B and oxalic acid, 3rd Australian New Zealand Soils Conference, 5 – 9 December 2004, University of Sydney, Australia. Published on CDROM. www.regional.org.au/au/asssi/, 8 pp (2004) http://www.regional.org.au/au/asssi/supersoil2004/s3/oral/1424_mustafag.htm
S. Lenore, Standard Method for the Examination of water and Wastewater (American Public Health Association, USA, 1998)
S.K. Mehta, S. Kumar, S. Chaudhary, K.K. Bhasin, Effect of cationic surfactant head groups on synthesis, growth and agglomeration behavior of ZnS nanoparticles. Nanoscale Res. Lett. 4, 1197–1208 (2009). https://doi.org/10.1007/s11671-009-9377-8
Funding
This research was funded by the Deanship of Academic Research (DAR) at The University of Jordan (UJ) (financial support project No. 1950 /2019). This work has been carried out during sabbatical leave granted to the author Abeer Al Bawab from the University of Jordan during the academic year (2017/2018).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Odeh, F., Abu-Dalo, M., Albiss, B. et al. Coupling magnetite and goethite nanoparticles with sorbent materials for olive mill wastewater remediation. emergent mater. 5, 77–88 (2022). https://doi.org/10.1007/s42247-022-00378-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42247-022-00378-8